Back to Journals » Journal of Pain Research » Volume 9
A double-blind, randomized, placebo-controlled pilot trial to determine the efficacy and safety of ibudilast, a potential glial attenuator, in chronic migraine
Authors Kwok YH, Swift JE, Gazerani P , Rolan P
Received 11 July 2016
Accepted for publication 3 August 2016
Published 31 October 2016 Volume 2016:9 Pages 899—907
DOI https://doi.org/10.2147/JPR.S116968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Yuen H Kwok,1 James E Swift,1 Parisa Gazerani,2 Paul Rolan1
1Discipline of Pharmacology, University of Adelaide, Level 5 Medical School North, South Australia, Australia; 2Department of Health Science & Technology, Aalborg University, Aalborg, Denmark
Background: Chronic migraine (CM) is problematic, and there are few effective treatments. Recently, it has been hypothesized that glial activation may be a contributor to migraine; therefore, this study investigated whether the potential glial inhibitor, ibudilast, could attenuate CM.
Methods: The study was of double-blind, randomized, placebo-controlled, two-period crossover design. Participants were randomized to receive either ibudilast (40 mg twice daily) or placebo treatment for 8 weeks. Subsequently, the participants underwent a 4-week washout period followed by a second 8-week treatment block with the alternative treatment. CM participants completed a headache diary 4 weeks before randomization throughout both treatment periods and 4 weeks after treatment. Questionnaires assessing quality of life and cutaneous allodynia were collected on eight occasions throughout the study.
Results: A total of 33 participants were randomized, and 14 participants completed the study. Ibudilast was generally well tolerated with mild, transient adverse events, principally nausea. Eight weeks of ibudilast treatment did not reduce the frequency of moderate to severe headache or of secondary outcome measures such as headache index, intake of symptomatic medications, quality of life or change in cutaneous allodynia.
Conclusion: Using the current regimen, ibudilast does not improve migraine with CM participants.
Keywords: chronic migraine, glia, ibudilast, headache, immune system
Introduction
Chronic migraine (CM) is a disabling and undertreated disorder1 which represents a clinically challenging problem that impacts significantly on the quality of life of affected individuals and imposes a large economic burden upon society due to lost productivity.2,3 Despite this burden, the pathophysiology behind migraine and especially the processes of migraine chronification remain poorly understood. A growing body of preclinical and clinical evidence suggests that acquired sensitization plays a significant role in the pathology of migraine and the process of chronification.4
Recent evidence supports the importance of neuronal–glial interactions as a potential mechanism for chronic pain. Glia are located in the central nervous system, and under basal condition, they perform housekeeping functions and maintain neural homeostasis.5 However, when glia are activated, they become immunoresponsive and modulate pain by releasing neuroexcitatory signals that can increase neuronal excitability,6,7 activate neighboring glia, and produce nociceptive mediators (such as nitric oxide, excitatory amino acids, and proinflammatory cytokines).8
Nonclinical models support a role of neuron–glia interactions in the initiation of migraine attacks, as glia have been shown to release a range of inflammatory cytokines and chemokines in animal models evoking some features of migraine.9 In nonclinical models, blockade of glial activation and proinflammatory mediators released by glia caused reduction in hypersensitivity and allodynia, respectively.10,11 Additionally, a clinical study has reported elevated levels of proinflammatory cytokines in the cerebrospinal fluid of CM participants.12 Such tonically increased glial activity may underlie the progression from episodic to CM.13 Furthermore, in a separate study, levels of the chemokine interleukin 8 were found to be elevated in the jugular venous blood of patients during a migraine attack.14 Interleukin 8 can be synthesized by glia in response to other proinflammatory mediators including tumor necrosis factor α, lending additional support to the neurogenic inflammation model of migraine.15
Thus, it seems likely that nociceptive signaling during migraine attacks releases mediators that activate glia. Activated glia then release a range of proinflammatory mediators such as cytokines, chemokines, reactive oxygen species, and prostaglandins, which not only facilitate pain transmission but also activate further glia, creating a positive feedback loop. After a stimulus is resolved, experimental evidence suggests that microglia may remain “primed,” entering a state in which they do not actively produce proinflammatory substances, yet they over-respond to subsequent stimuli, resulting in increased proinflammatory cytokine release and an exaggerated pain response.16 This could lead to an increase in migraine frequency as triggers that were previously subthreshold may now initiate headache.
Although glial involvement in the pathogenesis of migraine and in the transformation of episodic migraine to CM has been suggested by a number of authors,9,13,17–19 to our knowledge no treatments targeting this mechanism have been trialed specifically in high frequency migraineurs. However, encouraging results from an open-label retrospective study in participants with varying chronic daily headache phenotypes following treatment with the potential glial attenuator minocycline have been reported.20 This study, published only in abstract form, found a significant decrease in headache frequency following 2 months of minocycline administration when used as an adjunctive preventative medication.20 However, the efficacy of minocycline in animal studies, suggests that it is only effective before chronic pain is established and not subsequently,10 which may not be applicable to chronic pain patients.
Ibudilast is a nonselective phosphodiesterase inhibitor that has been used in Japan for 20 years to treat bronchial asthma,21 post-stroke dizziness,22 and ocular allergies.23 Ibudilast inhibits tracheal smooth muscle contractility24 and platelet aggregation,25 improves cerebral blood flow,22 and attenuates allergic reactions.26,27
As ibudilast is administered orally, is well tolerated, and has good blood–brain barrier permeability,28–30 it is a suitable candidate for the treatment of chronic pain. In nonclinical models, ibudilast attenuated microglial activation (reduced withdrawal behaviors), reduced production of proinflammatory cytokines, enhanced the production of anti-inflammatory cytokines, and improved the safety profile of opioids (reduced tolerance and attenuated reward and dependence).23,31–33 Moreover, ibudilast exhibited immunomodulatory actions in multiple sclerosis patients30 and also displayed inhibition of proinflammatory cytokines from peripheral leukocytes.34
In the present study, the efficacy of the putative glial attenuator ibudilast in participants with high frequency migraine was investigated. The study investigated the efficacy of ibudilast on the frequency of moderate to severe headache and other headache indices (such as headache index, headache-related questionnaires, and acute symptomatic medication intake) in CM patients over 8 weeks of ibudilast treatment compared with placebo treatment.
Methods
Study approval
Ethical approval was obtained from the Human Research Ethics Committee of the Royal Adelaide Hospital, Adelaide, South Australia. All the participants gave their written informed consent before the commencement of the study. The study was conducted at the Pain and Anaesthesia Research Clinic, Royal Adelaide Hospital, Adelaide, Australia, and in accordance with the Principles of International Conference on Harmonization Good Clinical Practice, the Declaration of Helsinki, and the Australian National Statement on Ethical Conduct in Research Involving Humans.
The study was of double-blind, randomized, placebo-controlled, two-period crossover design. Participants were randomized to initially receive either ibudilast or placebo treatment for 8 weeks. Subsequently, the participants underwent a 4-week washout period followed by a second 8-week treatment block with the alternative treatment (Clinicaltrials.gov NCT01389193).
Participant selection
Participants were recruited from June 2013 to May 2015. Criteria for CM were defined by the revised second edition of the International Classification of Headache Disorders (ICHD-II).35 Key inclusion criteria were:
- Men and women aged between 18 and 65 years
- Migraine with or without aura, as diagnosed according to the ICHD-II36
- Onset of migraine before 50 years of age
- Headache for ≥15 days per month
- Migraine-like headache for ≥8 days per month, as per the International Headache Society (IHS) guidelines37
Key exclusion criteria
Participants were excluded from the study on the basis of the following criteria: change in type or dose of migraine prophylactic medication in last 3 months, medication overuse headache as diagnosed according to the ICHD-II35, posttraumatic headache as diagnosed according to the ICHD-II36, another dominant chronic pain condition, an active inflammatory disease such as rheumatoid arthritis, a history of recent cerebrovascular disorder, inability to provide written informed consent, inability to read and write in English, presence of severe psychological/psychiatric disorders, recent history of significant trauma, recent history of drug or alcohol abuse, clinically significant findings on screening blood sample results, current malignancy, known hypersensitivity to ibudilast or excipients in Ketas® formulation, renal or hepatic impairment, pregnancy, lack of adequate contraception, and breastfeeding.
Study medication
The study used a delayed-release ibudilast product, Ketas®, which was obtained from Kyorin Pharmaceuticals Industries, Ltd (Tokyo, Japan). To blind the treatment allocation, the Ketas® capsule was overencapsulated with the space filled with microcrystalline cellulose. The placebo capsule was of the same size and color and filled with microcrystalline cellulose, so that the active and placebo medications were identical in appearance and weight. Each active capsule contained 10 mg ibudilast. Four capsules were self-administered by participants orally, twice daily for each 8-week treatment block. No treatment was administered during the washout.
Study schedule
4 weeks prior to baseline visit
At screening, potential participants underwent,
- Medical history including medication history
- Headache diagnosis based upon retrospective information from the participant confirmed using baseline headache diary data
- Completion of the Hospital Anxiety and Depression Scale
- Physical examination
- Urine pregnancy test for women of childbearing potential
- Urine drug screen to rule out the presence of nonprescribed drugs of abuse
- Blood samples to assess hematology (4 mL), biochemistry including renal function and hepatic function (8 mL).
After successful completion of the screening visit and confirmation of study eligibility, participants were provided with a headache diary to complete at least 4 weeks prior to the baseline visit to confirm study eligibility. Standardized education and instructions on how to complete the diary was provided by the experimenter, and the participants were required to fill in the headache diary daily. The headache diary recorded a comprehensive assessment of the headache experienced by participants and consisted of head pain characteristics, headache frequency, average headache intensity (11-point numerical rating scale), duration of headache (number of hours), and intake of symptomatic headache treatments (timing, type, and amount of medication consumed). The headache index was calculated by the summation of headache duration (hours) with headache intensity (11-point numerical rating scale). The dose of opioid-containing analgesics used by each patient was converted to oral morphine equivalent dose based on the local Royal Adelaide Hospital Pain Management Unit opioid conversion table.
Baseline visit
During the baseline visit, the headache diary was collected, and participants who were eligible to continue in the study were randomized to either ibudilast or placebo treatment in the first period. Participants were asked to complete two brief self-administered questionnaires. The 6-point Headache Impact Test (HIT-6) evaluated the impact of headache on the quality of life, reflecting upon the previous month,38 and the 12-point Allodynia Symptom Checklist (ASC-12) indicated whether, and quantified how, increased pain sensitivity impacted upon activities of day-to-day life.39
About 14 mL of blood was collected to assess safety blood profile (blood biochemistry [including the assessment of renal function], liver function tests and hematology). Participants were advised to take only the minimum amount of medications required to control their pain; however, they were not specifically informed to reduce their analgesic intake. Participants were asked to complete another headache diary for the 8-week treatment period. The study medication was dispensed to the participants prior to discharge.
Participants were required to complete the headache diary throughout the two treatment periods, during the washout phase and for 4 weeks after ceasing the study medication.
In study visits
Inspection of headache diary, assessment of adverse events, completion of questionnaires, and safety bloods were taken at weeks 2, 4, and 8 of each period. Figure 1 shows the flowchart of the study design.
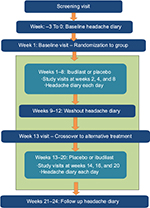  | Figure 1 Study overview. |
Statistical analysis
All the participants who received at least one dose of the study medication were included in the safety population. However, the efficacy data were only analyzed for completers, given the crossover nature of the trial.
Graphpad Prism 6.0 (GraphPad Software; San Diego, CA, USA) was used for all statistical analysis. The Wilcoxon matched pairs signed rank test was used to calculate the study medication adherence between the CM participants while in different treatment groups. Two-way analysis of variance tests with repeated measures and with Sidak adjustments were used to determine the differences between treatments in multiple comparisons were used to calculate frequency of moderate to severe headache experienced, headache index, frequency of symptomatic medication intake, average opioid intake, HIT-6 scores, and ASC-12 scores. Missing values were replaced by the last observed value for that variable (last observation carried forward). All results are presented as mean ± standard deviation (SD) unless stated otherwise. Significance was set at P<0.05.
Results
Demographics of CM participants
A total of 33 patients were randomized to treatment. Fourteen CM participants completed both the treatments of the study and returned completed headache diaries suitable for analysis. Four CM participants completed both the treatments of the study but did not return adequate diaries for analysis and therefore were excluded from the analysis (Figure 2).
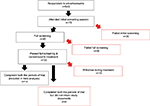  | Figure 2 Flow diagram of numbers of participants in different phases of the study. |
Fifteen participants (nine received ibudilast) did not complete the study and hence were also excluded from the analysis. Of these fifteen participants, two participants lost interest in the study; one was excluded due to work commitment; four were excluded because of loss to follow-up; one was excluded as that participant had changed his medication; one was excluded after receiving 1 week of treatment as she was found to be pregnant (she was on placebo); one was excluded after experiencing heart palpitation; four participants were withdrawn by the investigator because of worsening headaches; and one participant was excluded by the investigator as the patient experienced tingling sensation.
Demographics of the participants are listed in Table 1. The mean age was 46 years (SD 13). The study consisted of 11 men and 22 women. The average duration of headache was 21 years (SD 15). The mean daily morphine equivalent dose was 4.2 mg (SD10).
  | Table 1 Demographics of CM participants Abbreviations: CM, chronic migraine; HADS, Hospital Anxiety and Depression Scale; SD, standard deviation. |
Compliance to study medication and safety
In this study, all the participants had good adherence to study medications as demonstrated with the return of unused study capsules. There was no treatment group difference (P=0.6) with the number of capsules returned (placebo vs ibudilast [mean {SD}: 2.4 {2.4} vs 7.7 {15.6}]).
Adverse events experienced in the study were of mild intensity, and all the reported adverse events are listed in Table 2. Seventeen of the twenty-three (60%) CM participants who were on ibudilast treatments reported adverse events, whereas eight of the twenty-three (35%) CM participants reported adverse events during placebo treatments. The most frequent adverse event in both the groups was worsening migraine, reported by 4/28 under ibudilast and 2/23 on placebo. The next most frequently reported adverse event was nausea, reported by 4/28 under ibudilast and by no participant on placebo.
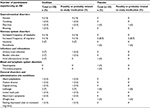  | Table 2 AEs reported by CM participants (n=33) during treatment Note: Values represent event experienced during two treatment periods. Abbreviations: AEs, adverse events; CM, chronic migraine. |
Headaches
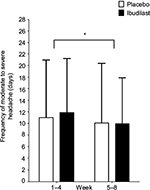
When CM participants were on ibudilast treatment, they did not experience reduced frequency of moderate to severe headache compared with placebo treatment (P=0.9) in either treatment weeks 1–4 (mean difference –0.9, 95% confidence interval [CI] –9.1 to 7.3) or treatment weeks 5–8 (mean difference 0.1, 95% CI –8.1 to 8.3). However, irrespective of treatments, in treatment weeks 5–8 of each block, there was a significant reduction (P=0.02) in the frequency of moderate to severe headaches (Figure 3).
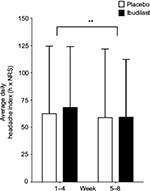
Likewise, when CM participants were on ibudilast treatment, they did not experience a reduction in headache index when compared with placebo treatment (P=0.9) in either treatment weeks 1–4 (mean difference –5.7, 95% CI –56.6 to 45.2) or treatment weeks 5–8 (mean difference –0.4, 95% CI –51.3 to 50.5). However, in treatment weeks 5–8, there was a significant reduction in the headache index (P=0.003) (Figure 4).
Acute symptomatic medication intake and self-reported questionnaires
No treatment differences were detected in the frequency intake of symptomatic medication (P=0.6) in either treatment weeks 1–4 (mean difference –2.6, 95% CI –11.7 to 6.6) or treatment weeks 5–8 (mean difference –1.9, 95% CI –11 to 7.3) (Figure 5A). The oral morphine equivalent was also calculated for the medication that was taken by the CM participants; however, no treatment differences were detected (P=1) in either treatment weeks 1–4 (mean difference –0.5, 95% CI –11.2 to 10.2) or treatment weeks 5–8 (mean difference 0.5, 95% CI –10.2 to 11.2) (Figure 5B).
Scores on the HIT-6 and ASC-12 questionnaires did not differ between the treatment groups during any study time period (baseline, weeks 2, 4, and 8 of treatment); P=0.3 and P=0.9, respectively (data not shown).
Discussion
Ibudilast caused no change in the frequency of moderate to severe headache when compared with placebo treatment. Furthermore, other headache indices such as headache index, frequency of acute symptomatic medication intake, HIT-6 scores or ASC-12 scores were not reduced when CM patients were on ibudilast treatment. Additionally, worsening migraine was reported at a higher frequency under ibudilast treatment rather than on placebo. However, given the small sample sizes used in this study, lack of statistical significance should be considered with caution, but there was no trend for a benefit with ibudilast. Interestingly, during treatment weeks 5–8 of each period, the frequency of moderate to severe headache and headache index for both the treatment groups were significantly reduced compared to treatment weeks 1–4. This observation could be an indicator of participants having a positive expectancy of the study drug.40
Even though ibudilast may not be an effective treatment for CM, this study further provides a safety profile of ibudilast in a homogeneous migraine cohort. The adverse events experienced by participants were mild and transient in nature, and most of the adverse events were resolved once ibudilast was halted.
Our results are in agreement with a recently published finding by our group, which found ibudilast to have no effect in reducing headache index or in reducing opioid intake in medication overuse headache patients.41 However, the study also measured toll-like receptor (TLR) reactivity from peripheral blood mononuclear cells and found ibudilast to significantly reduce the proinflammatory cytokine (interleukin 1β) levels from ex vivo stimulation with TLR-2 or TLR-4 agonists. This finding confirmed that ibudilast was likely to be inhibiting an inflammatory pathway, at least peripherally. However, the peripheral target may be different to the mechanism that is responsible for the headache or the treatment period may have been insufficient to reverse the long-term changes associated with CM or may not have been acting centrally.
It is unclear from this study whether glia are involved in CM. Ibudilast has demonstrated good central nervous system partitioning28 but as it was not found to have any effect in the clinical endpoints, it suggests that the mechanisms responsible for CM may be signaling through another pathway that is currently unidentified, or neurons may play a stronger role. Thus more research would be required to address whether glial activation is a likely contributor for CM. The recent demonstration of imaging of activated glia in patients with chronic low back pain provides a potential biomarker for future studies.42
Even though there have been reports of pro- and anti-inflammatory cytokines being a predictor of headaches, the examination of peripheral targets may not be adequate. In a CM study, elevation of tumor necrosis factor α was found in the cerebrospinal fluid but not in the plasma,12 indicating that peripheral biomarkers might not be helpful.
Limitations
The sample size for this study was small as the required number of participants could not be recruited within the time frame, so the study ended prematurely (intended target was 40 CM participants). This was due to the difficulty in finding CM participants who fitted the criteria, and this is common in research involving chronic headache patients.43,44
Conclusion
The present study showed ibudilast dosed at 40 mg twice daily for 8 weeks is not a promising treatment for CM. However, this study adds to the literature of the safety and tolerability of ibudilast in a CM cohort, which to our knowledge has not been investigated previously.
Acknowledgments
Funding for the clinical study was provided by the Migraine Research Foundation and partly by the Danish Research Council. Both the Migraine Research Foundation and Danish Research Council had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosure
PR holds a provisional patent on the use of ibudilast in medication overuse headache. The authors report no other conflicts of interest in this work.
References
Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. | ||
Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–334. | ||
Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149–156. | ||
Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. | ||
Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. | ||
Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. | ||
Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. | ||
Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. | ||
Thalakoti S, Patil VV, Damodaram S, Vause CV, Langford LE, Freeman SE, Durham PL. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. 2007;47:1008–1023. | ||
Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. | ||
Sweitzer SM, Colburn RW, Rutkowski M, DeLeo JA. Acute peripheral inflammation induces moderate glial activation and spinal IL-1beta expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. | ||
Rozen T, Swidan SZ. Elevation of CSF tumor necrosis factor alpha levels in new daily persistent headache and treatment refractory chronic migraine. Headache. 2007;47:1050–1055. | ||
Kraig RP, Mitchell HM, Christie-Pope B, Kunkler PE, White DM, Tang Y-P, Langan G. TNF-alpha and Microglial hormetic involvement in neurological health & migraine. Dose Response. 2010;8:389–413. | ||
Sarchielli P, Alberti A, Vaianella L, et al. Chemokine levels in the jugular venous blood of migraine without aura patients during attacks. Headache. 2004;44:961–968. | ||
Ehrlich LC, Hu S, Sheng WS, Sutton RL, Rockswold GL, Peterson PK, Chao CC. Cytokine regulation of human microglial cell IL-8 production. J Immunol. 1998;160:1944–1948. | ||
Hains LE, Loram LC, Weiseler JL, et al. Pain intensity and duration can be enhanced by prior challenge: initial evidence suggestive of a role of microglial priming. J Pain. 2010;11:1004–1014. | ||
Bartley J. Could glial activation be a factor in migraine? Med Hypotheses. 2009;72:255–257. | ||
Capuano A, De Corato A, Lisi L, Tringali G, Navarra P, Dello Russo C. Proinflammatory-activated trigeminal satellite cells promote neuronal sensitization: relevance for migraine pathology. Mol Pain. 2009;5:43. | ||
Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, Reuter U. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS One. 2011;6:e17360. | ||
Mendelson JT, Ailani J, Silberstein SD. Glial function inhibitors and headache [Abstract]. Cephalalgia. 2009;29 141. | ||
Kawasaki A, Hoshino K, Osaki R, Mizushima Y, Yano S. Effect of ibudilast: a novel antiasthmatic agent, on airway hypersensitivity in bronchial asthma. J Asthma. 1992;29:245–252. | ||
Fukuyama H, Kimura J, Yamaguchi S, et al. Pharmacological effects of ibudilast on cerebral circulation: a PET study. Neurol Re. 1993;15:169–173. | ||
Rolan P, Hutchinson M, Johnson K. Ibudilast: a review of its pharmacology, efficacy and safety in respiratory and neurological disease. Expert Opin Pharmacother. 2009;10:2897–2904. | ||
Souness JE, Villamil ME, Scott LC, Tomkinson A, Giembycz MA, Raeburn D. Possible role of cyclic AMP phosphodiesterases in the actions of ibudilast on eosinophil thromboxane generation and airways smooth muscle tone. Br J Pharmacol. 1994;111:1081–1088. | ||
Ohashi M, Ohkubo H, Kito J, Nishino K. A new vasodilator 3-isobutyryl-2-isopropylpyrazolo[1,5-a]pyridine (KC-404) has a dual mechanism of action on platelet aggregation. Arch Int Pharmacodyn Ther. 1986;283:321–334. | ||
Nishino K, Hara S, Irikura T. [Effect of KC-404 on allergic reactions types I-IV]. Nihon yakurigaku zasshi Folia pharmacologica Japonica. 1984;83:291–299. | ||
Ohashi M, Uno T, Nishino K. Effect of ibudilast, a novel antiasthmatic agent, on anaphylactic bronchoconstriction: predominant involvement of endogenous slow reacting substance of anaphylaxis. Int Arch Allergy Immunol. 1993;101:288–296. | ||
Ledeboer A, Liu T, Shumilla JA, et al. The glial modulatory drug AV411 attenuates mechanical allodynia in rat models of neuropathic pain. Neuron Glia Biol. 2006;2:279–291. | ||
Rolan P, Gibbons JA, He L, et al. Ibudilast in healthy volunteers: safety, tolerability and pharmacokinetics with single and multiple doses. Br J Clin Pharmacol. 2008;66:792–801. | ||
Barkhof F, Hulst HE, Drulovic J, Uitdehaag BM, Matsuda K, Landin R; MN166-001 Investigators. Ibudilast in relapsing-remitting multiple sclerosis: a neuroprotectant? Neurology. 2010;74:1033–1040. | ||
Mizuno T, Kurotani T, Komatsu Y, et al. Neuroprotective role of phosphodiesterase inhibitor ibudilast on neuronal cell death induced by activated microglia. Neuropharmacology. 2004;46:404–411. | ||
Suzumura A, Ito A, Yoshikawa M, Sawada M. Ibudilast suppresses TNFalpha production by glial cells functioning mainly as type III phosphodiesterase inhibitor in the CNS. Brain Res. 1999;837:203–212. | ||
Hutchinson MR, Lewis SS, Coats BD, et al. Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun. 2009;23:240–250. | ||
Feng J, Misu T, Fujihara K, et al. Ibudilast, a nonselective phosphodiesterase inhibitor, regulates Th1/Th2 balance and NKT cell subset in multiple sclerosis. Mult Scler. 2004;10:494–498. | ||
Headache Classification Committee; Olesen J, Bousser MG, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. | ||
Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Suppl 1):9–160. | ||
Silberstein S, Tfelt-Hansen P, Dodick DW, et al. Guidelines for controlled trials of prophylactic treatment of chronic migraine in adults. Cephalalgia. 2008;28:484–495. | ||
Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–974. | ||
Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. | ||
Bingel U, Wanigasekera V, Wiech K, Ni Mhuircheartaigh R, Lee MC, Ploner M, Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci Transl Med. 2011;3:70ra14. | ||
Johnson JL, Kwok YH, Sumracki NM, et al. Glial attenuation with ibudilast in the treatment of medication overuse headache: a double-blind, randomized, placebo-controlled pilot trial of efficacy and safety. Headache. 2015;55:1192–1208. | ||
Loggia ML, Chonde DB, Akeju O, et al. Evidence for brain glial activation in chronic pain patients. Brain. 2015;138:604–615. | ||
De Hertogh W, Vaes P, Devroey D, et al. Preliminary results, methodological considerations and recruitment difficulties of a randomised clinical trial comparing two treatment regimens for patients with headache and neck pain. BMC Musculoskelet Disord. 2009;10:115. | ||
Vernon H, Jansz G, Goldsmith CH, McDermaid C. A randomized, placebo-controlled clinical trial of chiropractic and medical prophylactic treatment of adults with tension-type headache: results from a stopped trial. J Manipulative Physiol Ther. 2009;32:344–351. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.