Back to Journals » Drug Design, Development and Therapy » Volume 16
A Dose-Response Relationship Study of Prophylactic Nalbuphine to Reduce Pain During the Awakening Period in Patients Undergoing Laparoscopic Total Hysterectomy: A Randomized, Controlled, Double-Blind Clinical Study
Authors Wang M , Wang D , Zuo J, Liu T, Niu Z , Xie J, Qi D
Received 31 December 2021
Accepted for publication 19 March 2022
Published 31 March 2022 Volume 2022:16 Pages 981—990
DOI https://doi.org/10.2147/DDDT.S356582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Min Wang,1,2,* Dongyue Wang,1,* Jingzhi Zuo,3 Tianyu Liu,4 Zheng Niu,5 Juan Xie,1 Dunyi Qi2
1Department of Anesthesiology, Jinshan Hospital of Fudan University, Shanghai, People’s Republic of China; 2Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China; 3Emergency Department, Yichang Central People’s Hospital, Yichang, Hubei, People’s Republic of China; 4Department of Anesthesiology, Peking University People’s Hospital, Beijing, People’s Republic of China; 5Department of Anesthesiology, Zhangjiagang First People’s Hospital, Zhangjiagang, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Dunyi Qi, Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China, Email [email protected]
Purpose: Prophylactic intravenous nalbuphine was administered to observe its median effective dose (ED50) in reducing pain after undergoing laparoscopic total hysterectomy. To investigate the effect of different doses of nalbuphine on postoperative analgesia and adverse reactions in patients.
Patients and Methods: The 120 patients undergoing laparoscopic total hysterectomy were divided into 6 groups: group C (control) and group P (5 different doses of nalbuphine) with 20 patients per group. The doses of nalbuphine in group P were in an equally proportional series (groups P1, P2, P3, P4, and P5 received doses of 0.280, 0.200, 0.140, 0.100, and 0.070 mg/kg, respectively), diluted to 20 mL with saline and administered 5 min before the induction of anesthesia. A similar volume (20 mL) of saline was administered to group C 5 min before the induction of anesthesia. The numeric rating scale (NRS) of patients during awakening and after surgery, the number of postoperative salvage analgesia, and the occurrence of postoperative adverse effects were recorded.
Results: The ED50 (95% confidence interval (CI)) of nalbuphine in preventing pain during the awakening period in patients calculated using the point-slope method was 0.125 (0.108, 0.145) mg/kg. NRS scores differed among the 6 groups at 30 min and 1 h after extubation (P < 0.001; P < 0.001). Pairwise comparisons between groups revealed that, at 30 min after extubation, compared with group P1, the NRS scores of groups P4, P5, and C were higher (P = 0.001, P < 0.001, P < 0.001); compared with group P2, groups P5 and C had higher NRS scores (P = 0.011, P = 0.001). At 1 h after extubation, the NRS scores of groups P1 and P2 were lower than that of group P4 (P = 0.046, P = 0.036). Compared with the control, only the group P1 had a lower cough score (P = 0.009) and there were no differences in the other groups. There were no differences in sedation score at 10 min after extubation, the incidence of adverse events at 24 h postoperatively, or the number of remedial analgesics at 24 h postoperatively (P > 0.05).
Conclusion: The ED50 (95% CI) of nalbuphine as a prophylactic in reducing pain during recovery was 0.125 (0.108, 0.145) mg/kg. Compared with the control, nalbuphine at doses of 0.140, 0.200, and 0.280 mg/kg prevented pain during the awakening period. Among these doses, 0.280 mg/kg was determined to be the best, the occurrence of cough was less during extubation and the postoperative analgesic effect was good. However, it is necessary to pay attention to the occurrence of adverse reactions.
Keywords: gynecology, laparoscopy, preventive analgesia, nalbuphine, ED50
Introduction
Benign lesions of the uterus are a common condition of the female reproductive system. Hysterectomy is the procedure that is often chosen when surgery is required for benign uterine lesions.1,2 An analysis of a retrospective study in 2017 reported benign lesions in 81.3% of the 223,000 patients who had their uterus removed.3 With the development of Enhanced Recovery After Surgery (ERAS), the surgical approach to many gynecological conditions has shifted from open to laparoscopic surgery.4 Laparoscopic surgery has the advantages of thorough exploration and small surgical wounds. However, surgical incisions, CO2 pneumoperitoneum, and remifentanil-induced hyperalgesia (RIH) can lead to pain and agitation during the awakening period,5,6 which not only makes anesthesia management more difficult but also renders patients susceptible to unintentional injury.7 Studies have shown that patients treated with gynecological laparoscopy are up to 80% more likely to experience post-procedural pain.8,9
Preventive analgesia refers to analgesic methods used at any time during the perioperative period to reduce pain and prevent nociceptive sensitization.10 Drugs commonly used for prophylactic analgesia include opioids and nonopioids.11 Previous studies have reported that opioids are effective in reducing pain during the awakening period; however, adverse effects such as pruritus, constipation, respiratory depression, and addiction are also likely.12 Nalbuphine is a synthetic, mixed opioid and both an agonist of κ receptor and an antagonist of μ receptor.13 It has good analgesic and sedative effects, mildly depresses the respiratory system, and is associated with good hemodynamic stability.14,15 Moreover, it has relatively fewer adverse effects and low addiction potential. Previous studies have used nalbuphine for prophylactic analgesia; however, only a few studies have focused on determining the appropriate dose of prophylactic nalbuphine for pain relief in patients during the awakening period.16 Therefore, in this study, we used the point-slope method as a trial design to calculate the ED50 of prophylactic nalbuphine for pain relief during the awakening period in patients undergoing laparoscopic total hysterectomy.17 The other aim of our study was to compare the effects of different doses of prophylactic nalbuphine versus saline during the operation, awakening period and 24 h postoperatively to provide a reference for the clinical use of nalbuphine.
The point-slope method was calculated as follows:
ED50 = log−1[Xm–i (∑P–0.5) + i/4 (1–Pm–Pn)]
When containing 0% and 100% response rates,
ED50 = log-1[Xm–i (ΣP–0.5)]
95% confidence interval for ED50 = log-1(logED50±1.96·S)
S = i·[(∑P–∑P2)/(n–1)]1/2,
where n is the number of test groups, Xm is the logarithm of the dose in the Pm group, i is the logarithm of the dose ratio between groups, P is the response rate for each group, Pm is the highest response rate, and Pn is the lowest response rate.
Materials and Methods
This study was approved by the Clinical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2020-KL092-02) and registered on the website of the China Clinical Trials Registry (registration number ChiCTR2000030375; Principal investigator: Min Wang, Date of registration: February 29, 2020) prior to patient enrollment. This study was conducted in accordance with the Declaration of Helsinki. The trial was conducted from September 10, 2020, to March 31, 2021, and all patients were enrolled in the trial after providing written informed consent. One hundred and twenty patients aged ≥ 45 years, American Society of Anesthesiologists (ASA) class I~II, weighing 50–70 kg, and undergoing laparoscopic total hysterectomy were included.
Patients with an operative time of ≥ 120 min or intraoperative bleeding ≥ 300 mL, a diagnosis of malignancy, a history of chronic pain, a history of opioid addiction, an inability to assess pain correctly, severe cardiopulmonary system disease, and those who had participated in other trials were excluded.
Patients were randomized into the following 6 groups: Group C (control) and Group P (trial group: divided into 5 groups, namely, P1, P2, P3, P4, and P5) with 20 patients per group. A statistical analyst who was not involved in the follow-up study, generated the randomization sequence using SPSS version 25.0, and was also responsible for producing the envelopes containing the subjects’ trial protocols daily. On the day of the procedure, the statistician handed the envelopes to the anesthesiologists, who used a 20-mL syringe to prepare the drug based on the grouping in the envelope. The prepared solutions were colorless and clear. Each syringe was labeled with a number. The subject, surgeon, and data follow-up recorder were unaware of the contents of each numbered syringe.
Patients were asked to visit the clinic before the procedure for the collection of basic information and introduction to the NRS.18 All patients were required to fast for 8 h and abstain from drinking for 2 h before the procedure. After entering the operating room, the peripheral veins were exposed, and standardized monitoring was performed including the noninvasive blood pressure (BP), pulse oxygen saturation (SpO2), and electrocardiogram. Oxygen was administered to the patient using a face mask at a flow rate of 5 L/min. Five minutes before induction, the anesthetist slowly administered the test drug intravenously. No other adjuvant drugs were used. The drugs used for induction were midazolam 0.05 mg/kg, sufentanil 0.4 μg/kg, rocuronium 0.6 mg/kg, and etomidate 0.3 mg/kg. Patients’ anesthesia index (Ai) and circulatory changes were observed. After the patients’ muscles relaxed, their trachea was intubated, mechanical ventilation was performed, and relevant parameters were adjusted as follows for the micropump: Vt 6–8 mL/kg, RR 10–12 times/min, FiO2 60%–100%, and inspiratory: expiratory ratio of 1:1.5. The initial dose was as follows: 4 mg/ (kg•h) propofol, 0.08 mg/ (kg•h) cis-atracurium, and 0.25 μg/ (kg•min) remifentanil, which led to the maintenance of Ai index values between 40 and 60 and PETCO2 between 35 and 45 mmHg. The dose of the pumped drug was adjusted based on patients’ intraoperative conditions. Pumping of muscle relaxants was stopped 30 min before the end of the operation and the pumping of anesthetics was stopped at the end of the suture.
All patients were operated upon by the same group of gynecological surgeons. During the operation, the patients’ blood pressure and heart rate (HR) were maintained within 20% of the baseline value. When the patients’ blood pressure and HR increased only slightly, and the Ai increased, the pump dose of propofol was increased by 0.4 mg/ (kg•h) and that of remifentanil by 0.05 μg/ (kg•min). If the opposite situation occurred during the operation, propofol and remifentanil doses were reduced accordingly. After the operation, patients were asked to open their eyes and their ability to recover spontaneous breathing (respiratory rate > 10 beats/min, SpO2 > 95% without oxygen inhalation) was determined. Upon correctly responding to the physician’s instructions, the tracheal tube was removed. Patients were observed for 30 min in the post-anesthesia care unit (PACU) after extubation. During the observation period in the PACU and during the postoperative follow-up period, flurbiprofen axetil injection was used to adjust analgesia based on their needs. If nonsteroidal anti-inflammatory drugs were ineffective in relieving pain, the opioids nalbuphine or fentanyl were used for analgesia. The administered doses were recorded.
The dose of the test drug was determined based on quantitative pharmacology and the point-slope method. The point-slope method requires patients to be divided into 5–8 groups with an equal number of subjects in each group. The dose ratio of drugs between groups is 1:0.60–1: 0.85 is considered suitable.17 Previous studies have reported that when the nalbuphine dose exceeds 0.3 mg/kg, there is no further increase in analgesia, but the adverse reactions increase.19 In clinical trials in China, the usual dose of nalbuphine is 0.2 mg/kg. Therefore, we divided patients into 5 groups with 0.2 mg/kg as the base, and no patient in any group received a dose higher than 0.3 mg/kg. The ratio between groups was 0.70. The final test doses that were selected were 0.280, 0.200, 0.140, 0.100, and 0.070 mg/kg. In the test group, nalbuphine was diluted with normal saline to 20 mL and injected intravenously 5 min before the induction of anesthesia. Patients in group C were given an equivalent volume (20 mL) of normal saline at the same time and via the same route.
The ED50 and its 95% confidence interval (CI) from the NRS score obtained 30 min after extubation were calculated. NRS scores were used to evaluate the degree of pain in patients at four time points, namely, 1, 4, 8, and 24 h after the operation. A score of 0 indicated that the patient had no pain, 1–3 indicated mild pain, 4–6 indicated moderate pain, and > 6 indicated severe pain. Among them, an NRS score ≤ 3 is classified as effective analgesia, and a score ≥ 4 is classified as postoperative acute pain. The cumulative amount of remifentanil and propofol pumped during the operation and the time for anesthesia and operation were recorded. During recovery from anesthesia, the cough score during extubation and the sedation score 10 min after extubation were recorded. The specific scoring standards of Minogue cough score are as follows: 1 point for no cough, 2 points for mild cough (1–2 times) and able to pull out the tracheal tube smoothly, 3 points for moderate cough (3–4 times), 4 points severe cough (5–10 times), 5 points for restlessness, where the tracheal tube could not be pulled out. The Ramsay score was as follows: 1 point indicates that the patient was awake, restless, and irritable; 2 points indicate that the patient was sedated and drowsy but responded well and cooperated; 3 points indicate that the patient was lethargic, easy to awaken, and could follow instructions; 4 points indicate that the patient was asleep and was difficult to awaken, but had eyelash reflex; 5 points indicate that the patient was asleep, the eyelash reflex was absent, and the call response was slow; 6 points indicate that the patient was asleep and did not wake up. When the score was greater than 5, it was classified as excessive sedation. The incidence of adverse reactions and the number of remedial analgesics used within 24 h after the operation were recorded.
Statistical Analysis
Based on the requirements of the point-slope method in quantitative pharmacology, each group of large-scale biological count tests requires the enrollment of 5–15 subjects. Previous studies suggest a requirement of 5–20 cases per group.20 In this study, a maximum of 20 patients was selected and a total of 120 patients were required. Conducted the post hoc test of sample size using the Cochran-Armitage test for proportional trend using PASS 11.0 software with a power of 0.90 and alpha of 0.05.21 One-hundred patients were equally allocated to 5 trial groups and 5 effective rates of 15%, 35%, 55%, 80%, and 90% were obtained for nalbuphine for pain prevention during the awakening period. A continuously corrected Z test was chosen to determine the efficacy of the linear trend and was calculated for a total sample size of 35 (7 per dose group). The sample size of 20 patients per group in this study was sufficient to calculate the median of the effective doses.
IBM SPSS 25.0 was used for all statistical analysis and the point-slope formula was used to calculate the ED50 of nalbuphine and its 95% CI. The normality of continuous data was evaluated using the Shapiro–Wilk test. If the quantitative information was normally distributed, it was reported as mean ± standard deviation (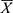 ± s). One-way ANOVA was used for comparisons between groups and repeated measures ANOVA was used for the comparison of repeated measures data. Quantitative data that did not conform to the normal distribution are expressed as median (M) and interquartile range (IQR). Kruskal–Wallis H-test was used for comparison between groups, and the generalized estimation equation was used for comparison of repeated measurement data. Bonferroni correction was used for post hoc comparisons. The count data was expressed as frequency (rate) and the test methods of χ2 or the Fisher exact probability were selected based on the specific situation. Inspection level: α = 0.05, P < 0.05 indicates the difference to be statistically significant.
± s). One-way ANOVA was used for comparisons between groups and repeated measures ANOVA was used for the comparison of repeated measures data. Quantitative data that did not conform to the normal distribution are expressed as median (M) and interquartile range (IQR). Kruskal–Wallis H-test was used for comparison between groups, and the generalized estimation equation was used for comparison of repeated measurement data. Bonferroni correction was used for post hoc comparisons. The count data was expressed as frequency (rate) and the test methods of χ2 or the Fisher exact probability were selected based on the specific situation. Inspection level: α = 0.05, P < 0.05 indicates the difference to be statistically significant.
Results
Consort plots are shown in Figure 1. From September 2020 to March 2021, a total of 264 patients undergoing laparoscopic total hysterectomy were screened. Apart from the inclusion and exclusion criteria, three patients were excluded due to operative time greater than 2 h, and one patient was excluded due to an intraoperative change in procedure. Another patient was enrolled to replace the excluded patient and was continued on that dose.18 The data from 120 patients were included in the analysis. The data in the six groups (P > 0.05) were comparable and there were no statistical differences in demographic data among them. The differences in outcomes between the administration of nalbuphine and saline before the induction of anesthesia were statistically significant (P < 0.05) in the prevention of moderate-to-severe pain (NRS score ≥ 4) in patients during the awakening period. Nalbuphine at doses of 0.140, 0.200, and 0.280 mg/kg was effective in preventing pain during the awakening period compared with the control (P < 0.05) (Table 1). By substituting the analgesic efficiency of the 5 groups in the test group into the point-slope formula, the ED50 (95% CI) for the prophylactic use of nalbuphine to reduce pain in the waking phase was calculated as 0.125 (0.108, 0.145) mg/kg.
 |
Table 1 Demographic Data of Patients and Intraoperative General Conditions |
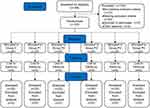 |
Figure 1 Flow diagram of the study. |
There were no significant differences in intraoperative propofol dosage between different nalbuphine dosage groups and the control group (P > 0.05). The difference in intraoperative remifentanil dosage among the 6 groups was statistically significant with a reduction in remifentanil dosage in the 0.280 mg/kg nalbuphine group compared with group C and the 0.100 mg/kg nalbuphine group (P < 0.001, P = 0.025) (Table 1).
Intergroup and temporal interactions existed for postoperative NRS scores in the 6 groups of patients (Wald χ2 = 73.630, P < 0.001), indicating different temporal trends in the different dose groups of nalbuphine. In terms of varying effects between groups, there were differences between the NRS scores in the 6 groups at 30 min and 1 h after extubation (Wald χ2 = 73.877, P < 0.001; Wald χ2 = 22.842, P < 0.001). Pairwise comparisons among groups revealed that at 30 min after extubation, compared with group P1, the NRS scores of groups P4, P5, and C were higher (P = 0.001, P < 0.001, P < 0.001); compared with group P2, groups P5 and C had higher NRS scores (P = 0.011, P = 0.001). At 1 h after extubation, the NRS scores of groups P1 and P2 were lower than that of group P4 (P = 0.046, P = 0.036) (Table 2).
 |
Table 2 NRS Scores at Different Time Points Within 24 Hours After the Operation |
There was a difference in cough scores at extubation among the 6 groups (P < 0.05), and when further compared, the differences between groups P1 and C were statistically significant (P = 0.009) (Table 3). There were no differences with respect to the number of patients requiring remedial analgesia within 24 h postoperatively among the 6 groups (P > 0.05), and none of the patients were administered opioid analgesics postoperatively (Table 4). There were no differences among groups in the incidence of adverse events postoperatively within 24 h among the 6 groups of patients (P > 0.05). None of the patients developed respiratory depression postoperatively. Among the adverse reactions, postoperative nausea and vomiting (PONV) had the highest incidence (Table 5).
 |
Table 3 Comparison of Cough Score During Extubation and Sedation Score 10 Minutes After Extubation |
 |
Table 4 Comparison of the Number of Postoperative Salvage Analgesia |
 |
Table 5 Comparison of the Incidence of Adverse Reactions After Surgery |
Discussion
The dose-effect curve of the drug was symmetrical and “S” shaped, with a large slope at the middle of the curve, ie, small changes in drug dose can cause large fluctuations in the positive rate. Therefore, it may be more clinically relevant to use ED50 as an indicator of pharmacodynamic evaluation than to use 95% of the effective drug dose. There are various methods of calculating ED50, and the point-slope method is one of the more practical methods of calculation. It requires uncomplicated test conditions and is easy to implement, simpler to calculate, and utilizes a rational test design. The ED50 (95% CI) of nalbuphine as an analgesic for prophylaxis during the awakening phase of patients was determined to be 0.125 (0.108, 0.145) mg/kg by substituting the effective rate into the point-slope formula. Compared with the control in this study, nalbuphine at doses of 0.140, 0.200, and 0.280 mg/kg prevented pain during the awakening period. Nalbuphine pushes at a dose of 0.280 mg/kg were associated with fewer cough episodes during extubation (Tables 1 and 3).
In patients undergoing laparoscopic gynecological surgery, irritation from pneumoperitoneum, perforation, and incision can cause pain and sensitize them to pain at the peripheral and central levels.22 Furthermore, postoperative visceral referred pain can occur when nerves are stretched or damaged by surgical equipment during surgery or when the peritoneum is irritated after CO2 absorption.23 We found that at most of time points pain scores were the highest in Group C postoperatively at 24 h and the lowest at nalbuphine doses of 0.280 mg/kg. The NRS scores of patients during the awakening period and within 24 h postoperatively decreased with increasing doses of nalbuphine (Table 2), suggesting that nalbuphine prophylaxis may not only reduce pain during the awakening period but also alleviate postoperative pain in patients.
To understand the underlying reasons, it is necessary to highlight that the uterus is mainly innervated by the sympathetic and parasympathetic nervous systems from the spinal cord. κ receptor agonists act on the spinal cord to inhibit the neuronal uptake of 5-hydroxytryptamine, enhancing the spinal analgesic pathway and stimulating opioid receptors on the neurons of the central nervous system to inhibit the transmission of action potentials in the nociceptive ascending pathway, thereby resulting in analgesia.24,25 Secondly, nalbuphine agonizes the opioid receptors in immune cells, activates their conformation, initiates intracellular signaling, and downregulates the function of the immune system.16 A study by Zhang et al concluded that nalbuphine prophylaxis is effective in controlling acute pain and regulating immune homeostasis in elderly patients in the early postoperative period after thoracotomy.26 Additionally, gene knockout experiments have shown that κ receptor agonists play an important role in regulating and treating visceral pain and they are superior to μ receptor agonists in alleviating visceral pain.27,28 We also found that patients in the 0.280 mg/kg nalbuphine group received significantly lower remifentanil compared with those in the control. Therefore, an intravenous bolus injection of nalbuphine before the induction of anesthesia can reduce the number of intraoperative opioids required, alleviate central sensitization caused by the sudden decrease in opioid concentrations in the body after remifentanil infusion is stopped, and decreased postoperative RIH.29 It can achieve the effect of preventive analgesia and relieve early acute pain in patients after the operation. Some researchers have found that local anesthetics injected at the trocar site, or a nerve block performed before surgery can reduce postoperative pain in patients undergoing laparoscopic surgery.30,31 If prophylactic nalbuphine is used in combination with these methods, better analgesia may be achieved. This study only focused on patients undergoing laparoscopic surgery, whereas total hysterectomy includes transabdominal, transvaginal, and laparoscopic-assisted surgery, and the surgical incision and postoperative pain caused by different methods vary.32–34 The efficacy of prophylactic nalbuphine for analgesia for other surgical modalities is uncertain.
There were no significant differences among the groups of patients who needed salvage analgesics after surgery (Table 4). Among them, group C had the largest number of patients who needed salvage analgesics after surgery. Although analgesics were added for these patients after surgery, the NRS score at each time point in the group was still higher than that in the trail group. We speculated that remedial additional analgesia, after nociceptive sensitization, peripheral and central sensitization, may not be as effective in patients who have not undergone prophylactic analgesia.
We found that the incidence of adverse reactions was highest in the 0.280 mg/kg nalbuphine dose group. The incidence of PONV in the six groups of patients was not significantly different (Table 5), which was contrary to the findings of Mao et al who suggested that nalbuphine may reduce the incidence of PONV by reducing the opioid dosage, decreasing central sensitivity to vomiting, and antagonizing the mu receptors.28 The reasons for these conflicting results may be that the subjects in our study were all women, which is an independent risk factor for PONV; thus, the effect due to nalbuphine was relatively small. Secondly, this finding may also be related to the sample size of each group in this trial; perhaps, different results may be obtained with larger sample sizes.
Limitation
Our study has several limitations. First, it only included female patients without other severe systemic diseases; thus, the results presented here cannot be extrapolated to patients with comorbidities. Second, the sample size in this paper was calculated using the point-slope method; thus, tests to determine postoperative acute pain indicators may lack the corresponding statistical efficacy.
Conclusion
The ED50 (95% CI) of nalbuphine as a prophylactic in reducing pain during recovery was 0.125 (0.108, 0.145) mg/kg. Compared with the control, nalbuphine at doses of 0.140, 0.200, and 0.280 mg/kg prevented pain during the awakening period. Among these doses, 0.280 mg/kg was determined to be the best; the occurrence of cough was less during extubation and the postoperative analgesia was good. However, it is necessary to pay attention to the occurrence of adverse reactions.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgments
This work was supported by the Department of Anesthesiology, Affiliated Hospital of Xuzhou Medical University. No commercial funding was received.
Disclosure
Min Wang and Dongyue Wang are co-first authors for this study. All authors report no conflicts of interest in this work.
References
1. Herrmann A, Torres-de la Roche LA, Krentel H, et al. Adhesions after laparoscopic myomectomy: incidence, risk factors, complications, and prevention. Gynecol Minim Invasive Ther. 2020;9(4):190–197. doi:10.4103/GMIT.GMIT_87_20
2. Novetsky AP, Boyd LR, Curtin JP. Trends in bilateral oophorectomy at the time of hysterectomy for benign disease. Obstet Gynecol. 2011;118(6):1280–1286. doi:10.1097/AOG.0b013e318236fe61
3. Blackwell RH, Kirshenbaum EJ, Shah AS, Kuo PC, Gupta GN, Turk TMT. Complications of recognized and unrecognized iatrogenic ureteral injury at time of hysterectomy: a population based analysis. J Urol. 2018;199(6):1540–1545. doi:10.1016/j.juro.2017.12.067
4. Orhan A, Ozerkan K, Kasapoglu I, et al. Laparoscopic hysterectomy trends in challenging cases (1995–2018). J Gynecol Obstet Hum Reprod. 2019;48(10):791–798. doi:10.1016/j.jogoh.2019.06.007
5. Clark NV, Moore K, Maghsoudlou P, et al. Superior hypogastric plexus block to reduce pain after laparoscopic hysterectomy: a randomized controlled trial. Obstet Gynecol. 2021;137(4):648–656. doi:10.1097/AOG.0000000000004329
6. An XJ, Liu RJ, Yang J, Hu XK, Wu GR, Chen JP. Effects of remifentanil gradual withdrawal on remifentanil induced postoperative hyperalgesia. Zhonghua Yi Xue Za Zhi. 2019;99(17):1298–1301. doi:10.3760/cma.j.issn.0376-2491.2019.17.005
7. Choi JJ, Kim K, Park HY, et al. CONSORT the effect of a bolus dose of dexmedetomidine on postoperative pain, agitation, and quality of recovery after laparoscopic cholecystectomy. Medicine. 2021;100(3):e24353. doi:10.1097/MD.0000000000024353
8. Sao CH, Chan-Tiopianco M, Chung KC, et al. Pain after laparoscopic surgery: focus on shoulder-tip pain after gynecological laparoscopic surgery. J Chin Med Assoc. 2019;82(11):819–826. doi:10.1097/JCMA.0000000000000190
9. Liu YM, Feng Y, Liu YQ, et al. Chinese Association for the Study of Pain: expert consensus on chronic postsurgical pain. World J Clin Cases. 2021;9(9):2090–2099. doi:10.12998/wjcc.v9.i9.2090
10. Horn A, Kaneshiro K, Tsui BCH. Preemptive and preventive pain psychoeducation and its potential application as a multimodal perioperative pain control option: a systematic review. Anesth Analg. 2020;130(3):559–573. doi:10.1213/ANE.0000000000004319
11. Hu J, Chen S, Zhu M, et al. Preemptive nalbuphine attenuates remifentanil-induced postoperative hyperalgesia after laparoscopic cholecystectomy: a prospective randomized double-blind clinical trial. J Pain Res. 2020;13:1915–1924. doi:10.2147/JPR.S257018
12. Zhu L, Cui Z, Zhu Q, Zha X, Xu Y. Novel opioid receptor agonists with reduced morphine-like side effects. Mini Rev Med Chem. 2018;18(19):1603–1610. doi:10.2174/1389557518666180716124336
13. Gress K, Charipova K, Jung JW, et al. A comprehensive review of partial opioid agonists for the treatment of chronic pain. Best Pract Res Clin Anaesthesiol. 2020;34(3):449–461. doi:10.1016/j.bpa.2020.06.003
14. Sadafule NN, Karhade SS. Comparative study of efficacy of preoperative nalbuphine hydrochloride and pentazocine lactate on hemodynamic response to tracheal intubation and postoperative analgesia. Anesth: Essays Res. 2018;12(1):218–222. doi:10.4103/aer.AER_168_17
15. Sun Z, Zhu Z, Yang G, Zheng H. The 95% effective dose of nalbuphine in patient-controlled intravenous analgesia for patients undergoing laparoscopic total hysterectomy compared to equivalent sufentanil. Medicine. 2020;99(22):e20424. doi:10.1097/MD.0000000000020424
16. Xi MY, Li SS, Zhang C, Zhang L, Wang T, Yu C. Nalbuphine for analgesia after orthognathic surgery and its effect on postoperative inflammatory and oxidative stress: a randomized double-blind controlled trial. J Oral Maxillofac Surg. 2020;78(4):528–537. doi:10.1016/j.joms.2019.10.017
17. Feng AM, Lu XH, Li J. Interactive effect between dexmedetomidine and propofol for sedation induction. J Clin Anesthesiol. 2018;34(5):429–431.
18. Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: a systematic review and meta-analysis. J Clin Anesth. 2021;68:110071. doi:10.1016/j.jclinane.2020.110071
19. Kubica-Cielińska A, Zielińska M. The use of nalbuphine in paediatric anaesthesia. Anaesthesiol Intensive Ther. 2015;47(3):252–256. doi:10.5603/AIT.2015.0036
20. Zhang CH, Ma WQ, Yang YL, Wang HM, Dong FT, Huang ZX. Median effective effect-site concentration of sufentanil for wake-up test in adolescents undergoing surgery: a randomized trial. BMC Anesthesiol. 2015;15:27. doi:10.1186/s12871-015-0003-2
21. Xiao F, Drzymalski D, Liu L, Zhang Y, Wang L, Chen X. Comparison of the ED50 and ED95 of intrathecal bupivacaine in parturients undergoing cesarean delivery with or without prophylactic phenylephrine infusion: a prospective, double-blind study. Reg Anesth Pain Med. 2018;43(8):885–889. doi:10.1097/AAP.0000000000000850
22. Zhou M, Wang L, Wu C, et al. Efficacy and safety of different doses of dezocine for preemptive analgesia in gynecological laparoscopic surgeries: a prospective, double blind and randomized controlled clinical trial. Int J Surg. 2017;37(Suppl 1):539–545. doi:10.1016/j.ijsu.2017.09.079
23. Fagotti A, Vizzielli G, Fanfani F, et al. Randomized study comparing use of THUNDERBEAT technology vs standard electrosurgery during laparoscopic radical hysterectomy and pelvic lymphadenectomy for gynecologic cancer. J Minim Invasive Gynecol. 2014;21(3):447–453. doi:10.1016/j.jmig.2013.12.001
24. Das A, RoyBasunia S, Mukherjee A, et al. Perineural nalbuphine in ambulatory upper limb surgery: a comparison of effects of levobupivacaine with and without nalbuphine as adjuvant in supraclavicular brachial plexus block - a prospective, double-blinded, randomized controlled study. Anesth: Essays Res. 2017;11(1):40–46. doi:10.4103/0259-1162.200225
25. Finley MJ, Happel CM, Kaminsky DE, Rogers TJ. Opioid and nociceptin receptors regulate cytokine and cytokine receptor expression. Cell Immunol. 2008;252(1–2):146–154. doi:10.1016/j.cellimm.2007.09.008
26. Zhang Y, Jiang Q, Li T. Nalbuphine analgesic and anti-inflammatory effects on patients undergoing thoracoscopic lobectomy during the perioperative period. Exp Ther Med. 2017;14(4):3117–3121. doi:10.3892/etm.2017.4920
27. Narver HL. Nalbuphine, a non-controlled opioid analgesic, and its potential use in research mice. Lab Anim. 2015;44(3):106–110. doi:10.1038/laban.701
28. Mao Y, Cao Y, Mei B, et al. Efficacy of nalbuphine with flurbiprofen on multimodal analgesia with transverse abdominis plane block in elderly patients undergoing open gastrointestinal surgery: a randomized, controlled, double-blinded trial. Pain Res Manag. 2018;2018:3637013. doi:10.1155/2018/3637013
29. Wu Z, Yu J, Lin Q, et al. Effects of an intraoperative intravenous bolus dose of dexmedetomidine on remifentanil-induced postinfusion hyperalgesia in patients undergoing thyroidectomy: a double-blind randomized controlled trial. Anesth Analg. 2021;132(2):320–328. doi:10.1213/ANE.0000000000005003
30. Hortu I, Turkay U, Terzi H, et al. Impact of bupivacaine injection to trocar sites on postoperative pain following laparoscopic hysterectomy: results from a prospective, multicentre, double-blind randomized controlled trial. Eur J Obstet Gynecol Reprod Biol. 2020;252:317–322. doi:10.1016/j.ejogrb.2020.07.007
31. Huang L, Zheng L, Zhang J, et al. Transmuscular quadratus lumborum block versus oblique subcostal transversus abdominis plane block for analgesia in laparoscopic hysterectomy: a randomised single-blind trial. BMJ open. 2021;11(8):e043883. doi:10.1136/bmjopen-2020-043883
32. Buzzaccarini G, Noventa M, D’Alterio MN, et al. vNOTES hysterectomy: can it be considered the optimal approach for obese patients? J Invest Surg. 2021;35:1–2.
33. Buzzaccarini G, Stabile G, Török P, et al. Surgical approach for enlarged uteri: further tailoring of vNOTES hysterectomy. J Invest Surg. 2021:1–2. doi: 10.1080/08941939.2021.1967528
34. Casarin J, Ielmini M, Cromi A, et al. Post-traumatic stress following total hysterectomy for benign disease: an observational prospective study. J Psychosom Obstet Gynaecol. 2020:1–7. doi:10.1080/0167482X.2020.1752174
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
