Back to Journals » Journal of Pain Research » Volume 15
A Delphi Study on the Management of Neuropathic Cancer Pain in Spain: The DOLNEO Study
Authors Pérez-Hernández C , Cánovas ML, Carmona-Bayonas A, Escobar Y , Margarit C, Mulero Cervantes JF , Quintanar T, Serrano Alfonso A , Virizuela J
Received 9 March 2022
Accepted for publication 21 June 2022
Published 2 August 2022 Volume 2022:15 Pages 2181—2196
DOI https://doi.org/10.2147/JPR.S365351
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amitabh Gulati
Concepción Pérez-Hernández,1 María Luz Cánovas,2 Alberto Carmona-Bayonas,3 Yolanda Escobar,4 César Margarit,5 Juan Francisco Mulero Cervantes,6 Teresa Quintanar,7 Ancor Serrano Alfonso,8 Juan Virizuela9
1Pain Unit, Hospital Universitario de la Princesa, Madrid, Spain; 2Anesthesia, Complexo Hospitalario Universitario de Ourense (SERGAS), Ourense, Spain; 3Hematology and Medical Oncology, Hospital Universitario Morales Meseguer, UMU, IMIB, Murcia, Spain; 4Medical Oncology, Hospital General Universitario Gregorio Marañon, Madrid, Spain; 5Pain Unit, Hospital General Universitario de Alicante, Alicante, Spain; 6Pain Unit, Hospital General Universitario Santa Lucía, Cartagena, Spain; 7Medical Oncology, Hospital General Universitario de Elche, Elche, Spain; 8Anesthesiology, Resuscitation and Pain Management, Hospital Universitari de Bellvitge, L’Hospitalet de Llobregat, Barcelona, Spain; 9Medical Oncology, Hospital Universitario Virgen Macarena, Sevilla, Spain
Correspondence: Concepción Pérez-Hernández, Pain Unit, Hospital Universitario de la Princesa, Calle de Diego de León 62, Madrid, 28006, Spain, Tel +34 915 20 22 00, Email [email protected]
Purpose: The objectives of this project were to assess the current situation and management of cancer-related neuropathic pain (CRNP) in Spain and to provide specific recommendations for the assessment, diagnosis and treatment of CRNP using a Delphi methodology.
Methods: This was a qualitative study that followed a Delphi methodology using a questionnaire with 56 statements that were grouped into 5 areas related to CRNP: prevalence and impact, pathophysiology, assessment and diagnosis, specific syndromes, treatment, and multidisciplinary approach. Based on the responses, the scientific committee prepared an algorithm and a recommended pathway for the management of CRNP.
Results: Seventy-nine physicians attended the meeting and completed the questionnaire. Consensus was reached for all statements relating to the prevalence and impact of CRNP. However, the perceptions of specialists from palliative care of the frequency and impact of CRNP differed from those of other specialists. A high degree of consensus was reached for all statements concerning the assessment and diagnosis of CRNP. Regarding specific syndromes, the only statement with a lack of consensus was that on the frequency of NP in patients undergoing radiotherapy. There were some disagreements regarding the multidisciplinary approach and referral criteria for the management of NP.
Conclusion: Our results show a large degree of agreement on the assessment, diagnosis and treatment of cancer-related neuropathic pain among the specialists involved in its management. There were, however, some disagreements regarding the multidisciplinary approach and referral criteria for the management of neuropathic pain.
Keywords: cancer-related neuropathic pain, prevalence, diagnosis, treatment, consensus
Plain Language Summary
The objectives of this project were to assess the current situation and how cancer-related neuropathic pain is diagnosed and treated in Spain and to provide specific recommendations for the assessment, diagnosis and treatment. For doing so, we asked the opinion on fifty-six specific issues to a group of seventy-nine physicians who deal with cancer-related neuropathic pain. Our results show a large degree of agreement on the assessment, diagnosis and treatment of cancer-related neuropathic pain among the specialists involved in its management. There were, however, some disagreements regarding the multidisciplinary approach and referral criteria for the management of neuropathic pain.
Introduction
Neuropathic pain, defined as “pain caused by a lesion or disease of the somatosensory nervous system”,1 is common in patients with cancer and has an important impact on the patient’s functioning. A systematic review that included 14 studies with a confirmatory evaluation of the sensory abnormality or diagnostic lesion—a requirement for the diagnosis of neuropathic pain—reported a prevalence of neuropathic pain among patients with cancer ranging from 19% as a conservative estimate to 39% when patients with mixed pain were considered.2 More recently, a systematic review of 29 studies reporting data on cancer-related neuropathic pain (CRNP) estimated a pooled prevalence of 31%.3 In patients with cancer pain, the presence of a neuropathic component is associated with a higher incidence of breakthrough pain, longer pain duration, higher pain intensity and greater interference with patients’ daily activities.4–6 Even after controlling for disease stage, cancer duration, radiotherapy, chemotherapy and comorbidities, the presence of CRNP is associated with a deterioration in quality of life.7 Compared to patients with pain of nociceptive origin, patients with CRNP exhibit greater analgesic requirements, worse performance status, and poorer physical, cognitive and social functioning.8
The etiology of CRNP is very heterogeneous and can broadly be categorized as disease-related (eg, tumor-related bone pain) or treatment-related neuropathic pain that can be secondary to surgery, radiotherapy or chemotherapy interventions.9,10 The adequate diagnosis of CRNP is key for establishing an optimal treatment strategy.11 However, the diagnosis of neuropathic pain in patients with cancer is complicated by the presence of coexisting processes, such as neurological disease or muscle spasticity, that may confuse the clinical picture, leading to underrecognition and, consequently, undertreatment of this clinical condition.10 This is further complicated in the case of chemotherapy-induced neuropathic pain (CINP) due to underreporting; therefore, adequate anamnesis is essential for identifying the origin of CRNP. Several studies have reported that patients with CRNP are not adequately treated, with a high proportion of patients not receiving adjuvant analgesics, less than half of patients receiving drugs from steps 2 and 3 of the WHO’s cancer pain ladder for adults, and in patients with mixed pain, a lack of treatment of the neuropathic component.5–7,12
The lack of specific clinical practice guidelines on the management of CRNP could have contributed to the abovementioned situation. In 2013–2014, a group of experts published their analysis of 9 European clinical practice guidelines that mentioned the management of CRNP.13–15 The authors reported great heterogeneity regarding the recommendations on diagnosis and assessment.14 Regarding treatment, the majority of guidelines based their recommendations on the extrapolation of data from noncancer publications without considering the specific situation of cancer patients,15 with the exception of some specific guidelines for CINP.16,17 Although the picture has improved and more recent guidelines on cancer pain devote a specific section to CRNP,18,19 only the European Society of Medical Oncology includes a treatment algorithm.18 Additionally, there is still a need for a comprehensive guide fully devoted to the management of CRNP. The American Society of Clinical Oncology also issued a guideline in 2020, but it is focused on CINP.20
The objectives of this project were to assess the current situation and management of CRNP in Spain and to provide specific recommendations for the assessment, diagnosis and treatment of CRNP using a Delphi methodology.
Materials and Methods
The DOLNEO (“DOLor NEuropatico Oncológico”; neuropathic pain in oncology) study was a qualitative study that followed a Delphi methodology, as explained below. The study was revised and approved by the Ethics Committee of the University Hospital “La Princesa” (Reference number 3789, 23-05-19, acta CEIm 10/19; Madrid, Spain).
Selection of Experts and Development of the Questionnaire
The scientific committee comprised two experts: a pain physician from a pain unit and a medical oncologist with broad experience in the management of cancer pain, including CRNP. Seventy-nine physicians from several specialties were selected by the scientific committee from the main hospitals throughout Spain, including physicians from pain units, medical oncology, radiation therapy and palliative care departments/units. They were invited to participate in the project via e-mail. They did not receive any incentive for their participation.
A literature search on CRNP was performed and revised by the scientific committee. Based on that revision and the key elements mentioned in a recent Spanish guideline for the interdisciplinary approach to cancer pain,21 they prepared a questionnaire containing 56 statements that were grouped into 5 areas: prevalence and impact, pathophysiology, assessment and diagnosis, specific syndromes, treatment and multidisciplinary approach to CRNP. The specific references from which the statements were extracted are shown in the results section. The questionnaire included a section requesting information on participant characteristics and background, including information on age, sex, geographic area, type of center (ie, public, private, mixed), years of experience, current medial specialty, whether they have had experience as a trainer on CRNP in the current year (2019), whether they have participated in any research on CRNP in the current year (2019) and the number of patients with CRNP they have seen in the last month. The second section of the questionnaire contained the 56 statements that had to be answered on a 5-point Likert scale: 1=fully disagree, 2=disagree, 3=neither agree nor disagree, 4=agree, 5=fully agree.
The Delphi Consensus Process
The Delphi method is a frequently used system to gather opinions in a structured way from a group of experts.22 The key characteristics of the method are the anonymous nature of the survey and that the participants receive feedback on their answers and may adjust their initial answers to that feedback using an iterative process.22
Two rounds of the survey were performed. For the first round, all participants attended a one-day meeting in Madrid (Spain). During the meeting, the scientific committee facilitated a discussion with all the attendees on the topics of the project that comprised all the items included in the questionnaire. After finalizing the meeting, the participants had to answer the questionnaire anonymously through a website specifically designed for the project. In the second round, the participants received via e-mail the results of the statistical analysis of the first round and a request to complete a second questionnaire that comprised only those questions where consensus (see definition below) was not reached in the first round.
The Algorithm and Pathway for the Management of CRNP
The final results of the consensus were reviewed by the scientific committee, who, based on the responses, prepared an algorithm for the management of CRNP and a recommended pathway for the management of these patients.
Statistical Analysis
Analysis of the questionnaire was performed using descriptive statistics. Thus, the absolute and relative frequencies for each answer were calculated. We considered that consensus was reached when at least 75% of the answers indicated that they fully agreed or agreed or fully disagreed or disagreed; this cut-off point for consensus has been used by other authors such as the American Society of Clinical Oncology.23 In addition, the median was also calculated to indicate the strength of the consensus. To compare some responses across the specialties involved in the study, the Kruskal–Wallis test was applied.
All analyses were performed using SAS, version 9.1.3 Service Pack 3.
Results
Participants
Seventy-nine physicians attended the meeting and completed the questionnaire. Although participants were working in 16 of the 17 geographic regions, one-fourth of them were working in Madrid. Participants were evenly distributed regarding sex and had a mean (SD) age of 44.7 (11.6) years. Most of them were working in a public (n=59, 74.7%) or mixed (n=13, 16.5%) center. The specialties involved among the 75 participants who answered that question were anesthesiologists/pain physician (38.7%), radiation oncology (25.3%), medical oncology (21.3%), palliative care (13.3%) and neurology (1.4%). The mean (SD) years of experience was 14.6 (10.3). In the current year (2019), 23 (29.1%) of the respondents had participated as a lecturer in training on CRNP, and 17 (21.5%) had participated in other research on CRNP. Regarding the number of patients with CRNP treated in the last month, 36 (45.6%) treated 0–10 patients, 26 (32.9%) treated 11–20 patients, 8 (10.1%) treated 20–30 patients and 4 (5.06%) treated more than 30 patients (in 5 [6.3%], this result was missing). The number of patients with CRNP treated in the last month was higher among respondents of the palliative care and pain units (data not shown).
Prevalence and Impact of CRNP
There was consensus on all the statements of this section. The strength of the consensus was somewhat weaker (ie, a median of 4 instead of a median of 5) for most of the questions related to prevalence (Table 1).
 |
Table 1 Results of the Delphi Consensus Process for the Statements on the Prevalence and Impact of Cancer-Related Neuropathic Pain |
Regarding the statement on the frequency of moderate to severe pain, respondents from palliative care units showed a greater degree of agreement, with a median of 5 on that statement compared to a median of 4 for the respondents of each of the other specialties; however, the distribution of the responses did not significantly differ across the specialties (data not shown). Respondents from palliative care and pain units (100%) agreed that CRNP has an impact on patients’ quality of life and worsens mood, decreasing their response to treatment and influencing the evolution of their disease, and the degree of agreement was lower in other specialties (p=0.0002).
Pathophysiology of CRNP
Consensus was reached on all 4 statements of this section, with a median of 5 on all statements, with the exception of the mitochondrial toxicity hypothesis as the main cause of chemotherapy-induced neuropathic pain (Table 2).
 |
Table 2 Results of the Delphi Consensus Process for Statements on the Pathophysiology of Cancer-Related Neuropathic Pain |
Assessment and Diagnosis of CRNP
There was consensus on all 8 statements, with a median of 5, except on the general statement on the difficulties associated with CRNP diagnosis due to the lack of standardized diagnostic tools; eleven (14.0%) disagreed with the presence of those difficulties (Table 3).
 |
Table 3 Results of the Delphi Consensus Process for the Statements on the Assessment and Diagnosis of Cancer-Related Neuropathic Pain |
Specific Syndromes of CRNP
In all but one of the statements regarding the specific syndromes, there was consensus (Table 4). The statement without consensus concerns the prevalence of radiotherapy-induced neuropathic pain, which stated that “of breast cancer patients undergoing radiotherapy treatment, 21–65% will develop chronic neuropathic pain”. Eighteen (25%) of the respondents disagreed with the statement. When analyzing this statement by specialty, the distribution of the responses was almost identical across all specialties, with a proportion of respondents who disagreed ranging from 19.2% among physicians from pain units to 27.8% among those from radiation oncology.
 |
Table 4 Results of the Delphi Consensus Process for the Statements on Specific Syndromes of Cancer-Related Neuropathic Pain |
Treatment for Patients with CRNP
The respondents agreed with all 8 statements regarding the treatment of CRNP, with a high degree of consensus on most of the statements (Table 5).
 |
Table 5 Results of the Delphi Consensus Process for the Statements on the Treatment of Cancer-Related Neuropathic Pain |
Multidisciplinary Approach and Referral Criteria for the Management of CRNP
There was consensus on all other statements about referral criteria (Table 6), with the exception of the statement that “their hospital facilities do not guarantee a correct approach to their patients with cancer pain”, with which 40% of respondents disagreed. When analyzed by treatment specialty, medical oncology respondents showed the highest proportion of disagreement (67%), whereas those from radiation oncology showed the lowest percentage (22%), although the differences across specialties were not statistically significant (p=0.1131).
 |
Table 6 Results of the Delphi Consensus Process for the Statements on the Referral Criteria for Cancer-Related Neuropathic Pain |
There were some other differences across specialties regarding the multidisciplinary approach to the management of CRNP. There was consensus that the “collaboration between the Pain Unit and the Medical Oncology Unit seems appropriate to address cancer pain” for medical oncology (93%), pain physicians (100%) and palliative care (80%) but not among respondents from radiation oncology (44%) (p<0.0001 for the comparison across specialties). Although the differences were not statistically significant across specialties, the proportion of agreement with the statement “I am prepared to take care of patients with cancer pain” was 75% for pain physicians compared to 94% for medical oncology, 88% for radiation oncology and 100% for palliative care (p=0.1468).
The Algorithm and Pathway for the Management of CRNP
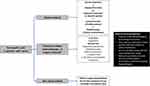
Based on the results, we propose a treatment algorithm and clinical pathway, which are presented in Figures 1 and 2, respectively. The treatment algorithm reflects that the pharmacological approach to neuropathic pain in cancer patients must be clearly differentiated depending on the etiology of the pain, distinguishing 3 scenarios. The first scenario is pain due to the disease itself, whose approach is mainly based on the treatment of the tumor, associated with opioids in first-line treatment and other drugs or adjuvant treatments such as corticosteroids. The second is neuropathic pain related with cancer treatment; in these cases, the first-line treatment would be duloxetine at a dose of 60 mg and second-line options comprised anticonvulsants (ie pregabalin, gabapentin), other antidepressants (eg venlafaxine, amitriptyline) or topical treatments (Capsaicin 8%, Baclofen + Amitriptyline + Ketamine). The third scenario is neuropathic pain occurring in the context of a patient with cancer, but not related with the disease itself or its treatment (eg postherpetic neuralgia, diabetic neuropathy); the treatment of these latter cases should follow the recommendations included in the clinical practice guidelines for neuropathic pain, with first lines of dual/tricyclic antidepressants and anticonvulsants, second line with topical treatments and third line opioids.
Discussion
Although consensus was reached for all statements relating to the prevalence and impact of CRNP, it was somewhat weaker for statements on prevalence. This probably reflects the different experiences of several specialists with neuropathic pain. In a recent systematic review, the raw prevalence of CRNP in 29 observational studies was 31%, whereas when evaluated in a survey among 137 physicians working in 50 Italian centers of palliative care reported in the same communication, the prevalence was 44%.3 In our study, we did not evaluate the perception of the respondents on the prevalence of CRNP; however, it is likely they considered it higher than what is reported in the literature. In any case, according to a systematic review, the prevalence of neuropathic pain in patients with cancer varies from 19% to 39% when including patients with mixed pain.2 On the other hand, in many cases, cancer is a long-lasting disease, and the prevalence of CRNP may differ substantially during the course of the disease, from the initial diagnosis through the treatment and finally in patients who survive or, on the contrary, require palliative care. In our view, further studies on the prevalence of CRNP throughout the course of the disease are needed.
The perceptions of specialists from palliative care on the frequency and impact of CRNP differs from that of other specialists, with a higher frequency of patients with CRNP, a higher frequency of moderate to severe pain and a greater impact on the quality of life and mood. These results are consistent with other studies on cancer pain. A study evaluating the prevalence and impact of breakthrough pain and its impact among patients with cancer found the highest prevalence among patients treated in palliative care units.24 In a study conducted of 156 patients with cancer, the authors reported that those with neuropathic pain hospitalized in the palliative care unit showed greater severity of symptoms of fatigue and depression than those hospitalized in general wards.25
A high degree of consensus was reached for all statements concerning the assessment and diagnosis of CRNP. However, it is interesting to note that whereas 80% of the respondents agreed that ascertaining the presence of neuropathic pain is not easy because of the lack of standardized tools, 14% disagreed with this notion. Possibly the more rigorous tool for diagnosing neuropathic pain is the grading system proposed by the Special Interest Group on Neuropathic Pain (NeuPSIG) of the International Association for the Study of Pain, which, using information from the history, clinical examination and confirmatory tests, categorized pain as possible, probable and definite.26 Previous criteria of the NeuPSIG have been adapted for cancer patients through a consensus process,27 but we are not aware of attempts to adapt the 2016 updated criteria. In fact, the NeuPSIG criteria have not been widely adopted, as their reliability and applicability in clinical practice have not been established.10 The DN4 (Douleur Neuropathique [Neuropathic Pain]-4 items), Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) and painDETECT (PDQ) are screening tools for neuropathic pain that have shown good accuracy in patients with cancer; it is believed that until the standardization of clinical diagnosis for CRNP is validated, these tools may be useful in clinical practice to identify potential cases of CRNP.11 This situation is consistent with the agreement of most respondents in our survey, indicating that there are no standardized diagnostic tools for NP diagnosis.
Regarding specific syndromes, the only statement with a lack of consensus was the following: “Of breast cancer patients undergoing RT treatment, 21–65% will develop chronic NP”, which was based on a literature review on CRNP.28 The development of neuropathic pain after the diagnosis of breast cancer is frequent (over 30% of the patients during the first year after diagnosis), and anxiety, arm symptoms, Stage III/IV cancer, breast-conserving surgery with axillary lymph node dissection, mastectomy with axillary lymph node dissection and damage to the intercostobrachial nerve have been identified as risk factors for the occurrence of neuropathic pain.29 The prevalence of CRNP is higher after treatment; thus, in a systematic review, Ilhan et al30 reported that among patients with breast cancer who reported pain after treatment, the estimated prevalence of neuropathic pain ranged from 33% to 58% using screening questionnaires and from 30% to 57% using NeuPSIG criteria; however, this systematic review does not specify figures for radiotherapy. It is possible that our lack of consensus reflects to some extent a lack of information on this topic. Other authors have reported that radiotherapy-induced neuropathic pain has scarcely been investigated.31
There were some disagreements regarding the multidisciplinary approach and referral criteria for the management of neuropathic pain. Thus, there was no consensus on the statement “current hospital facilities do not guarantee a correct approach to patients with cancer pain”, especially among medical oncologists. The lack of consensus among respondents from oncology radiotherapy with the statement “a collaboration between the Pain Unit and the Medical Oncology Unit seems appropriate to address cancer pain” is likely a consequence of not being one of the specialties involved in the statement. Interestingly, although all specialists reached consensus that they were prepared to take care of patients with cancer pain, respondents from pain units showed the lowest degree of agreement; we think that this finding suggests a perception of greatest need for a multidisciplinary approach from the perspective of the pain physician, a hypothesis that is supported by the fact that 100% of pain physicians agreed that “collaboration between the Pain Unit and the Medical Oncology Unit seems appropriate to address cancer pain”. Overall, we believe that these disagreements reflect the lack of a multidisciplinary and holistic approach to the management of CRNP, an approach that, in our view, should be promoted by the specialties involved.
Conclusions
Our main conclusions are as follows:
- There was a high degree of consensus on statements related to the prevalence and impact of CRNP among specialists.
- By consensus, the pathophysiology of CRNP is considered to be complex and largely unknown and coexists with other pathologies.
- It is important to determine the etiology of neuropathic cancer pain because its management differs depending on whether it is disease-related or treatment-related.
- Neuropathic cancer pain assessment and diagnosis are not easy, as there are no standard tools to diagnose this type of pain. However, there is consistency between clinical diagnosis and the results of screening tools, such as LANSS and DN-4.
- Neuropathic cancer pain prevention and early recognition are crucial to avoid serious and disabling forms, and for this purpose, it is essential to avoid or decrease neurotoxic events after cancer treatment and to determine the predictive factors of neuropathic cancer pain (female sex, youth, increased body mass index, more advanced cancer stage, perineural invasion, chemotherapy or invasive surgeries, genetic polymorphisms associated with increased pain sensitivity, depression, anxiety, stress, sleep disorders, low socioeconomic level, multifocal pain and intensity of perioperative pain).
- Chemotherapy-induced neuropathic pain is a limiting factor in cancer treatment. It is often underreported, underdiagnosed and undertreated. It can cause delays in the administration of a new cycle, reduction of cycle doses or even decreased therapy.
- Chemotherapy-induced neuropathic pain has a high prevalence early (first months) in treatment, and in almost 30% of patients, it becomes chronic (> 6 m); it is especially frequent in head and neck, gynecological, gastrointestinal, lung and urogenital tumors. Taxanes and vinca alkaloids frequently produce persistent polyneuropathy.
- There is no clear consensus on first-line treatment, which is a challenge for clinicians and patients. Duloxetine is a treatment that has shown sufficient evidence to recommend its use in oxaliplatin-related chemotherapy-induced neuropathic pain.
- Regarding new treatments, there are advances in topical treatments with baclofen + amitriptyline + ketamine, menthol and capsaicin 8%, the last one as a disease-modifying drug. There are also systemic drugs, such as angiotensin-II receptor antagonists and Toll-like receptor 4 (TLR4) receptor inhibitors.
- Collaborative work between pain and oncology units is recommended when addressing cancer-related pain, especially in patients with significant pain severity or decreased performance as well as those who would benefit from interventional pain management.
Abbreviations
CINP, chemotherapy-induced neuropathic pain; CRNP, cancer-related neuropathic pain, DN4, Douleur Neuropathique [Neuropathic Pain]-4 items; LANSS, Leeds Assessment of Neuropathic Symptoms and Signs; NeuPSIG, Special Interest Group on Neuropathic Pain; PDQ, painDETECT; SD, standard deviation.
Data Sharing Statement
All data are presented in the manuscript.
Ethics Approval and Informed Consent
The study was revised and approved by the Ethics Committee of the University Hospital “La Princesa” (Madrid, Spain). The study did not include patients but instead was a survey among physicians; therefore, informed consent was not required.
Acknowledgments
We thank Ana Esquivias and Manuela Rubio (Madrid, Spain) for the logistic coordination of the project. We would also like to thank all participants in consensus building: Alberto Carmona Bayonas (Murcia, Spain); Alejandro Falcon Gonzalez (Sevilla, Spain); Alejandro Gonzalez Forastero (Jerez, Spain); Alfonso Carregal Raño (Vigo, Spain); Alvaro Gandara del Castillo (Madrid, Spain); Amalia Sotoca Ruiz (Madrid, Spain); Ana Calin Lorca (Madrid, Spain); Andere Frias Capanaga (Bilbao, Spain); Andres Ancor Serrano Afonso; Angel Estuardo Plasencia Ezaine; Belinda Montalban Moreno (Madrid, Spain); Cesar Margarit Ferri; Concepcion Martin Iglesias (Riaño, Spain); Concepcion Perez Hernandez; Cristina de Miguel Sanchez; David Abejon Gonzalez; David Miguel Muñoz Carmona (Sevilla, Spain); Dulce Rodriguez Mesa; Elena Arregui Lopez (Ciudad Real, Spain); Enrique Cabrera Espinos (Lleida, Spain); Enrique Sanchez Jimenez; Esperanza Ortigosa Solorzano (Madrid, Spain); Estrella Uriarte Brizuela; Franc Pagan Ferrer; Francisco Javier Fuertes Velez (Bilbao, Spain); Gabriel Enrique Martinez Lopez; Gema Maria Marquez Garrido; Gorka Conejero Morga (San Sebastián, Spain); Ignacio Solis Navarro (Valladolid, Spain); Irene Garcia Cuartero (Albacete, Spain); Irene Riquelme Osado; Irene Ruperez San Emeterio (León, Spain); Iria Carou Frieiro (Pontevedra, Spain); Iris Violeta de la Rocha Vedia; Isabel Castillo Perez (Granada, Spain); Isabel Prieto Muñoz (Madrid, Spain); Ivan Diaz de Cerio Martinez (Santander, Spain); Javier Tomas Anchuelo Latorre (Santander, Spain); Joaquim Julia Torras (Badalona, Spain); Jose Juan Illarramendi Mañas; Jose Luis Gomez Palones; Jose Luis Herrero Burgos (Jerez, Spain); Jose Manuel Lopez-Millan Infantes (Sevilla, Spain); Juan Antonio Nuñez Sobrino (Madrid, Spain); Juan Antonio Virizuela Echaburu (Sevilla, Spain); Juan Carlos de la Pinta Garcia; Juan Carlos Quero Guillen; Juan Carlos Sierra Sanchez; Juan David Cardenas (Toledo, Spain); Juan Francisco Mulero Cervantes; Juan Jose Lozano Sanchez; Laura Diaz Paniagua (Madrid, Spain); Laura Ferrera Alayon (Las Palmas de Gran Canaria, Spain); Lidia Maria Castro Freitas; Lucio Gonzalez Montero; Luis Clemente Armendariz; Manuel Ignacio Algara Lopez (Barcelona, Spain); Manuel Jose Mejias Estevez; Margarita Martin Martin (Madrid, Spain); Maria Antonia Gomez Aparicio (Ciudad Real, Spain); Maria Ara Bermejo Marin; Maria Aranzazu Eraso Urien (Girona, Spain); Maria Candelas Madariaga Muñoz; Maria Carnero Gonzalez (Arganda del Rey, Spain); Maria Consuelo Nieto Iglesias; Maria Gorety Pazos Gonzalez (A Coruña, Spain); Maria Luisa Tarraso Gomez (Valencia, Spain); Maria Luz Canovas Martinez; Maria Olga Donnay Candil (Madrid, Spain); Maria Teresa Bovaira Forner (Valencia, Spain); Maria Teresa Quintanar Verduguez (Alicante, Spain); Mercedes Muñoz Fernandez (Madrid, Spain); Miguel Javier Salvador Bravo (Pamplona, Spain); Monica Araujo Vazquez (Pontevedra, Spain); Monica Rodriguez Galdeano (Albacete, Spain); Monir Kabiri Sacramento (Madrid, Spain); Montserrat Reche Garcia; Nazaret Cordero Franco (Talavera de la Reina, Spain); Noelia Martinez Jañez (Madrid, Spain); Pablo Villace Gallego (Sabadell, Spain); Patricia Lloreda Herradon (Madrid, Spain); Purificacion Martinez del Prado (Bilbao, Spain); Rafael Lopez Castro (Valladolid, Spain); Rafael Vergel Eleuterio (Murcia, Spain); Rocio Arenal Lopez; Rodolfo Chicas Sett (Las Palmas de Gran Canaria, Spain); Rosa Maria Prados Losa (Madrid, Spain); Sabina Cordoba Holt; Veronica Cañon Garcia (Santander, Spain); Vicente Antonio de Sanctis Briggs; Vladimir Ulises Marenco Arellano; Yolanda Escobar Alvarez (Madrid, Spain); Yolanda Lopez Sanchez (Tarragona, Spain); and Yosef Abdel-Kader Riego.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This project was funded by GRUNENTHAL PHARMA, S.A (Spain).
Disclosure
CPH has received consultancy fees or honoraria from Grunenthal, Ferrer, Kyowa, Teva, Boston Scientific, Takeda, Prim, Pfizer, and Medtronic, during the conduct of the study. YE has been advisory board member and/or has received speaker honorarium from Grünenthal, Ferrer, Takeda, Angelini, and Kiowa Kirin. CM has been advisory board member for Grunenthal/Aristo, has received speaker honorarium from Asacpharma, Grunenthal, Kiowa Kirim, Aristo, has received research grants to institution from Fundacion Navarro Tripodi, ISABIAL, and FIS. JFMF has received speaker honorarium from Grünenthal, Angelini, Kyowa-Kirin. TQ has received speaker honorarium from Grünenthal, Kiowa Kirin, and Esteve, during the conduct of the study. ASA has received speaker honoraria from Grünenthal, ESTEVE, Pfizer, Neuraxpharm, and Kyowa Kirin, has also been involved as a consultant and expert witness for Grünenthal and ESTEVE, and has received financial support for Congress attendance from Grünenthal and Neuraxpharm, outside the submitted work. The aforementioned authors report no other potential conflicts of interest in relation to this work and the remaining authors report no potential conflicts of interest in relation to this work.
References
1. Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59
2. Bennett MI, Rayment C, Hjermstad M, Aass N, Caraceni A, Kaasa S. Prevalence and aetiology of neuropathic pain in cancer patients: a systematic review. Pain. 2012;153(2):359–365. doi:10.1016/j.pain.2011.10.028
3. Roberto A, Deandrea S, Greco MT, et al. Prevalence of neuropathic pain in cancer patients: pooled estimates from a systematic review of published literature and results from a survey conducted in 50 Italian palliative care centers. J Pain Symptom Manag. 2016;51(6):1091–1102.e4. doi:10.1016/j.jpainsymman.2015.12.336
4. Reis-Pina P, Acharya A, Lawlor PG. Cancer pain with a neuropathic component: a cross-sectional study of its clinical characteristics, associated psychological distress, treatments, and predictors at referral to a cancer pain clinic. J Pain Symptom Manag. 2018;55(2):297–306. doi:10.1016/j.jpainsymman.2017.08.028
5. Bouhassira D, Luporsi E, Krakowski I. Prevalence and incidence of chronic pain with or without neuropathic characteristics in patients with cancer. Pain. 2017;158(6):1118–1125. doi:10.1097/j.pain.0000000000000895
6. Oosterling A, Te Boveldt N, Verhagen C, et al. Neuropathic pain components in patients with cancer: prevalence, treatment, and interference with daily activities. Pain Pract. 2016;16(4):413–421. doi:10.1111/papr.12291
7. Oh SY, Shin SW, Koh SJ, et al. Multicenter, cross-sectional observational study of the impact of neuropathic pain on quality of life in cancer patients. Support Care Cancer. 2017;25(12):3759–3767. doi:10.1007/s00520-017-3806-5
8. Rayment C, Hjermstad MJ, Aass N, et al. Neuropathic cancer pain: prevalence, severity, analgesics and impact from the European palliative care research collaborative-computerised symptom assessment study. Palliat Med. 2013;27(8):714–721. doi:10.1177/0269216312464408
9. Yoon SY, Oh J. Neuropathic cancer pain: prevalence, pathophysiology, and management. Korean J Intern Med. 2018;33(6):1058–1069. doi:10.3904/kjim.2018.162
10. Edwards HL, Mulvey MR, Bennett MI. Cancer-related neuropathic pain. Cancers. 2019;11(3):373. doi:10.3390/cancers11030373
11. Mulvey MR, Boland EG, Bouhassira D, et al. Neuropathic pain in cancer: systematic review, performance of screening tools and analysis of symptom profiles. Br J Anaesth. 2017;119(4):765–774. doi:10.1093/bja/aex175
12. Perez C, Sanchez-Martinez N, Ballesteros A, et al. Prevalence of pain and relative diagnostic performance of screening tools for neuropathic pain in cancer patients: a cross-sectional study. Eur J Pain. 2015;19(6):752–761. doi:10.1002/ejp.598
13. Piano V, Schalkwijk A, Burgers J, et al. Guidelines for neuropathic pain management in patients with cancer: a European survey and comparison. Pain Pract. 2013;13(5):349–357. doi:10.1111/j.1533-2500.2012.00602.x
14. Piano V, Verhagen S, Schalkwijk A, et al. Diagnosing neuropathic pain in patients with cancer: comparative analysis of recommendations in national guidelines from European countries. Pain Pract. 2013;13(6):433–439. doi:10.1111/papr.12018
15. Piano V, Verhagen S, Schalkwijk A, et al. Treatment for neuropathic pain in patients with cancer: comparative analysis of recommendations in national clinical practice guidelines from European countries. Pain Pract. 2014;14(1):1–7. doi:10.1111/papr.12036
16. Hershman DL, Lacchetti C, Dworkin RH, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2014;32(18):1941–1967. doi:10.1200/JCO.2013.54.0914
17. Jordan B, Margulies A, Cardoso F, et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO-EONS-EANO clinical practice guidelines for diagnosis, prevention, treatment and follow-up. Ann Oncol. 2020;31(10):1306–1319. doi:10.1016/j.annonc.2020.07.003
18. Fallon M, Giusti R, Aielli F, et al. Management of cancer pain in adult patients: ESMO clinical practice guidelines. Ann Oncol. 2018;29(Suppl 4):iv166–iv191. doi:10.1093/annonc/mdy152
19. Jara C, Del Barco S, Gravalos C, et al. SEOM clinical guideline for treatment of cancer pain (2017). Clin Transl Oncol. 2018;20(1):97–107. doi:10.1007/s12094-017-1791-2
20. Loprinzi CL, Lacchetti C, Bleeker J, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. 2020;38(28):3325–3348. doi:10.1200/JCO.20.01399
21. Pérez-Hernández C, Alonso A, Ramos A, Virizuela JA, Villegas F. Guía para el Abordaje Interdisciplinar del Dolor Oncológico; 2018. Available from: https://www.gado.es/.
22. Diamond IR, Grant RC, Feldman BM, et al. Defining consensus: a systematic review recommends methodologic criteria for reporting of Delphi studies. J Clin Epidemiol. 2014;67(4):401–409. doi:10.1016/j.jclinepi.2013.12.002
23. Loblaw DA, Prestrud AA, Somerfield MR, et al. American Society of Clinical Oncology clinical practice guidelines: formal systematic review-based consensus methodology. J Clin Oncol. 2012;30(25):3136–3140. doi:10.1200/JCO.2012.42.0489
24. Perez-Hernandez C, Blasco A, Gandara A, et al. Prevalence and characterization of breakthrough pain in patients with cancer in Spain: the CARPE-DIO study. Sci Rep. 2019;9(1):17701. doi:10.1038/s41598-019-54195-x
25. Ulas S, Eyigor S, Caramat I. Quality of life and neuropathic pain in hospitalized cancer patients: a comparative analysis of patients in palliative care wards versus those in general wards. Indian J Palliat Care. 2018;24(3):325–333. doi:10.4103/IJPC.IJPC_12_18
26. Finnerup NB, Haroutounian S, Kamerman P, et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157(8):1599–1606. doi:10.1097/j.pain.0000000000000492
27. Brunelli C, Bennett MI, Kaasa S, et al. Classification of neuropathic pain in cancer patients: a Delphi expert survey report and EAPC/IASP proposal of an algorithm for diagnostic criteria. Pain. 2014;155(12):2707–2713. doi:10.1016/j.pain.2014.09.038
28. Smith EM, Bridges CM, Kanzawa G, et al. Cancer treatment-related neuropathic pain syndromes–epidemiology and treatment: an update. Curr Pain Headache Rep. 2014;18(11):459. doi:10.1007/s11916-014-0459-7
29. Pereira S, Fontes F, Sonin T, et al. Neuropathic pain after breast cancer treatment: characterization and risk factors. J Pain Symptom Manag. 2017;54(6):877–888. doi:10.1016/j.jpainsymman.2017.04.011
30. Ilhan E, Chee E, Hush J, Moloney N. The prevalence of neuropathic pain is high after treatment for breast cancer: a systematic review. Pain. 2017;158(11):2082–2091. doi:10.1097/j.pain.0000000000001004
31. Pradat PF, Delanian S. Late radiation injury to peripheral nerves. Handb Clin Neurol. 2013;115:743–758.
32. Van den Beuken-Van Everdingen MH, De Rijke JM, Kessels AG, Schouten HC, Van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi:10.1093/annonc/mdm056
33. Caraceni A, Shkodra M. Cancer pain assessment and classification. Cancers. 2019;11(4):510. doi:10.3390/cancers11040510
34. Gutgsell T, Walsh D, Zhukovsky DS, Gonzales F, Lagman R. A prospective study of the pathophysiology and clinical characteristics of pain in a palliative medicine population. Am J Hosp Palliat Care. 2003;20(2):140–148. doi:10.1177/104990910302000213
35. Velasco R, Bruna J. Chemotherapy-induced peripheral neuropathy: an unresolved issue. Neurologia. 2010;25(2):116–131. doi:10.1016/S0213-4853(10)70036-0
36. Fallon MT. Neuropathic pain in cancer. Br J Anaesth. 2013;111(1):105–111. doi:10.1093/bja/aet208
37. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. 2014;155(12):2461–2470. doi:10.1016/j.pain.2014.09.020
38. Van den Beuken-Van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manag. 2016;51(6):1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
39. Urch CE, Dickenson AH. Neuropathic pain in cancer. Eur J Cancer. 2008;44(8):1091–1096. doi:10.1016/j.ejca.2008.03.015
40. Swarm RA, Paice JA, Anghelescu DL, et al. Adult cancer pain, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(8):977–1007. doi:10.6004/jnccn.2019.0038
41. Bennett GJ, Doyle T, Salvemini D. Mitotoxicity in distal symmetrical sensory peripheral neuropathies. Nat Rev Neurol. 2014;10(6):326–336. doi:10.1038/nrneurol.2014.77
42. Colvin LA. Chemotherapy-induced peripheral neuropathy: where are we now? Pain. 2019;160(Suppl 1):S1–S10. doi:10.1097/j.pain.0000000000001540
43. Pachman DR, Barton DL, Swetz KM, Loprinzi CL. Troublesome symptoms in cancer survivors: fatigue, insomnia, neuropathy, and pain. J Clin Oncol. 2012;30(30):3687–3696. doi:10.1200/JCO.2012.41.7238
44. Smith EML, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL. Assessing patient-reported peripheral neuropathy: the reliability and validity of the European organization for research and treatment of cancer QLQ-CIPN20 questionnaire. Qual Life Res. 2013;22(10):2787–2799. doi:10.1007/s11136-013-0379-8
45. Ferrier J, Pereira V, Busserolles J, Authier N, Balayssac D. Emerging trends in understanding chemotherapy-induced peripheral neuropathy. Curr Pain Headache Rep. 2013;17(10):364. doi:10.1007/s11916-013-0364-5
46. Glare PA, Davies PS, Finlay E, et al. Pain in cancer survivors. J Clin Oncol. 2014;32(16):1739–1747. doi:10.1200/JCO.2013.52.4629
47. Paice JA. Chronic treatment-related pain in cancer survivors. Pain. 2011;152(3 Suppl):S84–S89. doi:10.1016/j.pain.2010.10.010
48. Muller-Schwefe G, Ahlbeck K, Aldington D, et al. Pain in the cancer patient: different pain characteristics CHANGE pharmacological treatment requirements. Curr Med Res Opin. 2014;30(9):1895–1908. doi:10.1185/03007995.2014.925439
49. Watling CJ, Moulin DE. Neuropathic pain. In: Bruera ED, Portenoy RK, editors. Cancer Pain: Assessment and Management. New York, NY: Cambridge University Press; 2003:396–408.
50. Twycross R, Bennett M. Cancer pain síndromes. In: Sykes NN, Bennett M, Yuan C, editors. Cancer Pain: Clinical Pain Management. Bodmin, Cornwall: MPG Books; 2008:27–37.
51. Pérez P, Rodríguez MJ, Guerrero A, et al. Consenso experto sobre el uso clínico de los tratamientos por vía tópica en el manejo del dolor neuropático periférico. Rev Soc Esp Dolor. 2013;20(6):308–323. doi:10.4321/S1134-80462013000600005
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.