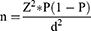Back to Journals » Research and Reports in Tropical Medicine » Volume 15
A Cross Sectional Study on the Bidirectional Interactions Between Leptospirosis and HIV Infection Among Patients from Maputo Central Hospital, Mozambique
Authors Comia IR , Manuel L, Miambo RD , Carimo AA , Manjate PDF, Maholela AE, Banze LR, Buene TP, Nhancupe N , Sousa IM , Benson CA, Schooley RT, Sacarlal J , Noormahomed EV
Received 8 November 2023
Accepted for publication 19 January 2024
Published 12 February 2024 Volume 2024:15 Pages 1—11
DOI https://doi.org/10.2147/RRTM.S445878
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Isac Rodrigues Comia,1– 3,* Leonardo Manuel,1– 3,* Regina Daniel Miambo,3,4 Awa Abdul Carimo,3,5 Percílio da Floca Manjate,3,5 Ana Edith Maholela,3,6 Lucas Raimundo Banze,2,3 Titos Paulo Buene,2,3 Noémia Nhancupe,2,3 Irina M Sousa,3,7 Constance A Benson,8 Robert T Schooley,9 Jahit Sacarlal,2 Emília Virgínia Noormahomed2,3,9
1Department of Research and Extension, Faculty of Health Sciences, Lúrio University, Nampula, Mozambique; 2Department of Microbiology, Faculty of Medicine, Eduardo Mondlane University, Maputo, Mozambique; 3Mozambique Institute for Health Education and Research (MIHER), Maputo, Mozambique; 4Department of Para-Clinics, Faculty of Veterinary, Eduardo Mondlane University, Maputo, Mozambique; 5Department of Medicine, Eduardo Mondlane University, Maputo, Mozambique; 6Department of Medicine, Maputo Central Hospital, Maputo, Mozambique; 7Department of Biological Sciences, Faculty of Sciences, Eduardo Mondlane University, Maputo, Mozambique; 8Department of Medicine, Division of Infectious Diseases and Global Public Health, University of California, San Diego, CA, USA; 9Department of Medicine, Division of Infectious Diseases, University of California, San Diego, CA, USA
*These authors contributed equally to this work
Correspondence: Emília Virgínia Noormahomed, Av. Salvador Allende, 702, Maputo, Mozambique, Tel +258828810907, Fax +25821304684, Email [email protected]
Introduction: This study aims to determine the baseline seroprevalence of leptospirosis, a zoonotic and neglected disease, in people living with HIV (PWH) in Maputo, Mozambique, and to evaluate the relationship between selected HIV-related factors that might influence risk of coinfection with leptospirosis, such as degree of immunosuppression, as assessed by CD4 cell count, World Health Organization (WHO) HIV/AIDS clinical stage and antiretroviral therapy (ART) intake.
Methods: This was a descriptive cross-sectional analysis of 157 PWH, aged over 18 years old, admitted to the Maputo Central Hospital, in Maputo, Mozambique, between March 2020 and October 2021. The study participants were recruited as a convenience sample regardless of the reasons for their admission. We collected sociodemographic and clinical data, including ART and WHO HIV/AIDS clinical stage, and blood for CD4 cell count and detection of Leptospira IgG antibodies using a commercial Kit ab247199 Leptospira IgG ELISA (www.abcam.com/ab247199) with sensitivity and specificity of 100% and 97.3%, respectively. Laboratory testing was performed at the Faculty of Medicine, Eduardo Mondlane University and Laboratory of Clinical Analysis, in Maputo.
Results: Participants were aged 18 to 72 years (median age 39 years; SD ± 10.5), the majority were female 100 (63.7%), from urban areas 138 (87.9%), with secondary-level education 80 (51%). The overall seroprevalence of Leptospira IgG antibodies was 40.1%. The median CD4 cell count was 385 cells/μl (02 to 2297; SD ± 378.47). Higher seroprevalence of Leptospira antibodies was found among participants with CD4 cell counts < 250 cells/μl (54.8%), WHO HIV/AIDS stage IV (70.2%) and those on ART (92%), though there were no statistically significant differences between groups with and without Leptospira antibodies.
Conclusion: Our study confirmed that Leptospira antibodies are highly prevalent in PWH in Maputo; however, Leptospira infection was not associated with the degree of immunosuppression, WHO HIV/AIDS clinical stage, or the use of ART. Our data support the need for routine screening for leptospirosis in PWH in Mozambique. Future studies are warranted to characterize the incidence and outcomes of symptomatic leptospirosis in this patient population and to identify circulating serovars and species in the country and region, as well as the implicated reservoirs.
Keywords: leptospirosis, co-infection Leptospira and HIV, Leptospira IgG, Mozambique
Introduction
Leptospirosis is a re-emerging zoonotic and neglected disease caused by the spirochetal bacteria of the genus Leptospira.1–4 Though the disease is widely distributed around the world, higher incidence is found in low-income countries where poor sanitation, heavy rainfall, floods, and close contact with infected animals prevail, all associated with limited infrastructure for diagnosis and lack of awareness of the disease among health professionals.5–7
Globally there are approximately, 1.03 million cases and 60,000 deaths per year8,9 due to leptospirosis, with highest incidence reported in Oceania (150.68 cases per 100,000, 95% CI 40.32–272.29), South-East Asia (55.54, 95% CI 20.32–99.53), Caribbean (50.68, 95% CI 14.93–87.58), and East sub-Saharan Africa (25.65, 95% CI 9.29–43.31).10 Studies conducted from sub-Saharan African countries including Nigeria, Senegal, Gabon, Democratic Republic of Congo, Kenya, and Ethiopia found a seroprevalence of leptospirosis ranging from 7.7% to as high as 47.5%.11 A recent systematic review of the prevalence of leptospirosis in the Southern African Development Community (SADC) which comprises 16 countries, including Mozambique, reported a pooled prevalence of 19%, with most of the studies conducted in Tanzania.7
Human infections are due to direct contact of skin lesions or mucosal membranes with infected urine or contaminated water or soil; those at high risk of infection include veterinarians, health-care professionals, agricultural workers, farmers, and swimmers.1,12 Although rats are the main reservoirs for the bacteria in urban slum areas12,13 livestock, pigs, and stray dogs can also play an important role in transmission.1,14,15
Multiple diagnostic tools for Leptospira infection have been developed over the years. Among them are the direct visualization of spirochetes in urine or blood under dark-field microscopy, serology for IgM and IgG antibody detection, Microscopic Agglutination Test (MAT) which is the gold standard, Cross Agglutination Absorption Test (CAAT), and the Polymerase Chain Reaction (PCR) for species differentiation.16 The sensitivity and specificity of these assays vary according to the onset of symptoms and disease stage (acute or chronic).17
Symptoms and signs of the disease may vary widely from symptomatic to subclinical (asymptomatic in 80% to 90% of cases) or self-limited anicteric febrile illness, leading to overlap with other febrile illnesses common in the region such as malaria, brucellosis, dengue, typhoid fever, babesiosis, and rickettsiosis.7,18,19 The acute stage of the disease may be accompanied by headache, myalgia, arthralgia, chills, nausea, abdominal pain, diarrhea, cough, conjunctivitis, and skin rashes, which may appear 2 to 20 days after exposure.13 Sub-acute and chronic complications as well as long-term sequelae may also occur.9 In about 10% of patients infected with pathogenic serovars, the symptoms may progress to fulminant leptospirosis, known as Weil’s disease, characterized by multiorgan dysfunction with pulmonary hemorrhage, renal and liver impairment.10,20 In children the disease is also associated with meningitis21 and case fatality rates for severe disease range from 10% to 30%.17,22–24
Studies of bidirectional interactions between Leptospira infection and HIV are scarce worldwide, especially from sub-Saharan Africa.25 Mozambique, along with many other Southern African Development Countries (SADC) such as South Africa (13.3%), Eswatini (27.9%), Zimbabwe (21.4%), Zambia (12.1%), and Tanzania (5.1%),26 ranks among the top 10 countries most affected by HIV; the general prevalence of HIV among adults is 12.1% with annual deaths of 51,000 in 2019.27,28 It is well known that people living with HIV (PWH) infection are more likely to contract some infectious diseases or have others worsened, presumably by HIV-associated immunosuppression or immune activation; these include tuberculosis, malaria, schistosomiasis, and toxoplasmosis.7,29–31 However, it is not yet clear if HIV-related factors may impact the acquisition or severity of leptospirosis.7
This study is the first to be conducted in Mozambique with the aim to determine the baseline seroprevalence of Leptospira IgG antibodies in PWH hospitalized in the Maputo Central Hospital, in Maputo, Mozambique, and to evaluate the bidirectional action between the two pathogens in relationship to the possible association of the disease with the degree of HIV-related immunosuppression, WHO HIV/AIDS clinical stage, and anti-retroviral treatment (ART) intake. Results obtained with this study will aid in filling the gaps in knowledge about this disease in PWH and provide valuable information about the dynamics of coinfection in this population. The ultimate goal of such studies is to support recommendations to health authorities and health-care providers regarding clinical diagnosis, treatment decisions, and preventive strategies for immunocompromised PWH in Mozambique.
Materials and Methods
Study Setting, Design, and Methods
Mozambique is a low-income country located in the Southeastern region of sub-Saharan Africa with an estimated population of 31.6 million and an adult literacy rate of 60.7%.31 Its geographical location predisposes the country to extreme weather events such as heavy floods, cyclones, storms, floods, and drought, which in turn exacerbate the burden of climate-sensitive diseases due to contamination of water and food.19 Maputo City, the country’s capital, has approximately 1.12 million inhabitants with half of the population residing in unplanned settlements, leading to high population density and poor access to basic safe drinking water and sewage drainage.32
We conducted a cross-sectional study between March 2020 and October 2021 in Maputo Central Hospital, the largest referral hospital in the country, which also serves as a teaching hospital for undergraduate students at Eduardo Mondlane University, Faculty of Medicine and for training of specialists and other health professionals such as nurses and physician technicians.33
Study Population, Sample Size
We recruited a convenience sample of 157 PWH hospitalized in the Department of Medicine at the Maputo Central Hospital. The sample size was calculated using the following formula of Thrusfield,34 using a 95% of confidence level and 5% of precision.
Where n is the sample size; z is the standard deviation (1.96); P is the estimated prevalence and d the significance level. A value of 11.5% was applied as a reference prevalence found in febrile patients in Mozambique.35
Recruitment and Data Collection
We recruited the study participants as a random convenience sample following their admission to the Maputo Central Hospital, Department of Medicine wards. Because the study took place during the highest period of SARS-COV-19 pandemic, the enrollment of participants was slowed due to restrictions imposed to circulate within the wards.36 The study participants were aged over 18 years, with documented HIV-1 infection regardless of their sex/gender or reasons for admission. Prior to enrollment, the principal investigators (IRC and LM), a nurse, or a research doctor explained the aims of the study to potential volunteers and obtained their written informed consent.
Further, demographic, and clinical data including age, sex, education, employment, contact with domestic animals, associated co-morbidities, WHO HIV/AIDS clinical stage, and antiretroviral treatment intake data were collected from clinical records by the research doctor and the principal investigators, using a questionnaire specifically designed for the purpose of study. All personal information of research subjects was treated with confidentiality, and principles of the Declaration of Helsinki were taken into consideration.
We use the WHO HIV/AIDS clinical stage to assess disease status, as this has generally been used in persons with confirmed HIV infection in resource limited settings where sophisticated laboratory testing is scarce and allows some prognostication about relative morbidity and mortality in PWH.37
Blood Collection and Laboratory Analysis
For each patient, we collected a total of 8 mL of blood sampled by venipuncture for serological testing of Leptospira IgG antibodies: 4mL into a gel + clot activator tube (Biota, Istanbul-Turkey) and 4 mL into a K3 EDTA vacutainer tube (Biota, Istanbul-Turkey) for CD4 cell count (performed at the laboratory of Parasitology, Faculty of Medicine, Eduardo Mondlane University, and at the Laboratory of Clinical Analyses (LAC) a private facility located in Maputo (Mozambique), respectively). The samples were transported to these laboratories in cooled boxes with ice.
Serological Testing for Leptospira IgG and CD4 Cell Counting
Blood samples for Leptospira IgG detection were centrifuged at 3000 rpm for 10 min, and the sera kept at −80°C until analyzed using a commercial Kit (ab247199 Leptospira IgG ELISA-www.abcam.com/ab247199) according to manufacturer’s instructions; samples were considered positive if values > 11 Units were obtained. This assay has sensitivity and specificity of 100% and 97.3%, respectively, and can cross react with Epstein–Barr virus (EBV) or Rheumatoid Factor antibodies. Blood samples were processed for CD4 T-cell count within 24 hr after collection using a standard flow cytometry, BD Bioscience FACSCount (BD FACSCount System) in the previously described Laboratory of Clinical Analyses (LAC).
Data Analysis
Sociodemographic and clinical data were entered into a Microsoft Excel spreadsheet and exported to the Statistical Package for the Social Sciences (SPSS) for analysis using the STATA software. Study participants were stratified by age group (18–28, 29–38, 39–49, and >50), CD4 cell count (<250, 250–500, >500 cell/µl) and WHO HIV/AIDS clinical stage (I, II, III, and IV). Descriptive analyses were performed using frequencies and percentages for categorical variable, and multivariate logistic regression analysis was performed to assess associations between Leptospira IgG infections, demographic and selected clinical variables, using Chi-square or Fisher’s exact test. A p-value of <0.05, 95% confidence interval was considered significant.
Results
Sociodemographic Characteristics and HIV Associated Factors of the Study Population
The median age among the 157 study participants was 39 years (SD±10.5; range 18–72 years). The sociodemographic and clinical data of the study participants are summarized in Table 1. Most of the participants were female (100; 63.7%), from urban areas (138; 87.9%), with secondary-level education (80; 51%), unemployed (84; 53.5%), with no access to treated water (118; 75.2%) and 87 (55.4%) owned domestic animals. Further, among those owning domestic animals, 61 (38.9%) owned dogs, 43 (27.4%) cats, 40 (25.5%) chickens, 6 (3.8%) pigs, and 1 (0.6%) cattle. CD4 T-cell counts were available for 95.5% of participants and the median CD4 cell count was 363.1 cells/ul (02 to 2297; SD ± 353.8); almost half (93; 45.5%) had CD4 cell counts of <250 cells/ul. The majority of study participants (114; 72.6%) were in WHO HIV/AIDS clinical stage IV, 146 (93%) were on ART and 101 (64.3%) were on prophylaxis with Trimethoprim-Sulfamethoxazole (TMP-SMX). Among those study participants on ART (146; 93.0%), more than half (77; 52.7%) had been on ART for more than 5 years.
 |
Table 1 Sociodemographic and HIV-Related Factors of Study Participants |
Leptospira Seroprevalence According to Sociodemographic and HIV-Related Characteristics
Table 2 summarizes the Leptospira seroprevalence according to demographic and HIV-related characteristics of study participants. Overall, of the 157 samples tested, 63 (40.1%) were found to be positive for Leptospira IgG antibodies. Higher seroprevalence was observed in females (61; 64.9%), in the age group of 29–38 years old (37; 39.4%), in patients from urban areas (82; 87.2%), with secondary school educational levels (48; 51.1%), unemployed (52; 55.3%), with domestic animals (56; 59.6%), and with no access to safe drinking water (61; 64.9%). However, there were no statistically significant differences reported between groups.
 |
Table 2 Leptospira Serology in Relation to Socio Demographic and HIV-Related Factors |
With regard to clinical factors associated with HIV, we found that IgG seroprevalence was higher among patients with CD4 cell counts <250/ul (34 of 62; 54.8%), WHO HIV/AIDS clinical stage IV (48 of 63; 76.2%), and those on ART (58 of 63; 92.1%); however, there were no statistically significant differences between groups. Furthermore, those patients who were on ART for more than 5 years had the highest seroprevalence of Leptospira antibodies (38 of 58; 60.3%) as compared to those who were on ART for less than 5 years (19 of 58; 32.8%). However, again this difference was not statistically significant. Our results also demonstrated that patients with CD4 cell counts <250/ul were two times more likely to be seropositive for Leptospira, but none of the clinical factors previously described was associated with seropositivity as shown on Supplementary Table 1.
Discussion
Our results demonstrated that Leptospira IgG antibodies were present in 40.1% of our study participants. Moreover, it was found that seroprevalence of Leptospira IgG was higher in PWH within the CD4 cell count group less than 250 cells/ul (54.8%), WHO HIV/AIDS clinical stage IV (70.2%) and on ART (93.6%), although these differences were not statistically significant. However, this finding suggests that leptospirosis is highly prevalent and may be an important cause of morbidity and mortality in our study participants. As far as we are aware, this is the first pilot exploratory study conducted in Mozambique with the aim to explore bidirectional interactions between Leptospira and HIV infection in PWH from Maputo. Our study revealed several important findings as follows.
Serology
We found that leptospirosis is highly prevalent in Mozambique but our seroprevalence may be an underestimate potentially due to lack of awareness of the disease, lack of diagnostic tools or resources to access them, and the burden of other febrile conditions prevalent in the country, with an emphasis on malaria. These draw more attention to health-care providers and other policymakers and stakeholders than leptospirosis, suggesting that undiagnosed leptospirosis may be an important but unrecognized cause of morbidity in our population.7
The seroprevalence of Leptospira found in our study is higher than that reported from other studies done in Mozambique among febrile patients.19,35 In these studies, the seroprevalence of Leptospira varied from 1.3% using the MAT assay to between 11.5% and 34.1% using ELISA IgM. We did not test for IgM antibodies due to financial limitations. However, these data raise the concern that if we had tested for IgM antibodies we might have found an even higher seroprevalence.
We also found that seroprevalence is higher when compared to seroprevalence reported in studies in PWH done in other sub-Saharan countries such as Tanzania and Zambia, where surveillance notifications are lacking and laboratory diagnosis is not established.11 Seroprevalence rates in those countries varied between 4.4% and 33% using the MAT test.38 Although IgG antibodies may indicate past infection, the high seroprevalence that we found suggests that the disease is spread more commonly in our setting. However, given this picture and the possibility of cross reactivity of the assay we used with Leptospira IgG antibodies and Epstein–Barr virus (EBV) or Rheumatoid Factor antibodies, it is important to discriminate active from past infections by testing IgM antibodies in immunocompromised patients.
Factors such as poor hygiene, environmental water contamination with faecal coliforms and other bacteria due to poor sanitary conditions, and the growing population density in urban areas without improvement in sanitary conditions39 may also play a very important role in the transmission of Leptospira and they must be taken into consideration in bacteria control.40 Furthermore, the increased spread of uncontrolled informal markets without appropriate hygienic conditions in food handling combined with the presence of rodents provides propitious conditions for establishment, and spread of this highly contagious disease. Exacerbating these scenarios is the regularly occurring flooding in Maputo, especially in suburban areas, and in the country in general, thought to be associated with climate change. The frequent flooding leads to contamination of drinking water sources and food, especially fresh vegetables sold at informal markets for displaced populations.19,32,35,41,42 For instance, a study done with the aim to assess the profile and frequency of parasites contaminating lettuce and cabbages sold in some selected markets in Maputo city found that 84% of samples were contaminated with parasites.32
Serology, Demographics and Selected HIV Related Factors
In general, there are few studies worldwide evaluating re-emerging infectious diseases like leptospirosis in PWH. In Mozambique, studies on leptospirosis are scarce. To date, there are only three studies published from febrile patients in which it was found that the seroprevalence of leptospirosis varied between 1.3% and 34.1% in febrile patients.19,35,43 However, neither study assessed the HIV serostatus of the study participants. Globally, some studies argue that PWH with greater degrees of immunosuppression do not have different outcomes with bacterial infections than those without HIV,44,45 although whether this is true of leptospirosis has not been addressed. A limited number of case reports have not been conclusive with regard to PWH presenting with severe or even mild symptomatology.46–49
Higher seroprevalence of Leptospira antibodies was found in PWH with CD4 cell counts <250 cells/ul (54.8%) in our study, though no significant differences were observed when compared to those with higher CD4 cell counts. This suggests that Leptospira infections may not be correlated with degree of immunosuppression among people with HIV. A study conducted in Tanzania also found no difference in immunosuppression status between patients infected by Leptospira and those not infected. Nevertheless, Leptospira IgG antibody itself does not indicate the presence of active disease, which was not the focus of our study.45
The majority of our study participants (72.6%) were categorized as WHO HIV/AIDS clinical stage IV. Of those who tested positive for Leptospira IgG, 46.8% had CD4 cell counts <250 cells/ul. Similar results were reported in a study done in the Kingdom of Saudi Arabia50 in 86% of their study participants had CD4 cell counts <200 cells/ul within the WHO clinical staging IV, and they concluded that the WHO clinical staging and classification of HIV/AIDS correlates well with CD4+ T-lymphocyte counts. Our results were not conclusive about the association between the degree of immunosuppression or WHO HIV/AIDS clinical stage and increased risk of infection with leptospirosis. This could be attributed to our small sample size and to reported co-infections with other opportunistic diseases such as tuberculosis (22.2%) and oral candidiasis (17.5%).
Interestingly, patients on ART for more than 5 years were more likely to test positive for Leptospira (93.3%) as compared to those who had been on ART for less than 5 years (53.4%), though the difference did not reach statistical significance when compared to other groups. A study from Zambia by Nombwende et al, found that PWH on ART had a higher risk of contracting leptospirosis than people without HIV.38 This may reflect better ascertainment as these patients have more contact with health units and may be more likely to be screened for co-infections and co-morbidities. It may also be that effective ART improves immune function and antibody production, which might increase detection of antibodies in general for various pathogens and reduce the risk of disease progression,51 although a study from Tanzania concluded that Leptospira infections were not associated with HIV-associated immunosuppression.45
Associated Risk Factors
In our study, a higher seroprevalence of Leptospira IgG was observed more frequently in females (61; 64.9%) and in the age group of 29–38 years old (37; 39.4%), though symptomatic Leptospira infections were not reported. In a study in Brazil that similarly evaluated Leptospira serology and risk factors for infection found lower seroprevalence (0.9%) in the age group 30–60 years old in PWH.52
Most of the seropositive PWH in our study owned dogs (38; 23%) or cats (19%), though the differences were not statistically significant when compared to those who did not own any animals. Studies conducted in the SADC region reported that cats are significant drivers of leptospirosis in households53 and exposure to cattle was identified as a risk factor for the transmission of the bacteria,54 although in this study only 1% of patients owned cattle. While the role of rats as the main reservoir globally and in Mozambique has been well described,55 the role of other animal species in the transmission of Leptospira needs further evaluation as part of the framework for developing better control measures.
Strengthens and Limitations
The strengths of our study reside in the fact that as far as we are aware, this is the first assessment done in Mozambique to define the seroprevalence of Leptospira IgG in PWH and to examine the effect of immunosuppression, the WHO HIV/AIDS clinical stage, ART therapy intake, and associations with some comorbid medical conditions in our cohort.
Key limitations of our work are as follows. First, we were unable to perform IgM testing nor PCR or MAT that could indicate active infection and the species of Leptospira implicated or the circulating serovars, due in part to disruption in supply chain and staff related to the COVID-19 pandemic.36 We also could not perform MAT assays, which are the gold standard for the diagnosis and identification of serovars. Second, we used an ELISA IgG assay, which has lower sensitivity compared with the MAT assay. Therefore, it is possible that the seroprevalence we detected among our study participants is underestimated. However, our results indicate that this neglected zoonotic disease constitutes a public health problem among the study participants. Third, since our study was done only in PWH and in a small population size, it is not possible to draw conclusions about the seroprevalence of Leptospira in HIV-uninfected patients in the general population. However, given the known environmental exposure risks and that we did not identify statistically significant HIV-related factors that contributed to higher seroprevalence, we can surmise that the background prevalence of infection in other populations in Mozambique might be similar to those we report based on their exposure to the same environment.
Conclusion
Our study confirmed that Leptospira infection is highly prevalent among people with HIV in Mozambique and that those with CD4 cell counts below 250 cells/µl, WHO HIV/AIDS clinical stage IV had higher seroprevalence of IgG Leptospira antibodies compared to other groups. The same trend was identified in PWH on ART for more than 5 years and on prophylaxis with TMP-SMX.
Although Leptospira IgG indicates past infection, our data provide valuable information on the circulation of these bacteria in Mozambique and a glimpse of animal exposure and environmental contamination that might result in higher leptospirosis infection rates. Due to the high seroprevalence identified in our study participants and possible implications that Leptospira infection can have in the severity of disease and outcomes in PWH, we recommend that PWH should be screened for this zoonotic neglected disease in our setting, particularly in the setting of undiagnosed febrile illness. Future studies of Leptospira IgM to detect active infections in both people with and without HIV infection, inclusion of higher numbers of participants and a follow-up of infected patients together with evaluation of soil and water contamination should be conducted in order to better clarify the bidirectional interactions of these two pathogens and the bacterial transmission dynamics using a One Health approach. Additional testing should also include appropriate diagnostic tools to identify circulating serovars and species in both humans and reservoirs. There is also a need for 1) sanitation improvement and implementation of preventive measures using a One Health approach, which involves both health and veterinary professionals; 2) limitation of environmental contamination of soil and water ponds by animal urine to reduce human exposure; and 3) raising awareness of the disease among health-care providers and other stakeholders and policymakers so that diagnostic tools are made available for routine screening of the disease in health units, particularly for immunocompromised patients to anticipate the correct treatment.
Institutional Review Board Statement
The study was approved by the Mozambique National Bioethics Committee of Health with the number 128/CNBS/2018 and by the administrative authorities from the Maputo Central Hospital.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Sharing Statement
The data sets used and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Consent for Publication
All authors consented to the publication of this article.
Acknowledgments
We are indebted to the study participants who consented to participate in this study and to the staff of the Department of Medicine at the Maputo Central Hospital, who helped identify study participants and supported collection of data.
Abstract of this paper was presented at the AFREhealth 6th Annual Symposium, 2023 in Maputo, Mozambique, https://afrehealth.org/2023symposium/list-of-posters by Comia IR, Manuel L, Miambo RD, Carimo A, Manjate PF, Maholela A, Banze L, Buene T, Nhacupe N, Sousa IM, Benson CA, Schooley RT, Sacalal J, Noormahomed EV.
Author Contributions
Jahit Sacalal and Emília Virgínia Noormahomed contributed equally as senior mentors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors read and approved the final version of the manuscript.
Funding
The research work and student fellowship (IRC and LM) were supported by the grant number D43TW010568 from the National Institutes of Health (NIH)- Fogarty International Center (FIC), titled Enhanced Advanced Biomedical Training in Mozambique (AEBTM). Additionally, RTS and EVN received support from the above-mentioned grant to support their efforts as PI and co-PI, respectively. RDM, IMS, LB, and TB received support from the above grant as mentors. NN, AC, PFM, AM, CAB, RTS, JS, and EVN received support from the grant number R25TW011216 also from NIH-FIC and PEPFAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Vet Microbiol. 2010;140(3–4):287–296. doi:10.1016/j.vetmic.2009.03.012
2. Picardeau M. Diagnosis and epidemiology of leptospirosis. Médecine Et Maladies Infectieuses. 2013;43(1):1–9. doi:10.1016/j.medmal.2012.11.005
3. Vincent AT, Schiettekatte O, Goarant C, et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl Trop Dis. 2019;13(5):e0007270. doi:10.1371/journal.pntd.0007270
4. Sohn-Hausner N, Kmetiuk LB, Biondo AW. One health approach to leptospirosis: human–dog seroprevalence associated to socioeconomic and environmental risk factors in brazil over a 20-year period (2001–2020). Trop Med Infect Dis. 2023;8(7):356. doi:10.3390/tropicalmed8070356
5. Mwachui MA, Crump L, Hartskeerl R, Zinsstag J, Hattendorf J. Environmental and behavioural determinants of leptospirosis transmission: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003843. doi:10.1371/journal.pntd.0003843
6. Naing C, Reid SA, Aye SN, Htet NH, Ambu S. Risk factors for human leptospirosis following flooding: a meta-analysis of observational studies. PLoS One. 2019;14(5):e0217643. doi:10.1371/journal.pone.0217643
7. Comia IR, Miambo RD, Noormahomed EV, et al. A systematic review and meta-analysis of the epidemiology of Leptospirosis in HIV uninfected and in people living with HIV from the Southern African development community. PLoS Negl Trop Dis. 2022;16(12):e0010823. doi:10.1371/journal.pntd.0010823
8. Picardeau M. Leptospirosis: updating the global picture of an emerging neglected disease. PLoS Negl Trop Dis. 2015;9(9):e0004039. doi:10.1371/journal.pntd.0004039
9. Guernier V, Allan KJ, Goarant C. Advances and challenges in barcoding pathogenic and environmental Leptospira. Parasitology. 2018;145(5):595–607. doi:10.1017/S0031182017001147
10. Costa F, Hagan JE, Calcagno J, et al. Global morbidity and mortality of leptospirosis: a systematic review. PLoS Negl Trop Dis. 2015;9(9):e0003898. doi:10.1371/journal.pntd.0003898
11. De Vries SG, Visser BJ, Nagel IM, Goris MGA, Hartskeerl RA, Grobusch MP. Leptospirosis in Sub-Saharan Africa: a systematic review. Inter J Infect Dis. 2014;28:47–64. doi:10.1016/j.ijid.2014.06.013
12. Goarant C, Trueba G, Bierque E, Thibeaux R, Davis B, de La Pena-Moctezuma A. Leptospira and Leptospirosis. Michigan State University; UNESCO; 2019.
13. Haake DA, Levett PN. Leptospirosis in humans. In: Leptospira and Leptospirosis. Springer; 2014;65–97.
14. Barragan V, Nieto N, Keim P, Pearson T. Meta-analysis to estimate the load of Leptospira excreted in urine: beyond rats as important sources of transmission in low-income rural communities. BMC Res Notes. 2017;10(1):1–7. doi:10.1186/s13104-017-2384-4
15. Costa A, Colocho RAB, Pereira CR, Lage AP, Heinemann MB, Dorneles EMS. Canine leptospirosis in stray and sheltered dogs: a systematic review. Anim Health Res Rev. 2022;23(1):39–58. doi:10.1017/S1466252321000190
16. Goarant C. Leptospirosis: risk factors and management challenges in developing countries. Res Rep Tropical Med. 2016;7(null):49–62. doi:10.2147/RRTM.S102543
17. Saito M, Nikaido Y, Matsumoto M, Ogawa M, Villanueva SY. The current status of diagnostic tools for leptospirosis. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai Shi. 2017;27(2):65–72.
18. Halliday JEB, Carugati M, Snavely ME, et al. Zoonotic causes of febrile illness in malaria endemic countries: a systematic review. Lancet Infect Dis. 2020;20(2):e27–e37. doi:10.1016/S1473-3099(19)30629-2
19. Mugabe VA, Inlamea OF, Ali S, et al. Surveillance for arboviruses and leptospirosis among non-malarial acute febrile illness outpatients in areas affected by cyclones idai and Kenneth in Mozambique. Front Trop Dis. 2023;4:1091545. doi:10.3389/fitd.2023.1091545
20. Mayxay M, Castonguay-Vanier J, Chansamouth V, et al. Causes of non-malarial fever in Laos: a prospective study. Lancet Glob Health. 2013;1(1):e46–e54. doi:10.1016/S2214-109X(13)70008-1
21. Abdelrahim NA, Fadl-Elmula IM, Hartskeerl RA, Ahmed A, Goris M. Are pathogenic Leptospira a possible cause of aseptic meningitis in suspected children in Sudan? Res Rep Tropical Med. 2021;12(null):267–274. doi:10.2147/RRTM.S339058
22. Lopes AA, Costa E, Costa YA, et al. Comparative study of the in-hospital case-fatality rate of leptospirosis between pediatric and adult patients of different age groups. Rev Inst Med Trop Sao Paulo. 2004;46(1):19–24. doi:10.1590/S0036-46652004000100004
23. Bismaya K, Dev P, Favas TT, Pathak A. Neuro-Leptospirosis: experience from a tertiary center of North India. Revue Neurol. 2022;179:238–243. doi:10.1016/j.neurol.2022.06.009
24. Chiu C-H, Chen P-C, Wang Y-C, et al. Risk of dementia in patients with leptospirosis: a nationwide cohort analysis. Int J Environ Res Public Health. 2019;16(17):3168. doi:10.3390/ijerph16173168
25. Amuche NJ, Emmanuel EI, Innocent NE. HIV/AIDS in sub-Saharan Africa: current status, challenges and prospects; 2017.
26. Simbayi L, Zuma K, Zungu N, et al. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2017: Towards Achieving the UNAIDS 90-90-90 Targets. HSRC Press; 2019.
27. Gona PN, Gona CM, Ballout S, et al. Burden and changes in HIV/AIDS morbidity and mortality in Southern Africa Development Community Countries, 1990–2017. BMC Public Health. 2020;20:1–14. doi:10.1186/s12889-020-08988-9
28. Langa I, Padama F, Nhancupe N, et al. The burden of T. solium cysticercosis and selected neuropsychiatric disorders in Mocuba district, Zambézia province, Mozambique. PLoS Negl Trop Dis. 2022;16(7):e0010606. doi:10.1371/journal.pntd.0010606
29. Noormahomed EV, Nhacupe N, Mascaró-Lazcano C, et al. A cross-sectional serological study of cysticercosis, schistosomiasis, toxocariasis and echinococcosis in HIV-1 infected people in Beira, Mozambique. PLoS Negl Trop Dis. 2014;8(9):e3121. doi:10.1371/journal.pntd.0003121
30. Banze L, Madureira AC, Zacarias BC, et al. Coinfection of HIV-1 with schistosoma spp. and with intestinal parasites in patients attending Boane health center, Maputo Province, Mozambique. EC Microbiol. 2021;17(5):3.
31. Noormahomed EV, Mandane A, Cuambe A, et al. Design and implementation of postgraduate programs in health in a resource-limited setting in Mozambique (The Lúrio University). Adv Med Educ Pract. 2021;12:399–412. doi:10.2147/AMEP.S291364
32. Sousa IM, Zucula L, Nhancupe N, Banze L, Zacarias B, Noormahomed EV. Assessment of parasitic contamination of lettuce and cabbages sold in selected markets in Maputo City, Mozambique. EC Microbiol. 2021;17(6):27–37.
33. Mocumbi AO, Carrilho C, Aronoff-Spencer E, et al. Innovative strategies for transforming internal medicine residency training in resource-limited settings: the Mozambique experience. Acad Med. 2014;89(8):S78–S82. doi:10.1097/ACM.0000000000000331
34. Thrusfield M. Chapter 17. Diagnostic testing. In: Veterinary Epidemiology.
35. Ribeiro P, Bhatt N, Ali S, et al. Seroepidemiology of leptospirosis among febrile patients in a rapidly growing suburban slum and a flood-vulnerable rural district in Mozambique, 2012–2014: implications for the management of fever. Inter J Infect Dis. 2017;64:50–57. doi:10.1016/j.ijid.2017.08.018
36. Noormahomed EV, Noormahomed S, Hlashwayo D, et al. Fostering sustainable biomedical research training in Mozambique: a spin-off of the medical education partnership initiative. Ann Glob Health. 2022;88(1):65. doi:10.5334/aogh.3684
37. Weinberg JL, Kovarik CL. The WHO clinical staging system for HIV/AIDS. AMA J Ethics. 2010;12(3):202–206.
38. Nombwende G, Jadhav M, Yambayamba KE, Korolova L, Kwenda J. Epidemiology of human leptospirosis in HIV patients attending anti-retro viral treatment in public hospitals and clinics in Kabwe Urban. Epidemiology. 2014;7:1.
39. van den Berg H, Quaye MN, Nguluve E, Schijven J, Ferrero G. Effect of operational strategies on microbial water quality in small scale intermittent water supply systems: the case of Moamba, Mozambique. Int J Hyg Environ Health. 2021;236:113794. doi:10.1016/j.ijheh.2021.113794
40. Khalil H, Santana R, de Oliveira D, et al. Poverty, sanitation, and Leptospira transmission pathways in residents from four Brazilian slums. PLoS Negl Trop Dis. 2021;15(3):e0009256. doi:10.1371/journal.pntd.0009256
41. Salamandane C. Intestinal parasites in commercial vegetables in the city of Maputo, Mozambique: is it a public health concern?; 2022.
42. Salamandane C, Fonseca F, Afonso S, Lobo ML, Antunes F, Matos O. Handling of fresh vegetables: knowledge, hygienic behavior of vendors, public health in Maputo markets, Mozambique. Int J Environ Res Public Health. 2020;17(17):6302. doi:10.3390/ijerph17176302
43. Collares-Pereira M, Gomes AC, Prassad M, et al. Preliminary survey of Leptospirosis and Lyme disease amongst febrile patients attending community hospital ambulatory care in Maputo, Mozambique. Cent Afr J Med. 1997;43(8):234–238.
44. Ganoza CA, Segura ER, Swancutt MA, Gotuzzo E, Vinetz JM. Mild, self-resolving acute leptospirosis in an HIV-infected patient in the Peruvian Amazon. American JTrop Med Hyg. 2005;73(1):67. doi:10.4269/ajtmh.2005.73.67
45. Biggs HM, Galloway RL, Bui DM, Morrissey AB, Maro VP, Crump JA. Leptospirosis and human immunodeficiency virus co-infection among febrile inpatients in northern Tanzania. Vector-Borne Zoonotic Dis. 2013;13(8):572–580. doi:10.1089/vbz.2012.1205
46. Nguyen DB, Chaparala S, Morel L, Bueno Y, Lovell RD. Weil’s Disease in an HIV-Infected Patient. Cureus. 2021;13(4):e14241. doi:10.7759/cureus.14241
47. Jones S, Kim T. Fulminant leptospirosis in a patient with human immunodeficiency virus infection: case report and review of the literature. Clin Infect Dis. 2001;33(5):E31–33. doi:10.1086/322645
48. Zylberberg H, Nebut M, Hagège H, Geslin P, Chousterman M. Severe leptospirosis in a patient with human immunodeficiency virus infection. Ann Med Interne. 1995;146(7):522.
49. Pai A. Mild self resolving acute leptospirosis in an HIV infected patient in south India. Ann Trop Med Public Health. 2013;6(2):261. doi:10.4103/1755-6783.116511
50. Edathodu J, Ali B, Alrajhi AA. CD4 validation for the World Health Organization classification and clinical staging of HIV/AIDS in a developing country. Inter J Infect Dis. 2009;13(2):243–246. doi:10.1016/j.ijid.2007.12.017
51. Menéndez-Arias L, Delgado R. Update and latest advances in antiretroviral therapy. Trends Pharmacol Sci. 2022;43(1):16–29. doi:10.1016/j.tips.2021.10.004
52. Brito FG, Menozzi BD, Mantovan KB, et al. Risk factors for leptospirosis and brucellosis in people living with human immunodeficiency virus who attended a referral hospital in southeastern Brazil. Rev Soc Bras Med Trop. 2021;54:e00762021.
53. Biscornet L, de Comarmond J, Bibi J, et al. An observational study of human leptospirosis in Seychelles. Am J Trop Med Hyg. 2020;103(3):999–1008. doi:10.4269/ajtmh.19-0228
54. Maze MJ, Cash-Goldwasser S, Rubach MP, et al. Risk factors for human acute leptospirosis in northern Tanzania. PLoS Negl Trop Dis. 2018;12(6):e0006372. doi:10.1371/journal.pntd.0006372
55. Comia I, Madureira AC, Schooley RT, Vieira ML, Noormahomed EV. Molecular detection of Leptospira spp. in rodents trapped in the Mozambique Island City, Nampula Province, Mozambique. EC Microbiol. 2018;14(12):813–821.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.