Back to Journals » International Journal of Women's Health » Volume 15
A Comprehensive Treatment Protocol for Endometriosis Patients Decreases Pain and Improves Function
Authors Shrikhande A, Patil S, Subhan M, Moody E, Natarajan J, Tailor Y, Mamsaang M, James N, Leishear K, Vyas R, Sandhu S , Ahmed T, Filart R, Daniel G, Kerin Orbuch I, Larish Y, Liu L
Received 10 May 2022
Accepted for publication 5 October 2022
Published 23 January 2023 Volume 2023:15 Pages 91—101
DOI https://doi.org/10.2147/IJWH.S365637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Allyson Shrikhande,1,2 Soha Patil,1,2 Merzia Subhan,1,2 Erika Moody,1,2 Janaki Natarajan,1,2 Yogita Tailor,1,2 Marjorie Mamsaang,1,2 Neha James,1,2 Kimberlee Leishear,1,2 Rakhi Vyas,1,2 Sandra Sandhu,1,2 Tayyaba Ahmed,1,2 Rosemarie Filart,1,2 Gabrielle Daniel,1,2 Iris Kerin Orbuch,3,4 Yaniv Larish,1,2 Lora Liu1,2
1Pelvic Rehabilitation Medicine Clinical Research Foundation, West Palm Beach, FL, USA; 2The Feinstein Institute for Medical Research, Manhasset, NY, USA; 3Providence St John’s Hospital, Santa Monica, CA, USA; 4Advanced Gynecologic Laparoscopy Center, Los Angeles, CA, USA
Correspondence: Allyson Shrikhande, Email [email protected]
Purpose: The purpose of this paper is to evaluate the efficacy of a multimodal, outpatient neuromuscular protocol in treating remaining sensitization and myofascial pain in endometriosis patients post-surgical excision.
Patients and Methods: A retrospective longitudinal study was conducted for women aged 22 to 78 with a history of surgically excised endometriosis. 60 women with an average duration of pain of 8.63 ± 7.65 years underwent a treatment protocol consisting of ultrasound guided trigger point injections, peripheral nerve blocks, and pelvic floor physical therapy for 6 weeks. Concomitant cognitive behavioral therapy once weekly for a total of 12 weeks was also undertaken. Pain intensity and pelvic functionality were assessed at new patient consults and 3-month follow ups using Visual Analogue Scale (VAS) and Functional Pelvic Pain Scale (FPPS).
Results: At new patient consults, average VAS and FPPS were 7.45 ± 2.11 (CI 6.92– 7.98) and 14.35 ± 6.62 (CI 12.68 − 16.02), respectively. At 3-month follow ups, average VAS and FPPS decreased to 4.12 ± 2.44 (CI 3.50– 4.73; p < 0.001) and 10.3 ± 6.55 (CI 8.64– 11.96; p < 0.001), respectively. Among FPPS categories, sleeping, intercourse, and working showed the highest statistical significance.
Conclusion: Data suggests the multimodal protocol was effective in treating the remaining underlying sensitization and myofascial pain seen in Endometriosis patients post-surgical excision, particularly in decreasing pain and improving function during work and intercourse.
Keywords: chronic pelvic pain, peripheral sensitization, central sensitization, myofascial dysfunction
Introduction
Endometriosis is a persistent and incurable disease that is associated with the proliferation and implantation of cells similar to the cells of endometrial glands and stroma that are found outside of the uterus.1 Endometriosis-related pain affects work, leisure, relationships, sexual well-being, and mental and emotional health,2 and this pain mechanism is complex, involving several biological, psychological, and social factors.3 Endometriosis costs the United States an estimated $78 billion annually.1 It is the leading cause of Chronic Pelvic Pain1 (CPP), a pain syndrome affecting 14–24% of women of reproductive ages.4 Diagnosis and treatment are complicated by the minimally understood etiology and co-existence of visceral pain syndromes such as Endometriosis which affects 70% of women with chronic pelvic pain.5 Relevant underlying factors in women with CPP with a history of endometriosis include myofascial and neuropathic pain.6
Myofascial dysfunction seen in the pelvis creates myofascial pain from myofascial trigger points. These trigger points are evident nodules on taut bands of muscle with a spontaneous response to stimuli, serving as a continuous nociception source. The visceral pain and inflammation seen in CPP patients causes muscle tightening and consequently hypertonic pelvic floor dysfunction which harbors an environment predisposed to activating/generating trigger points.7
Neuropathic pain refers to the triad of peripheral sensitization, central sensitization, and cross sensitization. Essentially, when pain is experienced for a long period of time, neuroplasticity leads to changes in the nervous system, causing the brain to perceive amplified pain signals.6,8 Sensitization of the nervous system is found in endometriosis patients secondary to 1) pro-inflammatory cytokines contributing to peripheral neurogenic inflammation and peripheral sensitization;9 2) chronic guarding in the pelvic musculature;10 and 3) upregulation of the central nervous system secondary to the peripheral nerve feedback loop, pain, and anxiety.11,12
Patients can still experience chronic pelvic pain and myofascial dysfunction after surgical removal of endometriosis.13 In fact, it has been observed that only half of patients experience a complete resolution of pain after surgery.14 We hypothesize that this is secondary to persistent myofascial pain and dysfunction, peripheral sensitization, and central sensitization. This study aimed to evaluate the efficacy of a multimodal, outpatient neuromuscular protocol in treating the remaining underlying sensitization and myofascial pain in Endometriosis patients post-surgical excision.
Materials and Methods
Participants
Sixty women between ages 22 to 78 with a diagnosis of Chronic Pelvic Pain and a history of excised endometriosis were the participants of this retrospective study. These women presented to an outpatient rehabilitation practice between February 2020 and May 2021. Demographics and clinical characteristics are presented in Table 1. Previous surgeries and medications tried are shown in Figures 1 and 2. Medications for central nervous system neuromodulation are shown in Table 2. Fifty-five women were started on CNS medications by our physiatrist and 5 women were continued on the CNS medications prescribed by their psychiatrist.
 |
Table 1 Demographics and Clinical Characteristics of Women with Chronic Pelvic Pain and Excised Endometriosis |
 |
Table 2 Current Medications for Central Nervous System Neuromodulation |
 |
Figure 1 Previous surgeries. |
 |
Figure 2 Previous medications. |
Inclusion Criteria
All women included had a history of chronic pelvic pain for at least 6 months and had completed a course of 6 weeks of pelvic floor physical therapy with persistent symptoms. Participants had endometriosis with central sensitization and myofascial pain following surgical excision. Prior to inclusion in the study, a full gynecological consultation with necessary workup by a gynecologist was performed. All participants underwent a detailed history and physical examination completed by one of eleven physiatrists as part of their pretreatment evaluation (Figure 3). Complete physical examination consisted of 1) lumbosacral exam to evaluate for joint pathology, coccydynia, and lumbar pain; 2) bilateral hip exam to uncover hip and pelvic girdle pain generators; 3) abdominal exam to check for trigger points in the external obliques and rectus abdominus and allodynia/hyperalgesia along the pudendal nerve and posterior femoral cutaneous nerve; and 4) internal pelvic floor examination involving palpating superficial and deep pelvic floor muscles to observe pelvic floor hypertonia and myofascial trigger points. These trigger points are evident bands within a muscle which often have pain patterns and twitch responses15 and are present in patients with sensitization.6 Presence of the allodynia/hyperalgesia, myofascial trigger points, and pelvic floor dysfunction during the physical examination warranted inclusion. All participants underwent the full treatment protocol, and there were no dropouts.
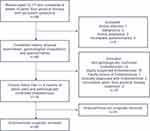 |
Figure 3 Study design. |
Exclusion Criteria
A full gynecological consultation with necessary workup by a gynecologist was performed to exclude infections and malignancy. Women who did not excise their endometriosis and did not have pathologically confirmed endometriosis were excluded. Women who did not participate in pelvic floor physical therapy during treatment, or who had incomplete Visual Analogue Scale or Functional Pelvic Pain Scale questionnaires, were also excluded.
Procedures
This study was approved by the International Review Board at the Feinstein Institutes for Medical Research (Northwell Health) for a protocol designed for chronic pelvic pain patients who failed to improve after completing a six-week course of pelvic floor physical therapy. The protocol consists of U/S guided trigger point injections to the pelvic floor muscles, peripheral nerve blocks, pelvic floor physical therapy, and cognitive behavioral therapy. In compliance with the Declaration of Helsinki and because the study involved retrospective information collection and analysis for research purposes, the need for consent was waived.
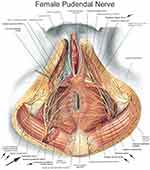
On U/S, myofascial trigger points can be seen as focal, hypoechoic regions with reduced vibration amplitude15 (Figure 4). With the patient lying in prone, using aseptic technique, a global injection by a flexible, 6-inch, 27-gauge needle to the iliococcygeus, pubococcygeus, and puborectalis was administered unilaterally from the subgluteal posterior approach once weekly for six weeks16 (Figure 5). Then, peripheral nerve blocks to the pudendal nerve at Alcocks’s canal were given.17 The patient was then flipped to the supine position, and nerve blocks to the posterior femoral cutaneous nerve 4 cm inferior to the ischial tuberosity were given.18 This was repeated at every visit, alternating right and left each time as an attempt to reduce peripheral neurogenic inflammation and attenuation of central sensitization regardless of laterality of pain. At the first visit, 2 mL of dexamethasone with 7 mL of 1% Lidocaine was placed around each nerve, on each side. At subsequent treatment appointments, saline was used in place of dexamethasone for nerve blocks. There are no side effects to the treatment protocol as a 27G needle is used and patients are premedicated with diclofenac 75 mg orally. After sitting on ice for 10 minutes, patients return to work the same day as this facilitates the healing process.16
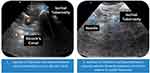 |
Figure 4 Ultrasound images of Alcock’s Canal and Obturator Canal. Work by Pelvic Rehabilitation Medicine. Reprinted with permission from Pelvic Rehabilitation Medicine. www.pelvicrehabilitation.com. |
The combination of concomitant pelvic floor physical therapy and cognitive behavioral therapy serve as contributors to the multimodal treatment protocol. Patients attended one-on-one pelvic floor physical therapy with a physical therapist at a facility of their choosing for 1 hour per week. They attended each session within 7 days after each injection. Pelvic floor physical therapy involves nerve gliding along the posterior femoral cutaneous and pudendal nerves, skin rolling along the lower abdomen and buttocks, visceral and scar tissue mobilization, and diaphragmatic breathing aimed at releasing the hypertonic pelvic floor muscles.19 Cognitive behavioral therapy consists of progressive muscle relaxation and behavioral activation strategies to target muscle tension and help patients interrupt the chronic pain cycle and related anxiety/depression.20,21
After completing the treatment protocol for 6 weeks and having follow-up visits, patients follow a neuromuscular re‐education home program guided by their physical therapist to maintain the progress made. Patients are also educated on lifestyle modifications, self-efficacy, and pain management practices such as stretching, diaphragmatic breathing, taking a warm bath, or using a muscle relaxant for any flare ups.
Outcome Measures
Pain intensity and pelvic functionality were assessed at new patient consults and 3-month follow ups using two self-assessment scales: Visual Analogue Scale (VAS) and Functional Pelvic Pain Scale (FPPS). A 0 to 10 scale for VAS measured average pain intensity over the last 24 hours where 0 meant no pain and 10 signified worst pain imaginable. Pelvic functionality using FPPS was measured using eight categories: sleeping, bowel, intercourse, walking, bladder, running, working, lifting. All categories are rated from 0 to 4 which mean normal function and brutal debilitation, respectively. Experimenter bias was limited by maintaining identical follow up questions for all patients.
Statistical Analysis
Data were analyzed using a paired t-test. This statistical analysis was used to compare the average values of outcome measures pretreatment and posttreatment to determine if there was a statistically significant difference after the treatment protocol. If the average decreased and the P value was less than 0.05, we concluded a statistical significance in that direction. Descriptive Statistics data are presented as mean ± standard deviation with a 95% confidence interval. All analyses were conducted using SPSS software version 26.
Results
The average VAS score at new patient consults was 7.45 ± 2.11 (CI 6.92–7.98). A statistically significant decrease to 4.12 ± 2.44 (CI 3.50–4.73) (P < 0.001) was seen at the 3-month follow ups. Similarly, the average total FPPS at new patient consults was 14.35 ± 6.62 (CI 12.68 −16.02) and it dropped to 10.3 ± 6.55 (CI 8.64–11.96) (P < 0.001) at the 3-month follow ups (Figure 6). Among FPPS categories, sleeping, intercourse, and working showed the highest statistical significance.
 |
Figure 6 Average Visual Analogue Scale (VAS) and Functional Pelvic Pain Scale (FPPS) before and after treatment. |
The average Functional Pelvic Pain (FPPS) scores for sleeping, intercourse, and working dropped by 0.77, 0.73, and 0.58 points when assessed at the 3-month follow ups. For the sleeping category, average score was 1.52 ± 1.28 (CI 1.19–1.84) at new patient consult and 0.75 ± 0.97 (CI 0.51–0.99) (P < 0.001) at 3-month follow up. For intercourse, average score at new patient consult was 2.32 ± 1.56 (CI 1.92–2.71) which decreased to 1.58 ± 1.52 (CI 1.20 to 1.97) (P < 0.001) at 3-month follow up. With working, average score at new patient consult was 2.27 ± 1.25 (CI 1.95 to 2.58) and at 3-month follow up reduced to 1.68 ± 1.05 (CI 1.42–1.95) (P < 0.001) (Figure 7). Table 3 illustrates these results and provides data for the other 5 FPPS categories.
 |
Table 3 Outcome Measures Results Table, Visual Analogue Scale (VAS) and Functional Pelvic Pain Scale (FPPS) |
 |
Figure 7 Functional Pelvic Pain Scale (FPPS) before and after treatment for most improved categories: sleep, intercourse, work. |
Discussion
This retrospective study investigated the effectiveness of an outpatient protocol in treating underlying sensitization and myofascial pain post-surgical excision of endometriosis. The neuropathic component includes peripheral sensitization and central sensitization. The primary outcome was measured through a decrease in VAS and FPPS scores of patients at 3-month follow up. Data suggests that the multimodal protocol was effective as the average VAS and FPPS scores decreased significantly by 3.33 and 4.05 points, respectively.
Endometriosis is a pro-inflammatory disease process that over time causes peripheral sensitization, central sensitization, and pelvic floor hypertonia.7 Despite surgical intervention of endometriosis, CPP persists in some women. This is because even after the endometriosis is removed from the body, the underlying myofascial pain and neurogenic pain which co-exists with endometriosis6,13 still needs to be addressed for patients to start feeling better.
In many Endometriosis patients, underlying myofascial pain and dysfunction is maintained by their ongoing pelvic floor spasm,6,22 the major area of chronic guarding secondary to the presence of Endometriosis in the pelvis.13 Pelvic floor hypertonia is more prevalent in patients with a history of pelvic surgery, trauma, or conditions such as endometriosis that cause myofascial pain.23,35 In this hypertonic state, nociceptors enable sensitization by producing neuropeptides that initiate neurogenic inflammation leading to peripheral sensitization.24 This recurrent stimulation of nociceptors creates a lowered activation boundary that is easier to surpass, thus provoking an exaggerated response to peripheral stimuli. Moreover, the recurrent nociceptor activation sends afferent signals to the spinal cord, causing functional and structural changes leading to central sensitization.21 This “long-term remodeling” of the central nervous system results in allodynia, hyperalgesia, and myofascial dysfunction/referred pain.6
The principle behind the treatment protocol is a three-prong approach to treating peripheral sensitization, central sensitization, and myofascial pain concomitantly, to break the pain cycle. This is achieved by programming the nervous system to return to a non-sensitized state. Peripheral sensitization is treated by reducing the neurogenic inflammation that stimulates incorrect firing of peripheral nociceptors.25 This includes 1) reversing neural ischemia by relieving pelvic floor hypertonia with trigger point injections ultimately relieving the myofascial peripheral nerve restrictions that contribute to the neural ischemia process;42 2) eliminating Substance P26 via using local peripheral nerve dexamethasone one time on each side; 3) diminishing the mast cell discharge of histamine27 via repetitive exposure of peripheral nerves to anti-inflammatory Lidocaine; and 4) desensitizing hyperactive peripheral nociceptors with repetitive exposure to an anesthetic.25 This decrease in overstimulation of peripheral nociceptors and neurogenic inflammation reverses the hyperexcitable pain processing and thus reverses central sensitization.28 Central Sensitization is also treated with weekly cognitive behavioral interventions including pain science education, mindfulness-based interventions and techniques that directly target thought and behavior change.21,29 Myofascial pain is treated with addressing the underlying myofascial trigger points that are seen in CPP patients also serve as a continuous source of nociception. U/S guided trigger point injections act not only to decrease neural ischemia but also reset the spastic pelvic floor.30 Releasing the tight muscles followed by a proper neuromuscular re-education allows for return to normal pelvic function.31,32
The significant improvement seen in the sleep category of the FPPS is related to the improvement in bladder symptoms which reduce nocturia. The peripheral nerves to the bladder are downregulated33,34 and the bladder is no longer irritated by lying on a hypertonic pelvic floor,35 so patients can avoid urinary frequency and get restful, unbroken sleep. Most of the participants experience dyspareunia: pain during, or after intercourse, due to its high prevalence in endometriosis patients.36,37 The pudendal nerve is considered as the main nerve involved in intercourse38 and the pelvic floor muscles are the main muscles involved in intercourse.39 Therefore, it makes sense that rehabilitating the pudendal nerve and the surrounding pelvic floor musculature with our outpatient multimodal protocol would decrease pain during and after intercourse and improve intercourse function. The category of working captures a patient’s ability to show up to work, which is frequently adversely affected by their pelvic symptoms.40 Lost productivity by absenteeism and presenteeism costs patients $15,737 annually in the United States.41 With our outpatient protocol reducing myofascial nerve compression and peripheral nerve inflammation through serial peripheral nerve blocks and pelvic floor physical therapy,42,43 patients can return to work unbothered by their pain symptoms, improving their productivity.
To provide better care management of chronic pelvic pain, the awareness of a comprehensive, evidence-based, treatment protocol is crucial.44 This study provides evidence for the efficacy of a comprehensive outpatient treatment protocol used when patients have incomplete resolution of their symptoms with pelvic floor physical therapy alone. Once combined with other treatment modalities such as ultrasound guided trigger point injections, peripheral nerve blocks, and cognitive behavioral therapy (CBT), patients showed significant improvements in both pain and function.
A limitation of this study is the retrospective design which does not allow for randomized control trials. The use of a placebo treatment would infringe the ethics and trust of our patients who are pursuing relief from their long-lasting pain. To gather in-depth insight into the clinical significance and quality-of-life improvements of our patients, a future consideration is to include PROMIS-29 and Female Sexual Function Index questionnaires as part of the outcome measures. Moreover, our follow ups occur 3 months after treatment which implies our outcomes may be short term. A future improvement is to gather data 6 months after treatment also.
Conclusion
Our study demonstrated that endometriosis related Chronic Pelvic Pain improves when the remaining underlying sensitization and myofascial pain seen in Endometriosis patients post-surgical excision are treated. The treatment protocol was effective for women aged 22 to 78 with a diagnosis of CPP and a history of excised endometriosis. This study favors the use of multimodal treatment modalities since patients showed improvements once pelvic floor physical therapy was combined with other treatment modalities.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sieberg CB, Lunde CE, Borsook D. Endometriosis and pain in the adolescent- striking early to limit suffering: a narrative review. Neurosci Biobehav Rev. 2020;108:866–876. doi:10.1016/j.neubiorev.2019.12.004
2. Della Corte L, Di Filippo C, Gabrielli O, et al. The burden of endometriosis on women’s lifespan: a narrative overview on quality of life and psychosocial wellbeing. Int J Environ Res Public Health. 2020;17(13):4683. doi:10.3390/ijerph17134683
3. Rossi V, Tripodi F, Simonelli C, Galizia R, Nimbi FM. Endometriosis-associated pain: a review of quality of life, sexual health and couple relationship. Minerva Obstet Gynecol. 2021;73(5):536–552. doi:10.23736/S2724-606X.21.04781-3
4. Romão AP, Gorayeb R, Romão GS, et al. High levels of anxiety and depression have a negative effect on quality of life of women with chronic pelvic pain. Int J Clin Pract. 2009;63(5):707–711. doi:10.1111/j.1742-1241.2009.02034.x
5. Tirlapur SA, Kuhrt K, Chaliha C, Ball E, Meads C, Khan KS. The ‘evil twin syndrome’ in chronic pelvic pain: a systematic review of prevalence studies of bladder pain syndrome and endometriosis. Int J Surg. 2013;11(3):233–237. doi:10.1016/j.ijsu.2013.02.003
6. Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shah J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet Gynecol. 2015;125(3):719–728. doi:10.1097/AOG.0000000000000663
7. Aredo JV, Heyrana KJ, Karp BI, Shah JP, Stratton P. Relating chronic pelvic pain and endometriosis to signs of sensitization and myofascial pain and dysfunction. Semin Reprod Med. 2017;35(1):88–97. doi:10.1055/s-0036-1597123
8. Kutch JJ, Ichesco E, Hampson JP, et al. MAPP research network: brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158(10):1979–1991. doi:10.1097/j.pain.0000000000001001
9. Liang Y, Xie H, Wu J, Liu D, Yao S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod Biol Endocrinol. 2018;16(1):122. doi:10.1186/s12958-018-0441-z
10. Shrikhande AA. The consideration of endometriosis in women with persistent gastrointestinal symptoms and a novel neuromusculoskeletal treatment approach. Arch Gastroenterol Res. 2020;1(3):66–72.
11. Li T, Mamillapalli R, Ding S, et al. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol Reprod. 2018;99(2):349–359. doi:10.1093/biolre/ioy035
12. Morotti M, Vincent K, Brawn J, Zondervan KT, Becker CM. Peripheral changes in endometriosis-associated pain. Hum Reprod Update. 2015;20(5):717–736. doi:10.1093/humupd/dmu021
13. Phan VT, Stratton P, Tandon HK, et al. Widespread myofascial dysfunction and sensitisation in women with endometriosis-associated chronic pelvic pain: a cross-sectional study. Eur J Pain. 2021;25(4):831–840. doi:10.1002/ejp.1713
14. Parra RS, Feitosa MR, Camargo HP, et al. The impact of laparoscopic surgery on the symptoms and wellbeing of patients with deep infiltrating endometriosis and bowel involvement. J Psychosom Obstet Gynaecol. 2021;42(1):75–80. doi:10.1080/0167482X.2020.1773785
15. Bonder JH, Chi M, Rispoli L. Myofascial pelvic pain and related disorders. Phys Med Rehabil Clin N Am. 2017;28(3):501–515. doi:10.1016/j.pmr.2017.03.005
16. Marcus NJ, Shrikhande AA, McCarberg B, Gracely E. A preliminary study to determine if a muscle pain protocol can produce long-term relief in chronic back pain patients. Pain Med. 2013;14(8):1212–1221. doi:10.1111/pme.12144
17. Bendtsen TF, Parras T, Moriggl B, et al. Ultrasound-guided pudendal nerve block at the entrance of the pudendal (Alcock) canal: description of anatomy and clinical technique. Reg Anesth Pain Med. 2016;41(2):140–145. doi:10.1097/AAP.0000000000000355
18. Tubbs RS, Miller J, Loukas M, Shoja MM, Shokouhi G, Cohen-Gadol AA. Surgical and anatomical landmarks for the perineal branch of the posterior femoral cutaneous nerve: implications in perineal pain syndromes. Laboratory investigation. J Neurosurg. 2009;111(2):332–335. doi:10.3171/2008.11.JNS081248
19. Natarajan J, Ahmed T, Patil S, et al. Pain and functionality improved when underlying neuromuscular dysfunction addressed in chronic pelvic pain patients. Neurourol Urodyn. 2021;40(6):1609–1615. doi:10.1002/nau.24726
20. Bonnema R, McNamara M, Harsh J, Hopkins E. Primary care management of chronic pelvic pain in women. Cleve Clin J Med. 2018;85(3):215–223. doi:10.3949/ccjm.85a.16038
21. Donat LC, Scrivani J, Shrikhande AA. Incorporating an integrative and holistic approach for chronic pelvic pain patients. J Womens Health Gyn. 2018;7:1–7.
22. Dos Bispo AP, Ploger C, Loureiro AF, et al. Assessment of pelvic floor muscles in women with deep endometriosis. Arch Gynecol Obstet. 2016;294(3):519–523. doi:10.1007/s00404-016-4025-x
23. Mabrouk M, Raimondo D, Del Forno S, et al. Pelvic floor muscle assessment on three- and four-dimensional transperineal ultrasound in women with ovarian endometriosis with or without retroperitoneal infiltration: a step towards complete functional assessment. Ultrasound Obstet Gynecol. 2018;52(2):265–268. doi:10.1002/uog.18924
24. Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi:10.1038/nn.3144
25. Tabuena DR, Solis A, Geraldi K, Moffatt CA, Fuse M. Central neural alterations predominate in an insect model of nociceptive sensitization. J Comp Neurol. 2017;525(5):1176–1191. doi:10.1002/cne.24124
26. McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation. A model for studying efferent actions of sensory nerves. Adv Exp Med Biol. 1996;410:453–462.
27. Yanagi H, Sankawa H, Saito H, Iikura Y. Effect of lidocaine on histamine release and Ca2+ mobilization from mast cells and basophils. Acta Anaesthesiol Scand. 1996;40(9):
28. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011l;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
29. Xu L, Zhang Y, Huang Y. Advances in the treatment of neuropathic pain. Adv Exp Med Biol. 2016;904:117–129.
30. Bartley J, Han E, Gupta P, et al. Transvaginal trigger point injections improve pain scores in women with pelvic floor hypertonicity and pelvic pain conditions. Female Pelvic Med Reconstr Surg. 2019;25(5):392–396. doi:10.1097/SPV.0000000000000581
31. Affaitati G, Costantini R, Tana C, Cipollone F, Giamberardino MA. Co-occurrence of pain syndromes. J Neural Transm. 2020;127(4):625–646. doi:10.1007/s00702-019-02107-8
32. Stein A, Sauder SK, Reale J. The role of physical therapy in sexual health in men and women: evaluation and treatment. Sexual Medicine Reviews. 2019;7(1):46–56. doi:10.1016/j.sxmr.2018.09.003
33. Hokanson JA, Langdale CL, Sridhar A, Grill WM. Stimulation of the sensory pudendal nerve increases bladder capacity in the rat. Am J Physiol Renal Physiol. 2018;314(4):F543–F550. doi:10.1152/ajprenal.00373.2017
34. Tai C, Shen B, Mally AD, et al. Inhibition of micturition reflex by activation of somatic afferents in posterior femoral cutaneous nerve. J Physiol. 2012;590(19):4945–4955. doi:10.1113/jphysiol.2012.239475
35. Gehrich AP, Aseff JN, Iglesia CB, Fischer JR, Buller JL. Chronic urinary retention and pelvic floor hypertonicity after surgery for endometriosis: a case series. Am J Obstet Gynecol. 2005;193(6):2133–2137. doi:10.1016/j.ajog.2005.07.013
36. Shum LK, Bedaiwy MA, Allaire C, et al. Sexual quality of life in women with endometriosis. Sex Med. 2018;6(3):224–233. doi:10.1016/j.esxm.2018.04.006
37. Orr NL, Noga H, Williams C, et al. Deep dyspareunia in endometriosis: role of the bladder and pelvic floor. J Sex Med. 2018;15(8):1158–1166. doi:10.1016/j.jsxm.2018.06.007
38. Aoun F, Alkassis M, Tayeh GA, et al. Sexual dysfunction due to pudendal neuralgia: a systematic review. Transl Androl Urol. 2021;10(6):2500–2511. doi:10.21037/tau-21-13
39. Ghaderi F, Bastani P, Hajebrahimi S, Jafarabadi MA, Berghmans B. Pelvic floor rehabilitation in the treatment of women with dyspareunia: a randomized controlled clinical trial. Int Urogynecol J. 2019;30(11):1849–1855. doi:10.1007/s00192-019-04019-3
40. Nnoaham KE, Hummelshoj L, Webster P, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366–373.e8. doi:10.1016/j.fertnstert.2011.05.090
41. Soliman AM, Yang H, Du EX, Kelley C, Winkel C. The direct and indirect costs associated with endometriosis: a systematic literature review. Hum Reprod. 2016;31(4):712–722. doi:10.1093/humrep/dev335
42. Prendergast SA, Weiss JM. Screening for musculoskeletal causes of pelvic pain. Clin Obstet Gynecol. 2003;46(4):773–782. doi:10.1097/00003081-200312000-00006
43. Plavnik K, Tenaglia A, Hill C, Ahmed T, Shrikhande A. A novel, non-opioid treatment for chronic pelvic pain in women with previously treated endometriosis utilizing pelvic-floor musculature trigger-point injections and peripheral nerve hydrodissection. J Inj Funct Rehabil. 2020;12(7):655–662. doi:10.1002/pmrj.12258
44. Pain CP. ACOG practice bulletin. Number 218. Obstet Gynecol. 2020;3:e98–109.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.