Back to Journals » Infection and Drug Resistance » Volume 16
A Hospital-Based and Cross-Sectional Investigation on Clinical Characteristics of Pediatric Streptococcus pneumoniae Isolates in Beijing from 2015 to 2021
Authors Lyu Z, Li J, Zhen J, Shi W, Meng Q, Zhou W, An J, Yao K, Dong F
Received 30 November 2022
Accepted for publication 14 January 2023
Published 26 January 2023 Volume 2023:16 Pages 499—508
DOI https://doi.org/10.2147/IDR.S398549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Zhiyong Lyu,1 Jing Li,2 Jinghui Zhen,1 Wei Shi,3 Qingying Meng,1 Wei Zhou,1 Jingyun An,1 Kaihu Yao,3,* Fang Dong1,*
1Department of Clinical Laboratory Center, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China; 2Department of Clinical Laboratory, Capital Institute of Pediatrics, Beijing, People’s Republic of China; 3Beijing Key Laboratory of Pediatric Respiratory Infection Diseases, Key Laboratory of Major Diseases in Children, Ministry of Education, National Clinical Research Center for Respiratory Diseases, National Key Discipline of Pediatrics (Capital Medical University), Beijing Pediatric Research Institute, Beijing Children’s Hospital, Capital Medical University, National Center for Children’s Health, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fang Dong; Kaihu Yao, Department of Clinical Laboratory Center, Beijing Children’s Hospital Affiliated to Capital Medical University, No. 56 Nan Lishi Road, Beijing, 100045, People’s Republic of China, Email [email protected]; [email protected]
Introduction: Streptococcus pneumoniae (S. pneumoniae) is a major pathogen causing death in children. Few studies have evaluated the importance of S. pneumoniae in the identified bacteria in clinical work. This retrospective study aimed to reveal the rank of S. pneumoniae in determined bacteria isolated from children in Beijing, China, as well as the antimicrobial resistance of this pathogen.
Methods: The number of specimen for bacterial culture and of bacterial species were cumulated and ranked based on the data of the two largest children’s hospitals in Beijing from 2015 to 2021. The temporal change of S. pneumoniae culture, as well as the clinical data of S. pneumoniae isolates were collected and analyzed. The minimum inhibitory concentrations of antimicrobial agents were determined by BD Phoenix 100 automated system or Vitek 2 automated system for antimicrobial susceptibility testing. The breakpoints recommended by CLSI were adopted.
Results: During the 7-year study period, a total of 45,631 bacterial isolates were cultured from 462,144 submitted specimens, in which S. pneumoniae was the third frequent agent following S. aureus and H. influenza, and accounting for 8.79% of the isolates (4011/45,631). In the 4011 S. pneumoniae isolates, 2239 and 997 ones were, respectively, isolated from sputum and bronchial lavage fluid. Most of S. pneumoniae strains were identified in winter (34.7%) and spring (26.1%), and were mainly isolated from patients under 5 years old (77.1%). Low susceptible rate (27.6%) of CSF isolates was determined to penicillin according to the parenteral meningitis breakpoints, while high susceptible rate (56.9%) of non-CSF isolates was obtained according to the parenteral non-meningitis breakpoints. The isolates showed low sensitivity to erythromycin and tetracycline (< 5%). All isolates were susceptible to vancomycin and linezolid.
Conclusion: The present results demonstrated that S. pneumoniae was one of the most commonly detected bacteria in current pediatric clinical tests, especially in young children under 5 years old, which emphasized the importance of prevention. Penicillin could still be the first empiric choice to treat non-meningitis pneumococcal infections, while erythromycin should not be involved in the treatment.
Keywords: Streptococcus pneumoniae, antimicrobial resistance, children, China
Introduction
S. pneumoniae is an opportunistic Gram-positive pathogen, normally colonizing in the nasopharynx, which can commonly infect young children, immune-deficient patients, and the elderly, responsible for millions of deaths worldwide. The carriage rate of S. pneumoniae in children is 27% in developed countries and 85% in developing countries.1 In common circumstances, it does not cause diseases, however, when the immunity is compromised, it can invade other parts or tissues and cause infections, including community acquired pneumonia, otitis media, sinusitis, bacteremia, osteomyelitis, meningitis and other diseases.2–4
Antibiotic administration is the main measure to treat S. pneumoniae infections. However, along with the continuous renewal of antibiotics and the unreasonable application of antimicrobial agents, S. pneumoniae strains resistant to antibiotics, especially multi-drug resistant strains continue to emerge and spread under the increased selection pressure. Penicillin has been used clinically to treat S. pneumoniae infections for more than half a century. However, since discovery of the first penicillin resistant S. pneumoniae strain in Australia in 1967, erythromycin, tetracycline, sulfamethoxazole, β-lactam and other antibiotic resistant strains are increasing, and the phenomenon of multiple drug resistance is becoming more and more serious.5–7
There is no national prevalence data of S. pneumoniae infection in China based on population investigation. However, S. pneumoniae culture has already been a part of regular work in clinical laboratories mainly for inpatient diagnosis. In the present study, the data of bacterial culture and some clinical information of S. pneumoniae isolates were collected from two clinical laboratories that belonged to the two biggest tertiary children’s hospitals in Beijing, from 2015 to 2021. The aim of this study was to evaluate the importance of S. pneumoniae in the current detectable bacteria, and the antibiotic resistance profiles of S. pneumoniae isolates in Beijing, China.
Materials and Methods
Study Sites
The present data were collected form two study sites. One is Beijing Children’s Hospital affiliated to Capital Medical university, the other is Children’s Hospital Capital Institute Pediatrics. Both of the two tertiary hospitals were ranked in top of all the domestic children’s hospitals with the most pediatric patients visiting in Beijing. Beijing Children’s Hospital was National Center for Children’s Health, China, with more than 3 million outpatients and 80,000 inpatients per year. Meanwhile, 2.3 million outpatients and 30,000 hospitalized children were treated at the Capital Institute of Pediatrics. The clinical information and laboratory examination results of the children were collected and analyzed retrospectively from the electronic information system.
Species Culture and Identification
Bacterial isolation and identification were performed in the two clinical laboratories of the hospitals. We collected the clinical information of the tested samples and bacterial identification results through the electronic recording system. In accordance with the National Guide to Clinical Laboratory Procedures, clinical specimens were inoculated into Columbia blood agar plate and incubated at 37°C and 5% CO2 for 24 hours. Sterile samples such as blood were injected into the blood culture bottle and cultured in BD BACTEC FX blood culture instrument, if the alarm was positive, the cultures were transferred to Columbia blood agar plate and incubated at 37°C and 5% CO2 for 24 hours. Optochin sensitivity test was used to identify the pneumococcal strains. All the Optochin sensitivity positive strains with an inhibition zone diameter of ≥ 14 mm were confirmed by MALDI-TOF MS (bio Mérieux, France) instrument.
The pneumococcal isolates analyzed in this study were collected from clinical laboratories. Our study was a retrospective study based on the clinical samples, which were part of the routine hospital laboratory procedures, and all the clinical samples in this study were not specifically isolated for this research. The Ethics Committees of Beijing Children’s Hospital affiliated to Capital Medical University and Children’s Hospital Capital institute Pediatrics exempted this study from review.
Antimicrobial Susceptibility Testing
Laboratories of the two hospitals utilized different systems to test the antimicrobial susceptibility of S. pneumoniae isolates, both based on detection of the minimum inhibitory concentrations (MICs). In Beijing Children’s Hospital, BD Phoenix 100 automated system was implemented to determine MICs of 2971 isolates. Meanwhile, Children’s Hospital Capital Institute used Vitek 2 automated system to measure MICs of 1040 strains. E-test stripes were used to reconfirm the results of penicillin, cefotaxime, and meropenem. Antibiotic susceptibility testing results were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (M100 29th edition). S. pneumoniae ATCC 49619 was included as a quality control strain in the whole testing process.
Statistical Analysis
Data were managed and analyzed with SPSS software v17.0 (SPSS Inc., Chicago, Illinois, USA). Antibiotic susceptibility results were analyzed using WHONET 5.6 software. Results were expressed as number of the patients or bacteria isolates, as well as proportion of the total number. Chi-square test or Fisher’s exact test were used to evaluate the differences between categorical variables. Difference among antibiotic susceptibility over time was determined using a chi-square test for trend. P value was calculated and considered statistically significant only when it was less than 0.05.
Results
Rank of S. pneumoniae in All the Isolated Bacteria Strains
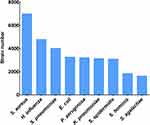
During the 7-year study period from 2015 to 2021, a total of 462,144 specimens were collected, among which 45,631 bacteria strains were identified. The top two common pathogens were S. aureus and H. influenza, accounting for 15.34% (7000/45,631) and 10.50% (4791/45,631), respectively. S. pneumoniae was the third frequent bacterium, accounting for 8.79% (4011/45,631) of the whole isolated strains (Figure 1).
Yearly and Seasonal Distribution
Among the 462,144 specimens, for which regular bacterial cultures were needed, we isolated 4011 S. pneumoniae strains, amounting to a positive rate of 0.87%. As shown in Table 1, the positive rate of S. pneumoniae strains was higher in the first four years, between 2015 and 2018 (P<0.05). The lowest positive rate occurred in 2020 (0.46%, 199/43,240) (P<0.05). In our seven-year study, the number of isolated S. pneumoniae strains was abundant and relatively stable from 2015 to 2018, around 700 to 750 per year. This annual number slightly decreased to 562 in 2019. However, the number of isolated strains sharply declined to 199 in 2020, followed by a small increase to 353 in 2021. In general, the year of 2017 (n=741) and 2020 (n=199) accounted for the highest and the lowest number of isolated S. pneumoniae strains, respectively. In terms of seasonal distribution, most S. pneumoniae strains were identified in winter (during December to February, n=1394, 34.7%) and spring (during March to May, n=1045, 26.1%), with the lowest number isolated from summer (during June to August, n=727, 18.1%).
 |
Table 1 Distribution of Collecting Year/Season and Specimen Types of the Isolated Strains |
Specimen Type Distribution and Positive Rate
Among the 462,144 specimens, the most common specimen type was venous blood (n=309,756, 67.2%), followed by sputum (n=54,287, 11.7%), cerebrospinal fluid (n=45,560, 9.9%) and bronchial lavage fluid (n=30,025, 6.5%). Among all the 4011 Streptococcus pneumoniae isolates, strains from respiratory tract were mainly collected from sputum (n=2239, 55.8%) and bronchial lavage fluid (n=997, 24.9%) (Table 1). The invasive pneumococcal disease (IPD)-causing strains comprised 7.2% (n=287) of the total isolates, which were isolated from venous blood (n=204), cerebrospinal fluid (n=58) and pleural effusion (n=20), joint fluid (n=3) and bone marrow (n=2). The positive rate of Streptococcus pneumoniae strains in ear secretion was the highest (9.01%, 77/854) (P<0.05), followed by nasopharynx swab (6.15%, 49/797), fester (5.24%, 191/3647), sputum (4.12%, 2239/54,287), eye secretion (3.45%, 26/754) and bronchial lavage fluid (3.32%, 997/30,025).
Characteristics of Patients with S. pneumoniae Infections
In this retrospective surveillance study, 4011 non-duplicated S. pneumoniae isolates were collected from the two tertiary hospitals from Jan 2015 to Dec 2021, with 74.1% (n=2971) of the isolates from Beijing Children’s Hospital affiliated to Capital Medical University. The number of male patients (59.3%, n=2378) was higher than the number of female patients (40.7%, n=1633). Among the total isolates, 77.1% were isolated from children under 5 years old (n=3091). With the increase of age, the number of children patients decreased gradually (Table 2). In our study, most of the strains were isolated from hospitalized patients (90.0%, n=3611), especially from pneumology department (43.8%, n=1757), Intensive Care Unit (9.9%, n=397), department of cardiology (9.8%, n=394), and general medicine department (8.3%, n=331).
 |
Table 2 Clinical Information of Children with the Isolated S. pneumoniae Strains |
Antimicrobial Susceptibility Testing of S. pneumoniae Isolates
The antimicrobial susceptibility results of the 4011 pneumococcal isolates to the tested antibiotics are shown in Table 3. According to the CLSI susceptibility breakpoints, all of the isolates were susceptible to vancomycin and linezolid. 27.6% (16/58) of the meningitis isolates and 56.9% (2251/3953) of the non-meningitis isolates were susceptible to penicillin. At the same time, the susceptible rates of meningitis strains and non-meningitis strains to cefotaxime, meropenem and trimethoprim-sulfamethoxazole were 36.2% vs 69.3%, 39.7% vs 33.7% and 29.3% vs 20.2%. On the whole, 56.5% and 68.8% of total isolates were susceptible to penicillin and cefotaxime, respectively. However, only 33.8% of the strains were susceptible to meropenem. Most of the isolates were sensitive to moxifloxacin (99.1%), levofloxacin (90.0%) and chloramphenicol (93.6%). In contrast, only a small part of the strains were sensitive to erythromycin (0.4%) and tetracycline (4.7%). Among non-meningitis strains, the prevalence of penicillin-susceptible, cefotaxime-susceptible, meropenem-susceptible and trimethoprim-sulfamethoxazole-susceptible strains exhibited an increasing trend across the seven years (P<0.05) (Figure 2).
 |
Table 3 Susceptibility of S. pneumoniae Isolates to Antimicrobial Agents (%) |
Discussion
The present 7-year retrospective study revealed that S. pneumoniae ranked third among all the isolated bacterial strains, following the S. aureus and H. influenza. In China, surveillance data from the ISPED program demonstrated that S. pneumoniae was one of the top three pathogens causing infections in children, from 2016 to 2020.8 In all-aged patients with acute respiratory infections, S. pneumoniae was the most prevalent bacterial pathogen in China, from 2009 to 2019.9 All these studies demonstrated that S. pneumoniae played an important role in bacterial isolates from pediatric patients in China.
These results showed that the positive rate of S. pneumoniae in the whole specimens was 0.87% in children patients, which was higher than a previous study from Korea, in which the positive rate was 0.15% based on the whole populations including both pediatrics and adults.10 This phenomenon could indicate that children exhibit higher infection rate of S. pneumoniae than adults. In all the specimen types, ear secretion which accounted for 0.18% of the total samples presented the highest positive rate (9.01%). Although the positive rate of ear secretion was higher than other sample types in the present study, it was much lower than previous reports,11,12 which demonstrated a positive rate of 26–36% in ear secretion samples. Furthermore, we noticed that the number of ear secretion specimen was relatively less than other specimen types, only 854 in 7 years. Because the acute otitis media (AOM) infection was often treated empirically and directly by antibiotics without performing further bacteria culture in outpatients, this result could indicate the common low vigilance in outpatient diagnosis. Therefore, the pathogen test should be further emphasized for outpatients in children, especially based on the current finding of high positivity of S. pneumoniae culture for AOM.
The proportion of pneumococcal isolates in children under 5 years old accounted for 77.1%, which was consistent with the reports concluded from data in developing countries by WHO,13 but it was relatively lower than the data (85%) reported in a multicenter retrospective study in mainland China.14 With development and maturation of the immune system, the positive rate of S. pneumoniae infection in children over 5 years old decreased concomitantly.15 Non-invasive strains accounted for 92.8% of the isolated strains, and the samples were mainly collected from sputum (55.8%) and bronchial lavage fluid (24.9%). Although the proportion of invasive strains was relatively low (7.2%), because of the high mortality of invasive pneumococcal disease (IPD), it should be paid more attention to these invasive strains clinically.16 Autumn and winter were the major seasons with diagnosis of S. pneumoniae infections, which was similar to a relevant report.17
Current guidelines recommend the use of broad-spectrum antibiotics for acute infections taking into account of normal etiologic pathogens, probably of S. pneumoniae infections.18 Penicillin, as a narrow-spectrum antibiotic, was usually recommended for the treatment of S. pneumoniae infections, which can also slow down the emergence of drug-resistant strains.19 In this seven-year study, we revealed a high Non-susceptibility rate of Streptococcus pneumoniae to penicillin (43.5%), which was in high level compared with other literature. Its Non-susceptibility rates differed from 29.3% in Europe to 47.6% in Asia-Pacific region for all years combined from 1997 to 2016.20 Since 2005, epidemiological studies in China showed that the antibiotic resistance of S. pneumoniae in pediatrics was becoming more and more serious.21 A previous study reported a significant increase, during the year of 2006 to 2007, of the resistance of S. pneumoniae strains isolated from children under 5 years old, admitted in four children’s hospitals in China, compared with the period from 2000 to 2002.22 The high resistance rate against multiple antimicrobial agents brings stress on the need for the empiric antibiotic treatment of the S. pneumoniae infections.23,24 In China, to combat drug resistance, the government has taken forceful measures to control the abuse of antibiotics since 2011. Fortunately, the sensitivity of Streptococcus pneumoniae to key antibiotics was increasing in children in recent years, which can be concluded from the susceptibility results of our study (Figure 2). In addition, the increasing sensitivity of S. pneumoniae to major antimicrobial agents was also confirmed by multicenter surveillance data from pediatric patients in China.8,25 However, in contrast to pediatric patients, the resistance of Streptococcus pneumoniae to common antibiotics was increasing over time in adult patients from 2009 to 2018, in a tertiary hospital in Beijing, China, with patients aged <18 years old excluded from study.26
Our study showed that the resistance rate to penicillin was 3.4%. In China, the resistance rate was higher than the studies in Antimicrobial Surveillance Network (CHINET, 0.56%, 2021), China Antimicrobial Resistance Surveillance System (CARSS, 0.9–2.7%, 2017–2020) and Asian Network for Surveillance of Resistant Pathogens (ANSORP, 1.4%).14 Worldwide, the resistance rate to penicillin varied. According to the literature, Japan (1.7–2.2%, 2013–2018) showed lower resistance rate to penicillin than ours.27 Many of the resistance rates from different countries were higher than our studies, such as Korea (9.8%),10 United States (14.8%),28 Ethiopia (17.5%)29 and Russia (26.1%).30 Although the resistance rate to penicillin was in low level across all over the country, it was in high level in Beijing. The reason could be interpreted by the fact that the hospitals had various specialist wards to accept children with serious diseases.
The third generation cephalosporins, such as cefotaxime and ceftriaxone, were commonly used for the treatment of S. pneumoniae infections to achieve a better therapeutic outcome.31 Lee et al reported that inappropriate use of ceftriaxone for the initial treatment of ceftriaxone-resistant isolates had a certain correlation with mortality.32 In our study, more the 60.0% of the non-meningitis strains were non-susceptible to cefotaxime. Our study suggested that susceptibility to penicillin was higher than sensitivity to cephalosporin. Therefore, penicillin seemed to have a better therapeutic effect than cephalosporin in treating pediatric infections caused by S. pneumoniae. There were significant differences in the susceptibility to multiple antibiotics between PSSP (penicillin-susceptible Streptococcus pneumoniae) and PRSP (penicillin-resistant Streptococcus pneumoniae). The resistance rate of PRSP to cefotaxime was significantly higher than that of PSSP in both meningitis and non-meningitis strains. Therefore, when treating the infections caused by S. pneumoniae, β-lactam antibiotics should still be considered as the initial empiric antibiotic agent, if the susceptibility test results suggested intermediate, the dosage should be increased appropriately. If the phenotype sensitivity results suggested resistance, it was necessary to pay attention to the multidrug resistance of PRSP and reasonably select antibiotics according to the antibiotic results.33,34
In our study, S. pneumoniae strains showed low susceptibility to meropenem. The study by Yu-Te Tsai et al showed that the susceptibility rate to meropenem was 85.2%,35 higher than ours. The reason for the difference in susceptibility rate might be the extensive application in severe infectious diseases.36–38
Our study demonstrated high resistance rates by S. pneumoniae strains to erythromycin, clindamycin and tetracycline, all of which were higher than 93.0%. The high resistance rates were consistent with previous reports.39 Due to the high resistance rate to erythromycin, clindamycin and tetracycline, these antibiotics were not suitable for the conventional treatment of S. pneumoniae infections.
Irrational use of antibiotics was the leading factor causing high levels of antibiotic resistance, as well as other factors including self-medication, and abuse of antibiotics in agriculture. All these actions could contribute to increasing antibiotic selective pressure, leading to resistant S. pneumoniae strain selection. In China, a campaign to curb the abuse of antibiotics and slow down the production of antibiotic-resistant strains was carried out in 2011.40 Because of the emergence of COVID-19, the government had taken great efforts to control the use of antibiotics, keep children to study in their households and reduce community contact, the number of S. pneumoniae isolates dramatically decreased since 2020. Although the resistance rates to several antibiotics decreased in these years, they were still in high level.
Several limitations exist in our study. First, the serotyping of S. pneumoniae is not regular clinical test, which is not available in the present analysis. Therefore, the potential effect of licensed pneumococcal conjugated vaccines could not be evaluated. Second, it was a hospital-based retrospective review, a few of data could not be made a check. Third, there were two different detection methods for antibiotic susceptibility, due to the limitations of testing methods, some strains isolated from Children’s Hospital Capital Institute Pediatrics in the early study could not distinguish PISP (penicillin-intermediate Streptococcus pneumoniae) and PRSP. The deficiencies need to be consummated in the future monitoring process.
Conclusion
In summary, we reported the distribution and antibiotic resistance profiles of S. pneumoniae in Beijing from 2015 to 2021. S. pneumoniae infection was common in infants and young children under 5 years old. Winter and autumn were the most common seasons for Streptococcus pneumoniae infections. Among patients, the proportion of boys was higher than that of girls. The most common specimen type was venous blood, followed by sputum, cerebrospinal fluid and bronchial lavage fluid. In the midst of the specimens, the average positive rate was 0.87%, with the highest positive rate in ear secretion. Our study thus revealed that penicillin, which was continuously effective in the treatment of pneumococcal diseases, could still be used as a first-line choice for empirical treatment. In terms of high-level β-lactam antibiotics, cefotaxime demonstrated better outcome than meropenem. In consideration of the high resistance rates of erythromycin, tetracycline and clindamycin, they were no longer suitable for empirical treatment in pneumococcal infections. Therefore, in clinical, we should pay much more attention to monitor antibiotic resistance, and use antibacterial agents reasonably.
Funding
This study was supported by the Natural Science Foundation of Beijing- Haidian Original Innovation Fund Joint Project [L202004].
Disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
1. World Health Organization. Pneumococcal vaccines WHO position paper--2012. Wkly Epidemiol Rec. 2012;87(14):129–144.
2. Yildirim I, Shea KM, Pelton SI. Pneumococcal disease in the era of pneumococcal conjugate vaccine. Infect Dis Clin North Am. 2015;29(4):679–697. doi:10.1016/j.idc.2015.07.009
3. Choe YJ, Choi EH, Lee HJ. The changing epidemiology of childhood pneumococcal disease in Korea. Infect Chemother. 2013;45(2):145–158. doi:10.3947/ic.2013.45.2.145
4. Amin AN, Cerceo EA, Deitelzweig SB, Pile JC, Rosenberg DJ, Sherman BM. The hospitalist perspective on treatment of community-acquired bacterial pneumonia. Postgrad Med. 2014;126(2):18–29. doi:10.3810/pgm.2014.03.2737
5. Mendes C, Marin ME, Quinones F, et al. Antibacterial resistance of community-acquired respiratory tract pathogens recovered from patients in Latin America: results from the PROTEKT surveillance study (1999–2000). Braz J Infect Dis. 2003;7(1):44–61. doi:10.1590/S1413-86702003000100006
6. Hsueh PR, Luh KT. Antimicrobial resistance in Streptococcus pneumoniae, Taiwan. Emerg Infect Dis. 2002;8(12):1487–1491. doi:10.3201/eid0812.020178
7. Oligbu G, Fry NK, Ladhani SN. The Pneumococcus and Its Critical Role in Public Health. Methods Mol Biol. 2019;1968:205–213.
8. Fu P, Xu H, Jing C, et al. Bacterial epidemiology and antimicrobial resistance profiles in children reported by the ISPED program in China, 2016 to 2020. Microbiol Spectr. 2021;9(3):e0028321. doi:10.1128/Spectrum.00283-21
9. Li ZJ, Zhang HY, Ren LL, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12(1):5026. doi:10.1038/s41467-021-25120-6
10. Kim JS, Jung BK, Kim JW, Kim GY. Prevalence and antimicrobial susceptibility of streptococcus pneumoniae isolated from clinical samples in the past 8 years in Korea. Biomed Res Int. 2021;2021:6615334. doi:10.1155/2021/6615334
11. Morris MC, Pichichero ME. Streptococcus pneumoniae burden and nasopharyngeal inflammation during acute otitis media. Innate Immun. 2017;23(8):667–677. doi:10.1177/1753425917737825
12. Casey JR, Kaur R, Friedel VC, Pichichero ME. Acute otitis media otopathogens during 2008 to 2010 in Rochester, New York. Pediatr Infect Dis J. 2013;32(8):805–809. doi:10.1097/INF.0b013e31828d9acc
13. Chandrasekharan S, Amin T, Kim J, et al. Intellectual property rights and challenges for development of affordable human papillomavirus, rotavirus and pneumococcal vaccines: patent landscaping and perspectives of developing country vaccine manufacturers. Vaccine. 2015;33(46):6366–6370. doi:10.1016/j.vaccine.2015.08.063
14. Wang CY, Chen YH, Fang C, et al. Antibiotic resistance profiles and multidrug resistance patterns of Streptococcus pneumoniae in pediatrics: a multicenter retrospective study in mainland China. Medicine. 2019;98(24):e15942. doi:10.1097/MD.0000000000015942
15. Smith DK, Kuckel DP, Recidoro AM. Community-acquired pneumonia in children: rapid evidence review. Am Fam Physician. 2021;104(6):618–625.
16. Jiang M, Wang X, Zhu L, et al. Clinical characteristics, antimicrobial resistance, and risk factors for mortality in paediatric invasive pneumococcal disease in Beijing, 2012–2017. BMC Infect Dis. 2022;22(1):338. doi:10.1186/s12879-022-07179-8
17. Song JY, Lee JS, Wie SH, et al. Prospective cohort study on the effectiveness of influenza and pneumococcal vaccines in preventing pneumonia development and hospitalization. Clin Vaccine Immunol. 2015;22(2):229–234. doi:10.1128/CVI.00673-14
18. von Specht M, Garcia Gabarrot G, Mollerach M, et al. Resistance to beta-lactams in Streptococcus pneumoniae. Rev Argent Microbiol. 2021;53(3):266–271. doi:10.1016/j.ram.2021.02.007
19. Jacobs MR. Clinical significance of antimicrobial resistance in Streptococcus pneumoniae. S Afr Med J. 2007;97(11 Pt 3):1133–1140.
20. Sader HS, Mendes RE, Le J, Denys G, Flamm RK, Jones RN. Antimicrobial susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific Region: results From 20 Years of the SENTRY Antimicrobial Surveillance Program (1997–2016). Open Forum Infect Dis. 2019;6(Suppl 1):S14–S23. doi:10.1093/ofid/ofy263
21. Yu S, Yao K, Shen X, Zhang W, Liu X, Yang Y. Serogroup distribution and antimicrobial resistance of nasopharyngeal isolates of Streptococcus pneumoniae among Beijing children with upper respiratory infections (2000–2005). Eur J Clin Microbiol Infect Dis. 2008;27(8):649–655. doi:10.1007/s10096-008-0481-y
22. Yao KH, Wang LB, Zhao GM, et al. Surveillance of antibiotic resistance of Streptococcus pneumoniae isolated from hospitalized patients with pneumonia in four children’s hospitals in China [in Chinese]. Chin J Contemp Pediatr. 2008;10(3):275–279.
23. Linares J, Ardanuy C, Pallares R, Fenoll A. Changes in antimicrobial resistance, serotypes and genotypes in Streptococcus pneumoniae over a 30-year period. Clin Microbiol Infect. 2010;16(5):402–410. doi:10.1111/j.1469-0691.2010.03182.x
24. Martin-Loeches I, Rodriguez AH, Torres A. New guidelines for hospital-acquired pneumonia/ventilator-associated pneumonia: USA vs. Europe Curr Opin Crit Care. 2018;24(5):347–352. doi:10.1097/MCC.0000000000000535
25. System CA. 2014至2017年中国儿童及新生儿患者细菌耐药监测研究 [Surveillance of bacterial resistance in children and newborns across China from 2014 to 2017]. Zhonghua Yi Xue Za Zhi. 2018;98(40):3279–3287. Chinese. doi:10.3760/cma.j.issn.0376-2491.2018.40.013
26. Zhao C, Yang S, Zhang F, et al. Antimicrobial resistance trends of the most common causative pathogens associated with community-acquired respiratory infections in China: 2009–2018. Infect Drug Resist. 2022;15:5069–5083. doi:10.2147/IDR.S374805
27. Tsuzuki S, Akiyama T, Matsunaga N, et al. Improved penicillin susceptibility of Streptococcus pneumoniae and increased penicillin consumption in Japan, 2013–2018. PLoS One. 2020;15(10):e0240655. doi:10.1371/journal.pone.0240655
28. Jones RN, Sader HS, Mendes RE, Flamm RK. Update on antimicrobial susceptibility trends among Streptococcus pneumoniae in the United States: report of ceftaroline activity from the SENTRY Antimicrobial Surveillance Program (1998–2011). Diagn Microbiol Infect Dis. 2013;75(1):107–109. doi:10.1016/j.diagmicrobio.2012.08.024
29. Sharew B, Moges F, Yismaw G, et al. Antimicrobial resistance profile and multidrug resistance patterns of Streptococcus pneumoniae isolates from patients suspected of pneumococcal infections in Ethiopia. Ann Clin Microbiol Antimicrob. 2021;20(1):26. doi:10.1186/s12941-021-00432-z
30. Wierzbowski AK, Karlowsky JA, Adam HJ, Nichol KA, Hoban DJ, Zhanel GG. Evolution and molecular characterization of macrolide-resistant Streptococcus pneumoniae in Canada between 1998 and 2008. J Antimicrob Chemother. 2014;69(1):59–66. doi:10.1093/jac/dkt332
31. Aspa J, Rajas O, de Castro FR. Pneumococcal antimicrobial resistance: therapeutic strategy and management in community-acquired pneumonia. Expert Opin Pharmacother. 2008;9(2):229–241. doi:10.1517/14656566.9.2.229
32. Lee HY, Wu TL, Su LH, et al. Invasive pneumococcal disease caused by ceftriaxone-resistant Streptococcus pneumoniae in Taiwan. J Microbiol Immunol Infect. 2018;51(4):500–509. doi:10.1016/j.jmii.2016.12.004
33. Lee MC, Kuo KC, Lee CH, et al. The antimicrobial susceptibility in adult invasive pneumococcal disease in the era of pneumococcus vaccination: a hospital-based observational study in Taiwan. J Microbiol Immunol Infect. 2020;53(6):836–844. doi:10.1016/j.jmii.2020.01.003
34. Cherazard R, Epstein M, Doan TL, Salim T, Bharti S, Smith MA. Antimicrobial resistant streptococcus pneumoniae: prevalence, mechanisms, and clinical implications. Am J Ther. 2017;24(3):e361–e9. doi:10.1097/MJT.0000000000000551
35. Tsai YT, Lee YL, Lu MC, et al. Nationwide surveillance of antimicrobial resistance in invasive isolates of Streptococcus pneumoniae in Taiwan from 2017 to 2019. J Microbiol Immunol Infect. 2021;55(2):215–224.
36. Choi SH, Park SJ, Jun JB, et al. Comparative in vitro activities of carbapenem antimicrobial agents against 264 penicillin-resistant Streptococcus pneumoniae isolates from Korea. Diagn Microbiol Infect Dis. 2007;58(1):141–143. doi:10.1016/j.diagmicrobio.2006.11.017
37. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev. 2012;25(4):682–707. doi:10.1128/CMR.05035-11
38. Nakano S, Fujisawa T, Ito Y, et al. Penicillin-binding protein typing, antibiotic resistance gene identification, and molecular phylogenetic analysis of meropenem-resistant Streptococcus pneumoniae serotype 19A-CC3111 Strains in Japan. Antimicrob Agents Chemother. 2019;63:9. doi:10.1128/AAC.00711-19
39. Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012;56(3):1418–1426. doi:10.1128/AAC.05658-11
40. Shi W, Li J, Dong F, et al. Serotype distribution, antibiotic resistance pattern, and multilocus sequence types of invasive Streptococcus pneumoniae isolates in two tertiary pediatric hospitals in Beijing prior to PCV13 availability. Expert Rev Vaccines. 2019;18(1):89–94. doi:10.1080/14760584.2019.1557523
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.