Back to Journals » International Journal of Nanomedicine » Volume 18
A Comprehensive Review of Small Interfering RNAs (siRNAs): Mechanism, Therapeutic Targets, and Delivery Strategies for Cancer Therapy
Authors Zhang J, Chen B, Gan C , Sun H, Zhang J, Feng L
Received 29 August 2023
Accepted for publication 29 November 2023
Published 13 December 2023 Volume 2023:18 Pages 7605—7635
DOI https://doi.org/10.2147/IJN.S436038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Jiaying Zhang,1,* Bo Chen,1,* Chunyuan Gan,1 Hongyan Sun,1 Jiaxin Zhang,2,3 Lin Feng1,4
1School of Mechanical Engineering and Automation, Beihang University, Beijing, 100191, People’s Republic of China; 2Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 3Institute of Liver Diseases, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 4Beijing Advanced Innovation Center for Biomedical Engineering, Beihang University, Beijing, 100191, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lin Feng; Jiaxin Zhang, Email [email protected]; [email protected]
Abstract: Small interfering RNA (siRNA) delivery by nanocarriers has been identified as a promising strategy in the study and treatment of cancer. Short nucleotide sequences are synthesized exogenously to create siRNA, which triggers RNA interference (RNAi) in cells and silences target gene expression in a sequence-specific way. As a nucleic acid-based medicine that has gained popularity recently, siRNA exhibits novel potential for the treatment of cancer. However, there are still many obstacles to overcome before clinical siRNA delivery devices can be developed. In this review, we discuss prospective targets for siRNA drug design, explain siRNA drug properties and benefits, and give an overview of the current clinical siRNA therapeutics for the treatment of cancer. Additionally, we introduce the siRNA chemical modifications and delivery systems that are clinically sophisticated and classify bioresponsive materials for siRNA release in a methodical manner. This review will serve as a reference for researchers in developing more precise and efficient targeted delivery systems, promoting ongoing advances in clinical applications.
Keywords: small interfering RNA, cancer, chemical modifications, delivery systems, bioresponsive materials
Graphical Abstract:
Introduction
Cancer, also called malignancy, is a group of diseases caused by the rapid multiplication and spread of malignant cells.1,2 According to statistics from the National Cancer Center, more than 100 types of cancer have been identified.3 The ability of malignant tumor cells to metastasize via the blood-lymphatic system and subsequent proliferation to invade numerous tissues and organs throughout the body complicates the treatment of ordinary surgical resection, which is the main reason for the high mortality rate in cancer.4–6
Traditional cancer therapeutics, including surgical resection, radiotherapy, and chemotherapy, are widely employed.7,8 Surgical resection utilizes advanced surgical instruments and imaging techniques to accurately remove non-metastatic tumor tissue.9,10 However, it struggles to eliminate metastatic tumor cells. Radiotherapy, often used as an adjunctive treatment prior to surgery, effectively eradicates tumor cells using high-energy radiation such as X or γ, with the aid of CT imaging techniques.11 Chemotherapy primarily combats tumors by impeding cell proliferation through the use of drugs. Nonetheless, it has notable side effects on normal proliferating cells and may induce drug resistance.12,13 To address the limitations of conventional therapies, various novel cancer treatments have been developed, such as tumor immunotherapy.14–17 Monoclonal antibody-based therapies targeting immune cells and pericyte therapy have proven to be highly effective approaches.17,18 Immune checkpoint blockade (ICB) therapies have made remarkable strides in cancer treatment, notably in melanoma19,20 and non-small cell lung cancer.21 Relay cell therapy involving the transfusion of chimeric antigen receptor T cells (CAR-T) has demonstrated efficacy in treating a range of chemotherapy-resistant leukemias. However, activated CAR-T cells can undergo division and proliferation, triggering a cytokine storm and leading to severe side effects, including fatalities.22–25
The introduction of precision medicine in 2015 marked a significant milestone in the field of healthcare, facilitated by advancements in genome sequencing and bioinformatics technology.26,27 This approach involves sequencing and analyzing the genomes of cancer patients to identify causative genes, thereby enabling the development of personalized cancer treatments for individuals. This personalized approach maximizes cancer treatment efficacy while minimizing toxic side effects.28,29 A promising avenue within precision medicine is the use of small interfering RNA (siRNA), a nucleic acid-based drug that holds potential for disease treatment by selectively silencing disease-related genes through sequence-specific binding.30–32 Notably, nucleic acid synthesis technology has enabled the precise, rapid, and cost-effective production of siRNA, with lower costs compared to small molecule and antibody drugs.33,34 In addition, the design of siRNA sequences allows for the targeting of previously considered undruggable genetic loci, overcoming a major challenge in personalized therapy.35 As a result, the siRNA modality has garnered significant attention due to its shorter research and development timeline, broader therapeutic scope, and versatility. In 2018, US Food and Drug Administration (FDA) approved the first siRNA therapeutic, ONPATTRO™ (Patisiran) (Alnylam Pharmaceuticals, Inc.), for the treatment of transthyretin-mediated amyloidosis.36,37 Currently, siRNA-based drugs are being utilized in the treatment of various diseases, including viral infections,38,39 genetic disorders,40–42 cardiovascular disease,43,44 and cancer.45–47 To date, four siRNA therapeutics have been approved by FDA, and more than 20 siRNA therapeutics have entered clinical trials48 (Table 1).
 |
Table 1 siRNA-Based Cancer Therapeutics in Clinical and Preclinical Trials |
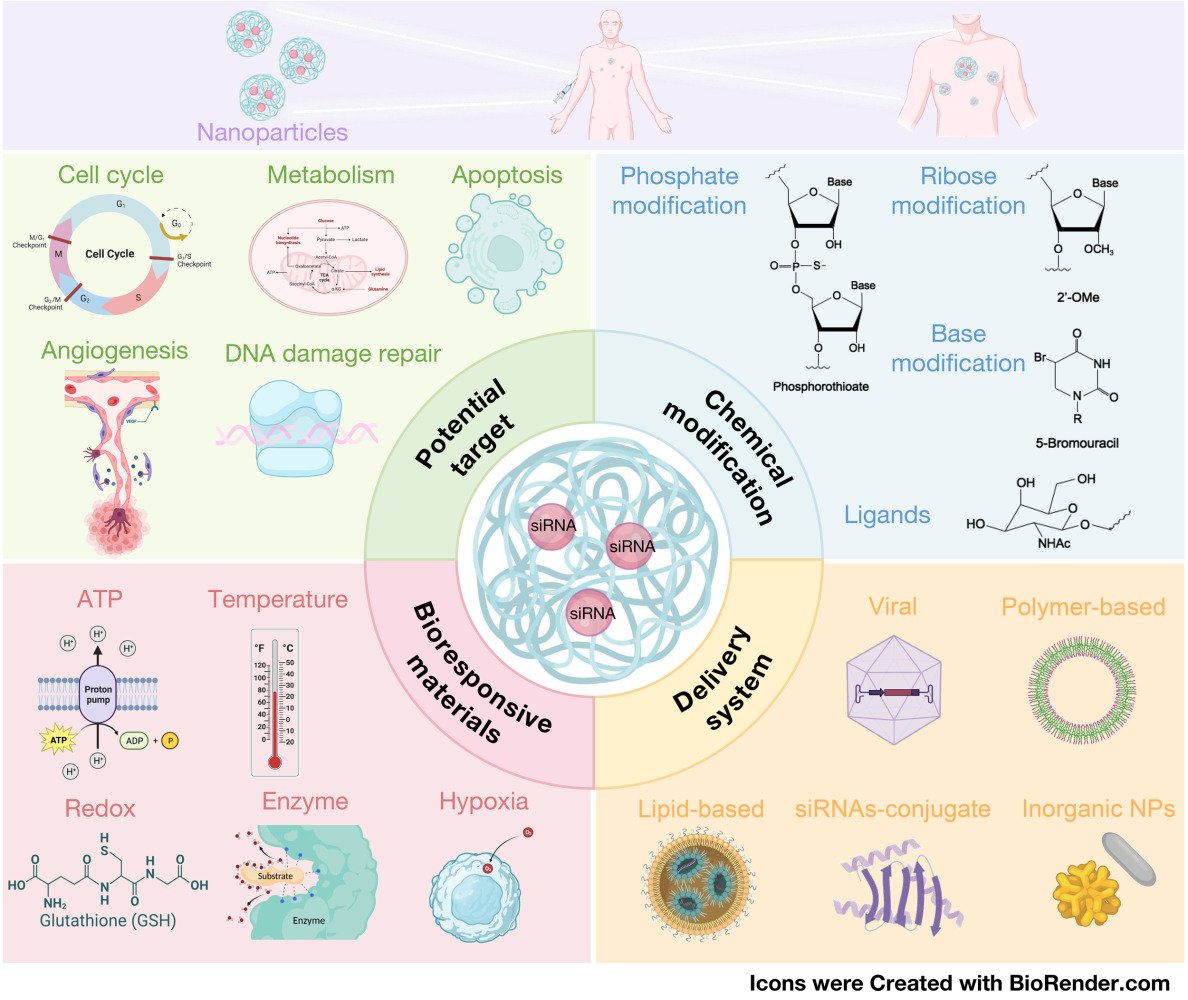
Despite the potential of siRNAs in drug development, there are several extracellular and intracellular barriers that limit their application in vivo,49,50 especially in systemic delivery. The extracellular barriers include degradation of naked siRNAs by endogenous nucleases in serum,51 rapid renal clearance due to their small size and molecular weight,52 activation of the innate immune system triggered by siRNAs through Toll-like receptors (TLR)-dependent or TLR-independent pathways,53,54 plasma protein sequestration and entrapment by the reticuloendothelial system (RES),55–57 and membrane impermeability due to their negative charge and high hydrophilicity.58 The intracellular barriers mainly include endosomal entrapment59,60 and off-target effects.61 To overcome these barriers, chemical modifications and/or delivery systems are essential to enhance siRNA bioavailability in target regions and therapeutic efficacy. Various chemical modifications have been extensively investigated and successfully employed to enhance the properties of siRNAs, including stability improvement, reduction of off-target effects, and mitigation of immunogenicity.62–64 These modifications can be categorized based on the specific sites of modification within the siRNA duplex. Examples include backbone modification,65 ribose modification,66,67 base modification,68,69 and terminal modification.70 When designing siRNAs, it is crucial to consider the modification type, position, and their impact on the charge of siRNA to ensure optimal gene silencing efficiency without compromising its effectiveness.71 Efficient delivery systems play a crucial role in delivering siRNAs to specific locations and facilitating cellular uptake, thereby enhancing their in vivo bioavailability. A diverse range of delivery strategies have been developed and widely utilized for siRNAs transportation, such as viral vectors,72–74 polymer-based delivery systems,75–77 lipid-based delivery systems,78,79 and conjugate delivery systems.80,81 Moreover, to exert the gene silencing function, siRNAs must be dissociated from the delivery vector and exist freely in the cytoplasm.82 Various bioresponsive materials have been employed to control siRNAs release under different conditions.83,84
Cancer cells possess a multitude of altered features compared to normal cells, offering numerous molecular targets for cancer therapy. Theoretically, siRNAs are capable of silencing all disease-related genes, including those associated with cancer. Therefore, siRNAs hold great promise for cancer treatment. This review aims to provide an overview of the mechanism of siRNA based on RNA interference (RNAi), highlight the advantages of siRNA in drug development, and explore potential targets for cancer therapy using siRNA therapeutics. Additionally, we summarize various chemical modifications, delivery systems, and bioresponsive materials utilized to enhance the properties of siRNA, improve gene silencing efficiency, and facilitate clinical application. Furthermore, we discuss ongoing clinical studies, potential challenges, and future prospect of siRNA therapeutics in cancer therapy.
siRNA and Potential Targets for Cancer Therapy
Mechanism of RNA Interference (RNAi)
RNAi is an important defense mechanism for eukaryotic cells, capable of degrading exogenously invading genetic material such as viruses.111 The mechanism of RNAi involves two main types: microRNAs (miRNAs) and small interfering RNAs (siRNAs), which are crucial for gene regulation. miRNAs are transcribed from endogenous genes, and their primary transcripts (pri-miRNA) are processed in the cell nucleus by Drosha and DGCR8 to form precursor miRNA (pre-miRNA). After being transported to the cytoplasm by Exportin 5, pre-miRNA is further processed by an enzyme called Dicer to remove the hairpin structure. The processed pre-miRNA is then loaded into the RNA-induced silencing complex (RISC) that comprises the Argonaute 1–4 (Ago1–Ago4) protein and separates from the complementary RNA strand. The miRNA-RISC complex binds to the 3’ untranslated region (UTR) of the target mRNA in an imperfectly complementary manner, ultimately leading to mRNA degradation or inhibition of mRNA translation.112 Conversely, siRNA-mediated RNAi initiates with the introduction of double-stranded RNA (dsRNA) into the cell. This dsRNA can originate from diverse sources, including exogenous genetic material such as viruses or synthetic siRNAs. Upon entry, the dsRNA is recognized and processed by Dicer, which cleaves the dsRNA into smaller fragments, typically around 20 nucleotides in length. These fragments are then loaded onto a protein complex known as the RISC. Within the RISC, one of the strands of the dsRNA, referred to as the guide strand, is selected to direct the complex to its target mRNA. The guide strand of the RISC binds to the complementary sequence on the target mRNA through base pairing. This interaction leads to the recruitment and activation of proteins, such as Argonaute 2 (Ago2), that facilitate the cleavage or degradation of the target mRNA. Consequently, the production of the corresponding protein is prevented or significantly reduced. RNAi serves as a powerful tool in research and exhibits potential therapeutic applications (Figure 1).113,114
Advantages of siRNAs for Cancer Therapy
RNAi encompasses three types of dsRNAs capable of inducing gene silencing: short hairpin RNA (shRNA), endogenous small RNA (miRNA), and siRNA. Among these, synthetic siRNAs are the most suitable for use as drugs due to their unique advantages over chemotherapeutic drugs and other anti-cancer agents, owing to the special RNAi mechanism.30,114–116 Four key points highlight the superiority of siRNA drugs: the specificity in silencing. siRNAs of 20 nucleotides in length can recognize any target gene with high specificity and minimal off-target effects due to the base complement pairing recognition mechanism. Second, the exceptional safety profile. siRNAs exert their post-transcriptional gene silencing effects exclusively in the cytoplasm, preventing nuclear entry and genome integration, thus minimizing the risk of host gene mutations. Third, the remarkable efficiency. Even with few fragments, siRNAs can induce significant gene silencing effects in cells. Fourth, the unlimited number of potential targets. Advances in molecular biology and whole genome sequencing have facilitated the establishment of large human genomic databases, cDNA libraries, and disease-causing gene databases. By simply designing siRNAs based on the mRNA sequence of the target gene, it becomes possible to obtain an siRNA capable of effectively silencing any disease-causing gene.30,116
Potential Targets for siRNAs Drugs for Cancer Therapy
Cancer cells exhibit substantial differences from normal tissue cells. Hanahan et al have comprehensively summarized the fourteen most important characteristics of cancer together with the corresponding therapy strategies.1,117,118 These traits include sustained proliferative signaling, loss of inhibition of proliferation, replicative immortalization, migration and metastasis, induction of angiogenesis, resistance to apoptosis, genomic susceptibility to mutation, evasion of immune attack, induction of cancer-promoting inflammation, derangement of energy metabolism, unlocked phenotypic plasticity, non-mutational epigenetic reprogramming, aging cells, and polymorphic microbiomes. While these altered traits contribute to cancer development and progression, they also offer a wealth of molecular targets for cancer therapy.
The targeted siRNA silencing of specific cancer pathogenesis and relevant target genes holds promise for interventions in cancer therapy. Figure 2 illustrates potential therapeutic targets for siRNA drugs, including: 1) cell cycle-related signaling pathways involving cyclins, cyclin-dependent kinases (CDKs), polo-like kinases (Plks), and mitosis-related molecules;119,120 2) cell proliferation-related signaling pathways involving protein kinase N3 (PKN3), ephrin type-A receptor 2 (EphA2), AKT1/2/3, and other molecules that promote cell proliferation;121–124 3) cancer metastasis-related signaling pathways, such as hepatocyte growth factor receptor (HGFR), transforming growth factor-β (TGFβ), chemokine receptor (CCR2, CXCR2) and molecules facilitating HCC metastasis;125,126 4) cancer angiogenesis-related signaling pathways involving vascular endothelial growth factors (VEGFs), vascular endothelial growth factor receptors (VEGFRs), matrix metalloproteinase 9 (MMP9), focal adhesion kinase (FAK), and other molecules that promote cancer angiogenesis;127 5) cancer cell anti-apoptotic proteins including Survivin, Bcl2, Bcl-xL, which inhibit cancer cell apoptosis;128 6) DNA damage repair-related molecules such as methyltransferase (MGMT), and poly(adenosine diphosphate-ribose) polymerase (PARP), which inhibit programmed cancer cell death;129 7) molecules associated with cancer immune escape, such as programmed cell death protein 1 (PD1) and its ligand (PDL1), cytotoxic T lymphocyte-associated protein 4 (CTLA4) and other immune test site molecules;15,130 8) genes related to cancer metabolism, such as glucose transporters (GLUTs), lactate dehydrogenase A (LDH-A), hypoxia-inducible factors (HIFs), glutaminases, fatty acid synthases;131 9) oncogenes, including c-myb, c-Myc, and RAS genes, which are closely associated with cancer development and tumor progression;132,133 10) In addition, there are cancer tyrosine kinase-related signaling pathways, such as epidermal growth factor receptor (EGFR), insulin-like growth factor 1 receptor (IGF-1R), Ras family proteins, BCR-ABL tyrosine kinases,134 and cancer cell multidrug resistance genes (MDRs).135,136
In cancer therapy, the abundance of potential targets necessitates careful selection based on the following principles: 1) Preferential expression in tumor cells, while being minimal, absent, or present at negligible levels in normal cells; 2) Key involvement in promoting tumorigenesis; 3) Preferably a broad-spectrum gene applicable to multiple tumor models. These principles include the Therapeutic Index and the Therapeutic Window, which together define the safety and efficacy of a treatment regimen.137,138 Differential expression of target genes in tumor cells and normal cells reduces damage to healthy tissue; The critical role of target genes in tumorigenesis increases the likelihood of successful treatment; In addition, the broad-spectrum nature of these genes facilitates the dissemination of therapeutic solutions and offers greater market potential. By considering these three factors, the selection of a safe and effective therapeutic target with broad applicability becomes possible.
Challenges in siRNA Drugs Development
Although siRNA offers significant advantages in cancer therapy, it is important to acknowledge the multitude of cancer-related genes that can be targeted using this approach. Nevertheless, the utilization of siRNA drugs in clinical cancer therapy encounters several obstacles that need to be overcome.
The first challenge lies in the inherent instability of siRNAs under physiological conditions, making them susceptible to rapid clearance. As shown in Figure 3, the physiological milieu harbors a multitude of nucleases, leading to the degradation of siRNAs upon entry into the bloodstream.139 Furthermore, phagocytes are abundant in tissues and organs such as blood, liver, and spleen, particularly in the mononuclear phagocytic system (MPS)/reticuloendothelial system (RES), including mononuclear macrophages, phagocytic cells, and neutrophils, which engulf siRNAs entering the body.57 Moreover, significant amounts of siRNA are efficiently eliminated during their circulation to the kidneys by glomerular filtration.140 The unmodified siRNAs have a short half-life in blood, lasting only a few minutes to one hour.139 Even if siRNA successfully reaches the target cells, it initially encounters the endosomes and lysosomes, which have an acidic environment with a pH range of 5–6 and an even lower pH of 4.5 within the lysosomes. These compartments contain a number of nucleases capable of rapidly degrading siRNAs.141
The second challenge concerns the cellular uptake of free siRNAs. Due to its hydrophilic nature and negative charge, siRNA encounters obstacles when attempting to cross the negatively charged hydrophobic cell membranes and enter cancer cells, resulting in charge repulsion and impermeability.57 The third hurdle involves the escape of siRNAs from endosomes. The assembly of siRNA into the RISC complex for gene silencing can only occur in the cytoplasm. However, siRNA faces limitations in freely penetrating biological membranes, requiring strategies to achieve efficient endosomal escape and release into the cytoplasm, creating a barrier to siRNA delivery.57,141 The fourth obstacle involves the immunogenicity of siRNAs. Toll-like receptors (TLRs), which are pattern recognition receptors expressed on immune cells, can recognize pathogen-associated molecular patterns, including CpG DNA and viral dsRNA. TLR3, TLR7, and TLR8 specifically recognize nucleotide sequences in siRNAs, such as the UG dinucleotide and 5’-UGU-3’ motifs. Upon injection into the body, siRNAs activate the innate immune system, resulting in the production of substantial cytokines.54,142
The fifth challenge focuses on the off-target effects of siRNAs. Although siRNAs, with a length of 20 nucleotides, have a high specificity in terms of base-complementary pairing, cells contain numerous long-stranded mRNAs and miRNAs. Incomplete complementary pairing of siRNAs can lead to the degradation of unintended mRNAs and miRNAs, causing non-specific gene regulatory effects. Furthermore, cells express various miRNAs to regulate gene expression, and these miRNAs can have sequences identical to the target mRNAs. Consequently, siRNAs not only degrade target mRNAs but also miRNAs, leading to unpredictable changes in gene expression.143
As previously mentioned, siRNA holds immense potential in cancer therapy but is accompanied by various challenges. Therefore, it is crucial to identify safe and efficient delivery strategies that can fully exploit the benefits of siRNA drugs in cancer therapy. In essence, siRNA delivery systems must fulfill the following requirements: 1) ensuring the serum stability of siRNA; 2) facilitating siRNA immune evasion; 3) attenuation of siRNA interactions with plasma proteins and phagocytes; 4) prevention of renal clearance; 5) enhancing the ability of siRNA to cross the vasculature and reach cancerous tissues; 6) facilitating cellular uptake of siRNA; 7) promoting siRNA escape from endosomes; 8) high biocompatibility and non-toxicity. Only when these conditions are met can efficient delivery of siRNA drugs be achieved.114 In the following section, we present a comprehensive overview of siRNA delivery strategies.
Delivery Strategies for siRNA Drugs
siRNA, when used as a therapeutic drug, faces challenges such as a short half-life, poor serum stability, and susceptibility to nuclease degradation. Furthermore, its negative charge hinders easy entry into tumor cells through the cell membrane and is prone to degradation by intracellular lysosomes. Consequently, the direct use of siRNAs as therapeutic drugs to treat diseases is challenging. To overcome these limitations, chemical modifications and nano-delivery systems have emerged as common strategies to facilitate the therapeutic application of siRNA drugs.
Chemically Modified siRNAs
While chemical modification does not serve as a delivery vehicle for siRNA, it does play a critical role in enhancing the inherent properties of siRNAs. Significant improvements in serum stability, immune evasion capability, and the assembly of siRNAs into RISC can be achieved through the use of appropriate chemical modifications.57,144 Therefore, it is imperative to discuss the impact of chemical modifications on siRNA delivery efficiency before introducing siRNA delivery vectors.
In general, siRNAs offer multiple sites that can be chemically modified, including the terminus, backbone, ribose moieties, and bases.30 Several commonly used methods for siRNA modification are shown in Figure 4. Experimental evidence has demonstrated that phosphorothioate (PS) modification enhances nuclease resistance, pharmacokinetic properties, and serum stability of the modified material. However, excessive PS modification can lead to serious toxic effects.145 Furthermore, 2’-fluoro (2’-F) or 2’-O-methyl (2’-OMe) modifications of siRNA ribose have been widely used in commercial siRNAs. These modifications improve serum stability, extend half-life, enhance RNAi ability, and reduce off-target effects.144 Other siRNA modification methods include the incorporation of locking nucleic acids (LNAs) at the 2’ and 4’ positions of methylene-linked ribose, as well as the replacement of phosphodiester nucleotides with phosphonothioate at the 3’ end of the RNA backbone.114 Additionally, modification of siRNA with the small molecule 2.4-dinitrophenol (DNP) not only reduces nuclease degradation but also facilitates cellular uptake by aiding cell membrane crossing.146
While numerous modification methods exist to enhance the stability and other properties of siRNAs, it is critical to strike a balance between the gene-silencing effect and safety when using chemical modifications. It should be noted that certain chemical modifications can affect the effectiveness of siRNAs. For instance, a boranophosphonate modification within the siRNA antisense strand may increase nuclease resistance but reduce the effectiveness of RNAi.147 As far as safety is concerned, the nucleotides generated by the degradation of regular siRNAs are indistinguishable from those naturally occurring in vivo. However, many chemical modifiers utilized are not native to the human body. Consequently, a critical evaluation of the safety of substances generated by the metabolism of these chemically modified siRNAs in humans is essential.
siRNA Delivery Systems
In addition to chemical modification, researchers have developed various types of delivery systems to facilitate the transport of siRNAs into target tissues and cells. Delivery systems can be broadly divided into viral and non-viral vectors. Viral vectors are known for their efficient nucleic acid delivery (Figure 5A); however, they possess certain limitations,148,149 such as: 1) the ability of some viruses to only deliver nucleic acids to dividing cells; 2) limited nucleic acid loading capacity (≤ 5 kb); 3) potential immunogenicity and toxicity; and 4) the risk of insertional mutagenesis. Given the extensive existing reviews on viral vectors, this article focuses on the systematic description of non-viral siRNA vectors (Figure 5B–E).
Lipid-Based siRNA Delivery Systems
Lipid-based siRNA delivery vectors include various types, including anionic liposomes, neutral liposomes, cationic liposomes, stable nucleic acid-lipid particles (SNALPs), and lipidoid nanoparticles (Figure 5B). Among these, cationic liposomes such as the commercial Lipofectamine have gained significant importance in DNA and RNA transfection, including siRNA transfection. In particular, Lipofectamine RNAiMAX has shown remarkable efficacy in achieving high transfection efficiencies at the cellular level. The mechanism underlying the use of cationic liposomes for cell transfection involves electrostatic interaction between positively charged lipids and negatively charged cell membranes, facilitating the phagocytosis of siRNA by cells.150
In terms of biocompatibility and pharmacokinetics, anionic or neutral liposomes generally outperform cationic liposomes due to the negatively charged nature of biological membranes. A remarkable neutral lipid, 1.2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC), has been utilized to improve the encapsulation efficiency of siRNA. In 2005, Landen et al developed a neutral liposome (siRNA-EphA2-DOPC) containing siRNA targeting the oncoprotein EphA2 using DOPC. Notably, within 48 hours after administration of this siRNA-EphA2-DOPC liposome to an in-situ mouse model of ovarian cancer, a significant downregulation of EphA2 expression was observed.109 Currently, siRNA-EphA2-DOPC is undergoing Phase I clinical trials at the M.D. Anderson Cancer Center (MDA). Recently, Sahu et al have developed a nanocarrier system composed of DOPC that can effectively stabilize mTOR siRNA and successfully deliver it (NL-mTOR siRNA). In vitro and in vivo experiments have demonstrated that NL-mTOR-siRNA can inhibit the growth and invasion of breast cancer and restore tumor morphology. Furthermore, the neutral liposomes enhance the accumulation of siRNA in breast cancer tissue, facilitate its distribution within the tumor, and enhance its anti-tumor potential.151 Ramos-Gonzalez et al employed a novel neutral DOPC liposome-siRNA system (L-siRNA) respiratory delivery strategy that effectively silenced WT1 in vitro B16F10 melanoma cells and in vivo lung metastatic melanoma mouse models, inhibiting cell proliferation, significantly reducing tumor weight, and delaying animal mortality.152
Although cationic liposomes may not exhibit the same level of biocompatibility as other liposome types and are prone to clearance by the mononuclear phagocyte system, their notable advantage lies in their high cellular uptake capacity. Therefore, cationic liposomes currently remain the preferred choice. Cationic lipids, such as dioleoyl-phosphatidylethanolamine and 1,2-dioleoyl-3-trimethyl propane (DOTAP), can be formulated into cationic liposomes that efficiently transport negatively charged siRNAs through electrostatic interactions.153 Sorensen et al successfully delivered TNF-α siRNA via DOTAP liposomes and effectively inhibited bacterial lipopolysaccharide (LPS)-induced mortality in a sepsis mouse model.154 Additionally, Balgobind et al developed a cationic lipid carrier system consisting of cholesteryl cytofectins, 3β-N-(N’,N’-dimethylaminopropyl)-carbamoyl cholesterol (Chol-T) or N,N-dimethylaminopropylaminylsuccinylcholesterylformylhydrazide (MS09), and neutral helper lipid dioleoylphosphatidylethanolamine (DOPE). The liposomes demonstrated effective transfection and gene silencing in cells overexpressing HER2/neu.155 Jarallah et al designed an efficient siRNA nanoliposome carrier using Genzyme Lipid 67 (GL67), DC-Chol, and DOPE lipids. Physicochemical characterization results showed that it exhibited a favorable impact on the metabolic activity and uptake rate of A549 cells.156 It is important to note that although liposomes are designed to possess a positive charge to facilitate cellular uptake, this positive charge also accelerates their clearance by mononuclear phagocytes. Therefore, strict control of the charge of cationic liposomes is required, typically achieved by maintaining an appropriate complex phosphorus ratio (N/P) of around 2 to 3.157
PEGylation of liposomes is an important method for enhancing drug delivery efficiency. By coating the liposome surfaces with a layer of polyethylene glycol (PEG), the particle size can be reduced, preventing aggregation and fusing during storage. In addition, PEGylation decreases clearance by the mononuclear phagocyte system and prolongs the blood half-life of liposomes.158 However, liposome PEGylation also has some disadvantages. For example, the spatial site-blocking effect and negative electrical properties of PEG can hinder cellular uptake, decrease fusion of liposomes with endosomal membranes, and inhibit endosome escape.158 These challenges can be addressed by rationally designing the PEG density and length on the liposome surface or by using pH-sensitive chemically bonded PEGs.159 While the optimal PEG density and length are still under investigation, pH-sensitive chemical bond-optimized PEG modification has demonstrated effectiveness.160 For instance, liposomes linked to PEG via oxime bonds remain stable at pH 7.4 in a neutral environment but exhibit accelerated siRNA release and improved gene silencing at pH 5.5 in an acidic environment. Additionally, HEMA-histidine-methacrylic acid (HEMA)-modified PEGylated liposomes exhibit stability under neutral conditions. In contrast, the 1.2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) liposome nucleus is positively charged, and upon entering the endosome, the imidazole and methacrylic acid residues become protonated in the acidic environment, converting the negatively charged PEG to a positive charge. This dissociation exposes the positively charged liposome nucleus, promoting fusion with the endosome and facilitating endosome escape.161
SNALPs (Stable Nucleic Acid-Lipid Particles) represent the most well-known lipid-based siRNA delivery vectors. Notably, the FDA-approved siRNA drug for Ebola treatment is delivered by SNALPs, a class of lipid nanoparticles encapsulating siRNA with a particle size of approximately 120 nm. SNALPs have a lipid bilayer structure consisting of a mixture of cationic lipids and pro-fusion lipids on the outer layer, while siRNA is encapsulated within the inner layer, with the surface of the lipid bilayer structure modified with PEGs.162 The presence of PEGs on the surface allows SNALPs to achieve prolonged circulation and passive enrichment at the tumor sites through the enhanced permeability and retention (EPR) effect. Once SNLAPs reach the tumor site, they can be rapidly taken up by tumor cells, facilitating efficient siRNA delivery. Alnylam Pharmaceuticals made a significant breakthrough by encapsulating two siRNAs (VEGF siRNA and KSP siRNA) simultaneously in SNALPs, naming the formulation ALN-VSP02. In April 2009, ALN-VSP02 entered a phase I clinical trial for advanced solid tumors, with an initial cohort of 28 patients showing good tolerance and antitumor activity following the administration of the six highest doses (1.25 mg/kg). Notably, one patient with endometrial cancer achieved complete remission.163 Tekmira Pharmaceuticals has also developed SNALP-Plk1 siRNA (TKM080301), which is currently undergoing phase I and Phase II clinical trials for various solid tumors and lymphomas.164 Furthermore, Abdel-Bar et al developed a stable SNALP formulation capable of co-delivering Dox and siCD47, synergistically enhancing tumor immunogenic cell death (ICD). The optimized SNALPs exhibited an encapsulation efficiency of over 65% for siRNA and Dox and improved serum stability. The combination of siCD47 and Dox-loaded SNALPs demonstrated potent anti-tumor activity in a tumor challenge model.165
Lipid nanoparticles (LNPs) are siRNA delivery vehicles composed of lipids, cholesterol, and PEGylated lipids with a structure like SNALPs, which also have a lipid bilayer structure with PEGylated surfaces and siRNAs encapsulated in the core.163 Akinc et al developed a rapid method for chemical synthesis of lipid-like libraries and performed a screen to identify the most effective lipid-like nanoparticles for siRNA delivery.166 One of the most promising lipid-like nanoparticle components was 98N12-5, with which siRNAs encapsulated with ApoB or FVII factors could achieve 75–90% gene silencing in non-human primate hepatocytes.
Polymer-Based siRNA Delivery Systems
Polymer-based delivery vehicles, also known as polymeric nanoparticles, are a class of solid biodegradable colloidal nanoparticles commonly used for drug delivery and are mainly classified into water-soluble polycationic nanoparticles and polymeric nanoparticles167 (Figure 5C).
Various water-soluble polycationic nanoparticles are available, including those derived from cyclodextrin polymers (CDP) and polyethyleneimine (PEI). Among these, CDP nanoparticles have shown promise for clinical delivery of siRNA drugs.77 A notable example is the CDP-based siRNA drug CALLA-01, developed by Calando Pharmaceuticals. CALLA-01 consists of four components: CDP, adamantane-PEG (AD-PEG), adamantane-PEG-transferrin (AD-PEG-Tf), and siRNA. These components self-assemble into nanoparticles, with the positively charged CDP and siRNA forming the particle core.168 AD-PEG incorporation creates a PEG shell on the particle surface, which increases particle stability and reduces clearance by the mononuclear phagocyte system. In addition, AD-PEG-Tf is coupled to transferrin, which specifically binds to the highly expressed transferrin receptor CD71 in tumor cells, thus enabling targeted drug delivery.169 In a study conducted by Wu et al, a polymer-siRNA nanoparticle (PEG-PEI/siRNA) was synthesized and evaluated as a non-viral carrier for siRNA targeting CD44v6 in gastric cancer cells. This nanoparticle exhibited a relatively high gene transfection efficiency and low cytotoxicity.170
The main polymer nanoparticles used in this area are derived from polycaprolactone (PCL), poly (lactic acid) (PLA) and poly (lactic acid glycolic acid) (PLGA).167 A notable study has reported a self-assembled micellar nanoparticle (MNP) called micelle plex, formed by the self-assembly of poly(ethylene glycol)-b-polycaprolactone)-b-poly(2-(2-oxo-1,3,2-dioxaphospholoyloxy)ethyl methacrylate) (mPEG-b-PCL-b-PPEEA) triblock polymer.171 The micelle plex consists of a hydrophobic core formed by the PCL block, the cationic hydrophilic phosphate PPEEA responsible for siRNA binding, and an outer PEG layer that stabilizes the particle. By delivering GFP siRNA, micelle plex effectively downregulates GFP expression in HEK293/GFP cells by 40%-70%. In addition, micelle plex has demonstrated the successful delivery of various siRNAs targeting tumor-associated genes, such as AC siRNA, HIF1 siRNA, and CDK4 siRNA, resulting in significant inhibition of tumor growth in various tumor models.172,173 Recently, Ma et al employed positively charged polydimethylaminoethyl methacrylate (PDMAEMA) and poly [oligo (ethylene glycol) methyl ether methacrylate] (POEGMA) to produce miktoarm star polymer nanoparticles. These star nanoparticles, when complexed with siRNAs targeting βIII-tubulin and Plk1, demonstrated efficient cellular uptake and endosomal escape. Upon nebulization, the star-siRNA nanoparticles inhibited lung tumor growth by accumulating in the lung and silencing the expression of βIII-tubulin and Plk1.174
PLA and PLGA are FDA-approved polymeric materials for medical use. Yang et al developed a PLA-based cationic lipid-assisted polymeric nanoparticle (CLAN) with double emulsified nanoparticles encapsulated with siRNA, named CLANsiRNA, prepared by block polymer PEG-PLA and cationic lipid N,N-bis (2-hydroxyethyl)-N-methyl-N-(2-cholesteryloxycarbonyl aminoethyl) ammonium bromide (BHEM-Chol).175 The particle size of CLANsiRNA was around 170 to 200 nm, and the incorporation of the cationic lipid BHEM-Chol significantly increased the encapsulation rate to over 90%. In a mouse model of MDA-MB-435s breast carcinoma, injection of CLANsiRNA encapsulated with Plk1 siRNA effectively inhibited tumor growth.175 In addition, Ghareghomi et al developed PLGA-based nanoparticles (NPs) functionalized with folic acid and loaded with magnetic nanoparticles for imaging. These NPs were co-loaded with Wtmn and hTERT siRNA to achieve efficient cytotoxicity and growth suppression in SKOV-3 cancer cells by targeting hTERT/telomerase activity, providing a potential combinatorial therapy for ovarian cancer.176
Dendrimers, a type of nanocarrier with a star-shaped or branched structure, are utilized for drug conjugation in tumor diagnosis and treatment and are extensively researched as potential nucleic acid carriers.177–181 Various dendrimers, such as poly(L-lysine) dendrimers (PLL), carbosilane dendrimers, polyethyleneimine (PEI) dendrimers, and poly(amidoamine) dendrimers (PAMAM), have been employed for siRNA delivery. Recent studies have demonstrated that these dendrimers effectively transport siRNA into cells and contribute to cancer treatment. Gorzkiewicz et al revealed that a novel PLL dendrimer, containing lysine and arginine or histidine residues, is capable of efficiently transferring siRNA into cells.182 Additionally, Olmo et al confirmed that Schiff-based carbosilane copper (II) metallodendrimers can deliver pro-apoptotic siRNAs Mcl-1 and Bcl-2 into human breast cancer cells (MCF-7), enhancing the anti-cancer effect of siRNA.183 Furthermore, Pan et al discovered that generation 4 PAMAM (G4 PAMAM), conjugated with polyethylene glycol (PEG)-modified 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (PEG2K-DOPE), can deliver MDR-1 siRNA and the drug DOX to MDR cancer cells through the EPR effect, reducing the P-gp and drug resistance of MDR cancer cells.184 Chen et al synthesized a bola-amphiphilic PAMAM dendrimer, demonstrating its potential for delivering siRNA drugs to tumors and cancer cells and treating various cancer models.185
siRNA-Conjugate Delivery Systems
siRNA-conjugate delivery systems involve the direct coupling of siRNA to a delivery material, resulting in a single-component drug delivery system with specific characteristics. The initial approach to siRNA conjugation involved coupling siRNA to a lipophilic molecule like cholesterol. However, various coupling systems have been developed. These include the coupling of siRNA to polymers, peptides, antibodies, nucleic acid aptamers, or small molecules35,186 (Figure 5D).
Lipophile-siRNA conjugates, in which cholesterol is coupled to the 3’ end of the siRNA sense strand via a pyrrolidone bond, not only increase the transfection efficiency of siRNA in vitro but also enhance the pharmacokinetics of siRNA in vivo.187 In 2007, Wolfrum et al investigated the use of high-density lipoprotein (HDL) to further optimize cholesterol-siRNA conjugates, resulting in an 8- to 15-fold increase in siRNA gene silencing effect in vivo.188 Recently, Masimov et al developed chitosan nanoparticles conjugated with HDL to shield siRNAs from enzymatic degradation. These nanoparticles possess the capability to target siRNAs in liver cancer cells expressing SR-B1 receptors.189 Moreover, antibody-siRNA-coupled delivery systems, leveraging the specificity of antibodies, enable targeted delivery of siRNA. For instance, by coupling a targeting antibody against the transferrin receptor CD71 to siRNA via biotin-strand affinity action, siRNA can cross the blood-brain barrier and inhibit the expression of reporter genes in intracranially transplanted rat brain tumor cells.190 Wang et al reported novel photoresponsive antibody-siRNA conjugates (PARCs), consisting of anti-programmed death-ligand 1 antibody (α-PD-L1) and siRNA targeting intracellular PD-L1 mRNA, linked by a photo-cleavable linker. This complex enables tumor-specific light-induced siRNA delivery and photo-activated immunogene therapy.191 Similarly, nucleic acid aptamer-siRNA-coupled delivery systems, utilizing the specificity of nucleic acid aptamers for protein binding, serve as targeted siRNA delivery systems. For example, the coupling of nucleic acid aptamers targeting prostate-specific membrane antigen (PSMA) to siRNA through biotin-strand affinity action successfully increases siRNA transfection efficiency in PSMA-highly expressed cells.192 Besides, Yang et al developed a multifunctional RNA nanoparticle, EGFRapt-3WJ-Alexa647-siKRASG12C, capable of delivering siRNA targeting KRASG12C to non-small cell lung cancer (NSCLC) cells. The EGFR-targeting RNA aptamer (EGFRapt) further enhances specificity for the tumor.193
Cell-penetrating peptides (CPPs) are used as siRNA coupling agents to facilitate the crossing of cell or endosomal membranes, thereby improving siRNA transfection efficiency or endosomal escape. An example of a CPP is the TAT transcription activator protein derived from the HIV-1 virus. TAT can be coupled to the 3’ end of the siRNA antisense strand using sulfosuccinimidyl-4-(p-maleimidophenyl) butyrate and other heterobifunctional bridge bonds. This coupling strategy significantly improves the intracellular delivery efficiency of siRNA.194 Yang et al proposed the use of graphene nanoparticle-loaded siRNA to silence Rictor molecules, which are essential for the mammalian rapamycin (mTOR) complex 2 (mTORC2) complex. They modified a CPP to treat breast cancer, leading to enhanced gene delivery to tumor cells.195 In a study by Cai et al, a CPP-modified metal-organic framework nanoplatform named PEG-CPP33@ORI@survivin siRNA@ZIF-90 (PEG-CPP33@NPs) was designed for targeted co-delivery of oridonin (ORI), a naturally occurring antitumor active component, and survivin siRNA in vivo.196 However, it is important to note that CPP-siRNA couplers may pose potential side effects due to the membrane-penetrating ability and immunogenicity of CPP.197 Additionally, Ma et al constructed a nanobubble modified with CPP-conjugated DOX and CPP-conjugated c-myc siRNA, along with asparagine-glycine-arginine peptide (NGR) modification. When combined with ultrasound, the CPP- and NGR-modified formulation led to enhanced drug accumulation in tumors and demonstrated potent anti-tumor effects.198
In addition to the aforementioned siRNA-coupled delivery systems, one of the most promising clinical applications is the N-acetylgalactosamine-siRNA (GalNAc-siRNA)-coupled delivery system. This system exhibits a high affinity for the asialoglycoprotein receptor (ASGPR) present on the hepatocyte surface, enabling hepatic targeting of siRNA.199 Khan et al developed a safe, effective, and biocompatible formulation called GalNAc@PEG@siRNA-PLGA, which encapsulates survivin siRNA in GalNAc-modified polyethylene glycolized PLGA nanocouples (NCs). They evaluated the synergistic antitumor effects of this formulation for targeted delivery to mice with hepatocellular carcinoma.200 Li et al established a GalNAc-modified nanocarrier formulation containing 5’-FU and siRNA targeting the VEGFs. This formulation exhibited significant anti-metastatic activity against C5WN1-HCC cells and effectively suppressed tumorigenic and pulmonary metastases with a tumor suppression rate of 96%.201 Additionally, Alnylam Pharmaceuticals has developed a trivalent GalNAc-siRNA delivery system to enhance liver-targeting capabilities. Three siRNA drugs, namely ALN-TTRsc, ALNPCS, and ALN-AT3, have been prepared by coupling trivalent GalNAc with TTR siRNA, PCSK9 siRNA, or AT siRNA, respectively. These drugs have entered clinical trials for the treatment of thyrotropin amyloidosis, hypercholesterolemia, and hemophilia.114 Recently, Porter et al investigated the potential of GalNAc-conjugated siRNA, known as SLN124, for treating beta-thalassemia and myeloproliferative neoplasms. SLN124 exhibits liver-targeting properties and can effectively silence the TMPRSS6 gene, resulting in increased endogenous hepatic phospholipid synthesis. The initial phase I clinical trial in human subjects assessed the safety and tolerability of escalating single doses of SLN124 (1.0, 3.0, and 4.5 mg/kg) in healthy volunteers. The study revealed dose-dependent impacts on iron metabolism and red blood cell markers, with sustained effects lasting up to 56 days following a single dose.202
Inorganic Nanoparticle siRNA Delivery Systems
Inorganic materials offer the opportunity to develop nanocarriers with controllable size and morphology. These materials possess unique properties that contribute to the carriers’ favorable characteristics, including excellent biocompatibility, non-immunogenicity, non-toxicity, ease of scale-up, and straightforward surface functionalization (Figure 5E).
Gold nanoparticles (AuNPs) have garnered significant attention as inorganic nanocarriers due to their favorable physicochemical properties. Yi et al successfully obtained sub-50 nm modified glucose nanoparticles (Glu-NPs) through a two-step bottom-up self-assembly process involving unimer polyion complexes (uPICs) and AuNPs. These Glu-NPs show the potential for systemic delivery of siRNAs to cancer stem-like cells (CSCs)-rich breast cancer models. The study shows that Glu-NPs specifically recognize CSCs in MDA-MB-231 breast cancer spheroid culture models and enhance antitumor effects in in situ breast tumor models.203 Baghani et al developed a delivery system using trimethyl-chitosan (TMC)-coated AuNPs as nanocarriers capable of transporting EGFR-siRNA into breast cancer cells. These nanoparticles were efficiently taken up by breast cancer cells and exhibited a significant gene silencing effect on target genes, providing a potential therapeutic strategy for breast cancer treatment.204 However, it is important to note that gold nanoparticle-based carriers also have limitations, including low encapsulation efficiency, poor storage stability, and slow in vivo escape.
Mesoporous silica nanoparticles (MSNPs) have been widely employed for nucleic acid delivery due to their robust loading capacity and biodegradability. In a study conducted by Mora-Raimundo et al, MSNPs were utilized to deliver SOST siRNA and osteostatin, resulting in increased expression of frontal osteogenesis-related genes, improved bone microarchitecture, and alleviated osteoporosis symptoms.205 Kumar et al developed a nanocarrier based on MSNPs by modifying them with poly-l-arginine (PLR) and PEG to endow siRNA binding ability and enhance biocompatibility. They then conjugated the nanocarrier with AS1411 aptamer for cancer cell targeting. The multifunctional nanocarrier demonstrated excellent target specificity, and the co-delivery of DOX and siRNA against BCL-2/BCL-xL showed strong potential in overcoming drug resistance in triple-negative breast cancer cells.206 Alternatively, superparamagnetic iron oxide nanoparticles (SPIONs), such as Fe2O3 or Fe3O4, exhibit superparamagnetic magnetism at specific sizes and can serve as siRNA carriers for targeted delivery to specific tissues or tumors in the presence of an external magnetic field. To facilitate electrostatic interactions with anionic siRNAs, surface-engineered cationic compound modifications are required for SPIONs. For instance, PEI- or PEG-coated iron oxide nanoparticles ranging from 20 to 100 nm have demonstrated superior siRNA delivery activity.207–211 In a recent study, Chung et al introduced a delivery system based on SPIONs to deliver siRNA targeting the O6-methylguanine-DNA methyltransferase (MGMT) gene, aiming to enhance the sensitivity of glioma cells to the alkylating drug temozolomide. The SPIONs were coated with polymer and conjugated with the targeting ligand chlorotoxin and cell-penetrating polyarginine (R10), thereby enhancing the transfection efficiency of siRNA.212
Calcium phosphate (CaP) nanoparticles have several advantages in terms of biocompatibility, ease of preparation, and high affinity for RNA. Furthermore, they exhibit rapid solubilization at low pH within early cellular lysosomes after endocytosis, leading to increased osmotic pressure and the subsequent release of encapsulated siRNA into the cytoplasm. This unique characteristic has been utilized to enhance the transfection efficiency of CaP nanoparticles. Huang et al demonstrated that lipid-coated CaP nanoparticles exhibit elevated Ca2+ concentrations upon cellular internalization.213,214 In addition, the incorporation of PEG-phospholipids and the targeting ligand anisamide into lipid-coated CaP nanoparticles resulted in a significant improvement in siRNA delivery compared to previous formulations (40-fold in vitro and 4-fold in vivo). Kara et al synthesized two arginine-modified CaP nanoparticles with different chemical and morphological characteristics to serve as targets for survivin and cyclin B1 silencing through specific siRNA vectors. Using A549 non-small-cell lung cancer cells, Kara et al demonstrated that CaP-Arg-siRNA-mediated inhibition of these genes led to a substantial reduction in cell growth and the induction of apoptosis.215 Furthermore, Hsieh et al proposed a novel immunotherapy strategy for treating glioblastoma by developing a CaP-based delivery system that overcomes the blood-brain barrier (BBB). This system encapsulates the NO donor and siRNA targeting PD-L1 within CaP cores, coats them with a lipid bilayer, and modifies them with the CXCR4 antagonist peptide.216
Carbon nanotubes (CNTs), typically ranging from 1–2 nm in diameter and 50 nm to 1 cm in length, have been shown to effectively deliver siRNA when fabricated by functionalization methods. Siu et al non-covalently functionalized carbon nanotubes with lipopolymers (DSPEPEG) and PEl to facilitate siRNA delivery to target cells.217 They observed successful hepatic GAPDH siRNA uptake and gene silencing in a mouse model. Zhao et al succeeded in self-assembling peptide lipids (PL) and sucrose laurate (SL) on CNTs, leading to the construction of bifunctional lipid-coated single- and multi-walled CNT delivery systems (SCNT-PS and MCNT-PS). After 21 days of treatment with SCNT-PS/siRNA, certain tumors regressed completely. Furthermore, SCNT-PS and MCNT-PS nanoparticles showed no significant toxic effects on cells at concentrations up to 60 μg/mL.218 Besides, Wen et al introduced a delivery system based on CNTs using multiwalled carbon nanotubes (MWNTs) to co-deliver sorafenib and siRNA targeting EGFR. This co-delivery system demonstrated a positive anti-tumor effect on liver cancer in both in vitro and in vivo experiments.219
Other siRNA Delivery Systems
In addition to the aforementioned siRNA delivery systems, there are several other potential systems that have not found wide application. Examples include exosomes, oligonucleotide nanoparticles (ONP), RNAi microsponges, and electro/photoporation.
Exosomes, 40–100 nm in size, are natural vesicles released from cellular multivesicular vesicles fused to the cell membrane. They serve as carriers of various types of RNA, including coding and non-coding RNAs, between cells.220 Isolated exosomes can be used as delivery vehicles for siRNA.221 Alvarez et al developed brain-targeted siRNA delivery vectors using exosomes derived from dendritic cells.222 First, they expressed the rabies virus glycoprotein (Rabies glycoprotein) fused to the Lamp2b protein of dendritic cells. Subsequently, dendritic cell exosomes were isolated, and siRNAs were loaded into the exosomes through electrotransfer.222 By administering exosome-siRNA via tail vein injection in mice, specific delivery of siRNA to neuronal cells was achieved, resulting in over 60% down-regulation of target gene expression in the mouse cortex, midbrain, and striatum.222 Recently, Huang et al developed an exosome-based delivery system using exosomes derived from primary patient cells. The exosomes were loaded with siRNAs targeting CCDC80, a potential target involved in tumor metastasis and chemoresistance. The exo-siCCDC80 demonstrated ideal biocompatibility and biosafety. The delivery of exo-siCCDC80 showed excellent antitumor effects and increased chemotherapy sensitivity in multiple animal tumor models.223 Since exosomes are vesicles secreted by cells themselves, they are completely non-toxic and non-immunogenic.221
Oligonucleotide nanoparticles (ONPs) are three-dimensional nanostructures formed by hybridization of complementary DNA fragments, offering structural programmability, spatial addressability, and biocompatibility. To enhance stability, Ponnuswamy et al employed oligo-lysine-PEG to wrap DNA nanostructures, thereby improving their resistance to low salt denaturation and nuclease degradation.224 Nucleic acid nanostructures hold great promise as carriers for siRNA drugs. Various methods have been employed to load nucleic acid nanostructures with nucleic acid drugs. It has been reported that pre-designed functional nucleic acid sequences can be assembled into DNA dendrimers for siRNA delivery.225 Additionally, DNA origami structures, such as DNA tetrahedral nanostructures developed by Lee et al, can serve as multivalent nanocarriers for siRNA drugs.226 Lee et al developed tumor-targeted folate-ONP by modifying ONP with trivalent folic acid (folate). By administering folate-ONP loaded with luciferase siRNA via tail vein injection into tumor-bearing mice expressing luciferase, the expression of the luciferase gene in the tumor was downregulated by 60% without inducing a significant immune response.226 Additionally, Xu et al developed octahedral DNA origami frameworks (OctDOFs) to shield siRNAs from RNase degradation and protein binding. This strategy effectively reduces connective tissue growth factor (CTGF) and heat shock protein 72 (HSP72), sensitizing cancer cells to chemo-photothermal therapy.227 Furthermore, Ding et al developed a novel siRNA delivery system with excellent thermal stability and physiological stability against enzymatic degradation.228 They achieved this by grafting DNA onto the linear polymer polycaprolactone (DNA-g-PCL), utilizing functional siRNA on a cross-linked strand, and forming spherical nanogels by nucleic acid hybridization.
RNAi microsponges consist entirely of cleavable RNA strands and are only processed by the cells’ RNA machinery after cellular uptake. This conversion of stable hairpin RNA into siRNA provides inherent protection during delivery and translocation to the cytoplasm.229 Lee et al hybridized linear single-stranded DNA, including luciferase-resistant siRNA, with short DNA strands and sealed the cut in the circular DNA using T4 DNA ligase. Closing the rolling circle transcription (RCT) of the circular DNA generated multiple tandem repeats of the hairpin RNA structure, forming a spherical sponge-like structure. The incorporation of a single RNAi microsponge enables the delivery of over 500,000 copies of siRNA to the cell.229 Roh et al proposed a novel method called Multi-RNAi-MS to produce multiple components of multimeric siRNA by simultaneous self-assembly and dense packing into composite sponge-like porous microstructures using RCT.230
The delivery of siRNA by electroporation involves the application of short electrical pulses to temporarily modify the permeability of tissue and cell membranes.231 Yang et al developed the Rolling Microneedle Electrode Array (RoMEA), which combines closely spaced microneedle electrodes with a rolling structure. RoMEA utilizes parallel circular blades with microneedle arrays as electrodes, applying synchronized electric fields while rolling to enable large-scale in vivo tissue electroporation on irregular living tissue surfaces. This innovative approach facilitates effective siRNA delivery and gene silencing.232 Shokouhi et al introduced an electroactive nanoinjection (ENI) platform with vertically configured conductive nanotubes for rapid TRIOBP siRNA delivery into cells, effectively achieving gene silencing. This system is non-viral, reusable, and operates at low voltage.233 Additionally, Wayteck et al proposed a nanoparticle-sensitized photoporation technique for transfecting siRNA into primary cytotoxic T lymphocytes (CTL). A photothermal effect is generated by irradiating gold nanoparticles (AuNPs) attached to the cell surface with laser light. This effect leads to a transient permeation of the cell membrane by causing the evaporation of water near the AuNPs and the formation of water vapor nanobubbles (VNBs). Rapid expansion and collapse of the VNBs generate high-pressure shock waves, resulting in local membrane damage and the formation of reversible pores in the cell membrane. These pores allow direct access for siRNA to the cytoplasm, thus enabling efficient transfection.234 Xiong et al successfully downregulated PD1 expression by embedding light-sensitive iron oxide nanoparticles (IONPs) into biocompatible electrospun nanofibers and using photothermal effects for cellular photopuncture. This photothermal nanofiber effectively delivered siRNA to adherent and suspended cells, such as embryonic stem cells, without affecting cell proliferation or phenotype.235
Bioresponsive Materials
In the context of siRNA drugs, the stable and efficient delivery of siRNA to the target site is crucial for therapeutic effectiveness. It is necessary for siRNA to be effectively accumulated at the focal site within a nano-delivery system. However, in order to exert its gene silencing effect, the siRNA must be completely dissociated from the delivery vehicle and exist freely in the cytoplasm.82 Therefore, drug release behavior also plays a crucial role in determining the therapeutic efficacy of siRNA. Smart bioresponsive materials offer a solution for controlled drug release by responding to specific biological signals, environmental factors, or pathological conditions within the organism. These materials can undergo physical, chemical, or biological changes triggered by factors such as pH, redox potential, enzymes, oxygen levels, temperature, or ATP levels.83,84 These changes can lead to dissolution, contraction, dissociation, or degradation of the delivery system, as illustrated in Figure 6.
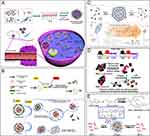 |
Figure 6 Bioresponsive materials. (A) Schematic diagram of siRNA delivery systems with dual tumor-targeting and pH-responsive capabilities, which can break through biological barriers and penetrate deep into tumors to achieve better tumor therapeutic effects. International Journal of Nanomedicine 2022;17:953–967’ used with permission from Zhang XY, Qin B, Wang M et al. Dual pH-responsive and tumor-targeted nanoparticle-mediated anti-angiogenesis siRNA delivery for tumor treatment. Int J Nanomedicine. 2022;17:953–967. Copyright 2022 Dove Medical Press Ltd.236 (B) Schematic diagram of reduction-sensitive PEG-SS-PEI-PE and GSH-mediated PEG de-shielding, and proposed mechanisms for intracellular stimulus-sensitive siRNA delivery. Reproduced with permission from Mutlu Agardan NB, Sarisozen C, Torchilin VP. Redox-triggered intracellular siRNA delivery. Chem Commun (Camb). 2018;54(49):6368–6371.237 Copyright 2018, The Royal Society of Chemistry. (C) Schematic diagram of triazolium amphiphiles capable of binding siRNA and releasing the nucleic acid payload via enzyme-responsive. Reproduced with permission from Hollstein S, Ali LMA, Coste M et al. A triazolium-anchored self-immolative linker enables self-assembly-driven siRNA binding and esterase-induced release. Chemistry. 2023;29(8):e202203311.238 Creative Commons CC BY license. Copyright 2022, The Authors, published by Wiley-VCH GmbH. (D) Schematic diagram of hypoxia-induced siRNA uptake and silencing using a nanocarrier consisting of PEG2000, azobenzene, PEI (1.8 kDa), and DOPE units (named PAPD). Reproduced with permission from Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed Engl. 2014;53(13):3362–3366.239 Copyright 2014, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (E) Schematic diagram of self-assembly of PEI1.8k-PBA/PEI1.8k-α-CD supramolecular polymer and release of siRNA through multimer disassembly under ATP stimulation. Reproduced with permission from Jiang C, Qi Z, Jia H et al. ATP-responsive low-molecular-weight polyethylenimine-based supramolecular assembly via host-guest interaction for gene delivery. Biomacromolecules. 2019;20(1):478–489.240 Copyright 2019, American Chemical Society. |
pH-Responsive Biomaterials
pH-responsive biomaterials are collectively referred to as materials that can change in response to variations in pH levels in their environment. The pH responsiveness of these materials primarily arises from the protonation of ionizable groups or acid degradation of chemical bonds within the material, which leads to the dissolution, shrinkage, dissociation, and degradation of the material. Consequently, pH-responsive biomaterials offer the potential for targeted drug delivery at different levels and precise release at specific sites. Common pH-responsive materials include polymers synthesized from acrylic acid, methacrylic acid, maleic anhydride, and N,N-dimethylaminoethyl methacrylate.241–245 In a recent study, Zhang et al developed a multifunctional siRNA delivery system (CHCE/siRNA nanoparticles) by self-assembly of histidine- and cholesterol-modified carboxymethyl chitosan with anti-EGFR antibodies. This system exhibited both tumor-targeting and pH-responsive capabilities, enabling effective treatment by overcoming biological barriers236 (Figure 6A). Wang et al developed an ultra-pH-responsive peptide nanocarrier that can dynamically assemble in response to pH changes in the tumor microenvironment and intracellular lysosomal environment, thereby efficiently delivering siRNA into cancer cells. The carrier exhibits high transfection efficiency and targeting specificity, exerting high toxicity to cancer cells while showing low toxicity to normal cells.246
Redox-Responsive Biomaterials
Based on the redox reaction, nanocarriers can regulate drug release under reducing or oxidizing conditions. Intracellular glutathione (GSH) has been observed to have a concentration ranging from 2–10 mM, significantly higher than extracellular levels. To exploit this disparity, researchers have proposed a novel redox-sensitive micellar nanopreparation named PEG-SS-PEI-PE (PSSPD). This preparation utilizes a triple conjugate of polyethylene glycol, polyethyleneimine, and phosphatidylethanolamine. Upon exposure to increased GSH levels in tumor cells, the PEG shield is removed, facilitating the release of siRNA inside the cells237 (Figure 6B). In a recent study, Li et al developed an innovative redox-responsive polyprodrug nanoplatform for targeted siRNA delivery and synergistic cancer therapy. The platform consists of a polyprodrug of 10-hydroxycamptothecin (polyHCPT) as the core, lipid-PEG as the shell, and surface modification with lactobionic acid (LA). Elevated GSH concentrations disrupt the disulfide bonds in polyHCPT, leading to the release of HCPT molecules and Bcl-2 siRNA (siBcl-2), thereby inducing apoptosis through HCPT and synergistically inhibiting tumor growth by silencing the anti-apoptotic gene via siBcl-2.247 It has also been found that reactive oxygen species (ROS) are prevalent in conditions such as tumors, stroke, and atherosclerosis.248–250 To exploit the redox potential differences (eg, glutathione) and ROS groups (eg, hydrogen peroxide, hydroxyl radicals) in diseased tissues and cells, researchers have developed various polymers based on disulfide bonds, thioethers, and selenides as nanocarriers for targeted siRNA release at the site of the lesion. These advances aim to enhance drug targeting and improve therapeutic efficacy.247,251–256
Enzyme-Responsive Biomaterials
The utilization of enzyme-responsive materials for siRNA release represents an innovative strategy that enables precise control over siRNA release. This approach offers several advantages, including accurate release control, which helps prevent premature release and potential side effects in non-target tissues. This strategy shows promise by improving therapeutic efficacy and minimizing damage to healthy tissue. Hollstein et al performed a study on triazolium-based amphiphiles that demonstrated their ability to bind to siRNA and facilitate enzyme-responsive release of the nucleic acid payload. Upon the addition of esterases, the linker undergoes cascade degradation, preventing the formation of complexes with neutral triazole compounds and resulting in the release of negatively charged siRNA238 (Figure 6C). Additionally, Shi et al designed a novel siRNA carrier called polyfluorinated polyarginine (PFC-PR), which is responsive to both oxidoreductases and enzymes. PFC-PR responds to the overexpression of tissue protease B and elevated levels of glutathione in cancer cells. This response enhances the release of siRNA in cancer cells, allowing for targeted delivery and effective cancer therapy.257
Hypoxia-Responsive Biomaterials
In various human body lesions, such as tumors, myocardial ischemia, and vascular diseases, localized sites experience a depleted oxygen environment. To address this, prodrugs and molecular probes designed specifically for these conditions are extensively employed in disease diagnosis and treatment.258,259 A commonly used oxygen-depleted group is 2-nitroimidazole (2-NI), which is hydrophobic under normal physiological conditions. However, in the depleted oxygen environment, 2-NI is reduced in the presence of nitroreductase to form the hydrophilic compound 2-aminoimidazole (2-AI), leading to nanoparticle depolymerization and subsequent drug release.239,260 Another commonly used depleted oxygen-sensitive group is azobenzene, which Torchilin et al used as a linking group between PEI and PEG. In the absence of oxygen, the azobenzene bond breaks, leading to the detachment of PEG and the exposure of the positive charge of PEI. The alteration improves the cellular uptake of siRNA while reducing its potential toxic effects239 (Figure 6D).
Thermo-Responsive Biomaterials
Temperature serves as a convenient and effective trigger, as thermal stimulation can dilate blood vessels and facilitate drug release. Certain polymers possess temperature-sensitive properties, such as poly-N-isopropylacrylamide (NIPAM). These temperature-sensitive polymers exhibit a low critical solubility temperature (LCST). Below the LCST, hydrogen bonding occurs between the polymer and water molecules, resulting in a water-soluble swollen state. However, as the temperature rises, the hydrogen bonds break, causing the polymer to become insoluble and collapse. Exploiting this phase change behavior in drug delivery systems enables controlled drug release at 40°C.261 In a study by Honda et al, a thermos-responsive copolymer was developed by combining siRNA with N-isopropylacrylamide (NIPAAm) and hydrophilic N,N-dimethylacrylamide (DMAA). This copolymer undergoes a coil-to-globule transition, allowing for temperature-controlled interaction between siRNA and intracellular proteins involved in gene silencing.262
ATP-Responsive Biomaterials
Adenosine-triphosphate (ATP) serves as a molecular currency for intracellular energy delivery and is more abundant intracellularly than extracellularly. ATP can either compete for adsorption to drug binding sites to trigger drug release263–266 or disrupt the structure of the carrier by inducing conformational changes.267 One study reported ATP-responsive nanomicelles modified with phenylboronic acid (PBA) for the controlled release of siRNA. The findings revealed that under normal physiological conditions, siRNA can be encapsulated within the micelles through electrostatic interactions. The PBA component binds to the ribose of siRNA, stabilizing the micelles. However, when the micelles reach tumor sites with elevated ATP levels, ATP competitively binds to PBA, leading to the disruption of the micelle structure and subsequent release of siRNA.265 In another study investigating ATP-triggered siRNA release, a supramolecular assembly was constructed using low molecular weight polyethyleneimine (LMW-PEI) through host-guest interactions between PEI1.8k-α-CD and PEI1.8k -PBA. This assembly facilitated the cellular uptake of siRNA through ATP-dependent endocytosis240 (Figure 6E).
Summary and Outlook
The utilization of siRNA has proven to be highly effective in inhibiting the expression of cancer-related genes and reducing the proliferation of cancer cells. By targeting genes associated with cancer metastasis, signaling pathways, drug sensitivity, and apoptosis, siRNA can significantly decrease the malignancy of cancer and improve patient prognosis. In this review, we introduce the mechanism of RNAi and potential targets for siRNA drugs in cancer treatment and summarize the current clinical trials of siRNA therapeutics for cancer. Furthermore, recent advancements in various chemical modifications, delivery systems, and bioresponsive materials that facilitate the stability, safety, bioavailability, and controlled release of siRNAs, are discussed. The review provides a comprehensive overview of siRNA therapeutics and guidance for the investigation and clinical application of siRNA drugs.
siRNA holds promise for the future of disease treatment, particularly those caused by the dysregulation of specific genes. Molecular therapies utilizing siRNA have shown potential for addressing abnormal gene expression or mutations associated with various conditions, including cancer, viral infections, genetic disorders, and pain management. Despite the immense potential of siRNA therapy, the clinical utilization of siRNA-based drugs remains constrained. This can be attributed to the inherent challenges of siRNA itself and the delivery systems. An ideal siRNA delivery system should possess the following functions: 1) maintaining siRNA stability in circulation, 2) improving siRNA’s pharmacokinetic characteristics, extending its half-life in the body, and enhancing its distribution in target tissues, 3) promoting cellular uptake of siRNA, 4) facilitating siRNA escape from endosomes, 5) being non-toxic or low in toxicity and non-immunogenic, and 6) exhibiting high transfection efficiency and achieving efficient in vivo delivery of siRNA across cell culture and animal models. The key challenge in achieving the aforementioned functions lies in overcoming physiological barriers within the body and successfully delivering the drugs to the site of the lesion. Advances in nanotechnology have contributed to the maturation of siRNA technology as scientists strive to overcome the safety and delivery obstacles associated with siRNA-based drugs.268
Currently, nanodrug delivery primarily relies on external forces, such as magnetic interactions and ultrasound, to overcome physiological barriers. However, the efficiency of this delivery method remains low.269 In nature, organisms exhibit a diverse array of intricate and sophisticated dynamic systems. For example, multicellular organisms actively transport substances across cells by utilizing their own energy. Cellular scaffolding proteins employ microtubules as tracks to facilitate intracellular substance delivery. Bacterial rotary motor proteins enable flagella to move directionally, resembling propellers. Viral DNA packaging motor proteins assist in loading DNA during replication processes. All of these energy mechanisms are dependent on the biological energy ATP. Regrettably, these systems have not yet been directly applied to siRNA nanodrug delivery. Nevertheless, with the continuous development of new materials and formulation technologies, it is anticipated that siRNA will soon overcome the limitations of the delivery system and find extensive use in tumor treatment.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Beijing Municipal Fund for Distinguished Young Scholars (Grand No. JQ22022), and National Key R&D Program of China (Grant No. 2022YFF1502000). We appreciate the contribution of the online tool BioRender (https://biorender.com/) to help us create excellent drawings.
Disclosure
The authors declare no conflicts of interests in this work.
References
1. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
2. Roy S, Banerjee P, Ekser B, et al. Targeting lymphangiogenesis and lymph node metastasis in liver cancer. Am J Pathol. 2021;191(12):2052–2063. doi:10.1016/j.ajpath.2021.08.011
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin. 2022;72(1):7–33 doi:10.3322/caac.21708.
4. Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi:10.1038/457036b
5. Gray J. Cancer: genomics of metastasis. Nature. 2010;464(7291):989–990. doi:10.1038/464989a
6. Ganesh K, Massague J. Targeting metastatic cancer. Nat Med. 2021;27(1):34–44. doi:10.1038/s41591-020-01195-4
7. Ji B, Wei M, Yang B. Recent advances in nanomedicines for photodynamic therapy (PDT)-driven cancer immunotherapy. Theranostics. 2022;12(1):434–458. doi:10.7150/thno.67300
8. Zhang C, Liu X, Jin S, Chen Y, Guo R. Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer. 2022;21(1):47. doi:10.1186/s12943-022-01530-y
9. Van Rijssen LB, Rombouts SJ, Walma MS, et al. Recent advances in pancreatic cancer surgery of relevance to the practicing pathologist. Surg Pathol Clin. 2016;9(4):539–545. doi:10.1016/j.path.2016.05.002
10. Ishihara S, Otani K, Yasuda K, et al. Recent advances in robotic surgery for rectal cancer. Int J Clin Oncol. 2015;20(4):633–640. doi:10.1007/s10147-015-0854-z
11. Grimes DR. Radiofrequency radiation and cancer: a review. JAMA Oncol. 2022;8(3):456–461. doi:10.1001/jamaoncol.2021.5964
12. Dias MP, Moser SC, Ganesan S, Jonkers J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat Rev Clin Oncol. 2021;18(12):773–791. doi:10.1038/s41571-021-00532-x
13. Nussinov R, Tsai CJ, Jang H. Anticancer drug resistance: an update and perspective. Drug Resist Updat. 2021;59:100796. doi:10.1016/j.drup.2021.100796
14. Ramaswami R, Harding V, Newsom-Davis T. Novel cancer therapies: treatments driven by tumour biology. Postgrad Med J. 2013;89(1057):652–658. doi:10.1136/postgradmedj-2012-131533
15. Khalil DN, Smith EL, Brentjens RJ, Wolchok JD. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13(5):273–290. doi:10.1038/nrclinonc.2016.25
16. DePeaux K, Delgoffe GM. Metabolic barriers to cancer immunotherapy. Nat Rev Immunol. 2021;21(12):785–797. doi:10.1038/s41577-021-00541-y
17. Pan R, Ryan J, Pan D, Wucherpfennig KW, Letai A. Augmenting NK cell-based immunotherapy by targeting mitochondrial apoptosis. Cell. 2022;185(9):1521–1538 e1518. doi:10.1016/j.cell.2022.03.030
18. Zhang Y, Vu T, Palmer DC, et al. A T cell resilience model associated with response to immunotherapy in multiple tumor types. Nat Med. 2022;28(10):2219–2219. doi:10.1038/s41591-022-01997-8
19. Robert C, Long G, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med;2014. 372. doi:10.1056/NEJMhle1403384
20. Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in advanced melanoma. N Engl J Med. 2015;372(26):2521–2532. doi:10.1056/NEJMoa1503093
21. Rizvi NA, Mazieres J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a Phase 2, single-arm trial. Lancet Oncol. 2015;16(3):257–265. doi:10.1016/S1470-2045(15)70054-9
22. Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev. 2019;34:45–55. doi:10.1016/j.blre.2018.11.002
23. Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19(3):185–199. doi:10.1038/s41573-019-0051-2
24. Ma S, Li X, Wang X, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. 2019;15(12):2548–2560. doi:10.7150/ijbs.34213
25. Schubert ML, Schmitt M, Wang L, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021;32(1):34–48. doi:10.1016/j.annonc.2020.10.478
26. Bahcall O. Precision medicine. Nature. 2015;526(7573):335. doi:10.1038/526335a
27. Jameson JL, Longo DL. Precision medicine--personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–2234. doi:10.1056/NEJMsb1503104
28. Koch L. Genetic screen: a network to guide precision cancer therapy. Nat Rev Genet. 2016;17(9):504–505. doi:10.1038/nrg.2016.105
29. Mateo J, Steuten L, Aftimos P, et al. Delivering precision oncology to patients with cancer. Nat Med. 2022;28(4):658–665. doi:10.1038/s41591-022-01717-2
30. Hu B, Zhong L, Weng Y, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5(1):101. doi:10.1038/s41392-020-0207-x
31. Yoon J, Shin M, Lee JY, Lee SN, Choi JH, Choi JW. RNA interference (RNAi)-based plasmonic nanomaterials for cancer diagnosis and therapy. J Control Release. 2022;342:228–240. doi:10.1016/j.jconrel.2022.01.012
32. Buchholz F. RNA interference on target. Nat Methods. 2006;3(9):659. doi:10.1038/nmeth0906-659
33. Beaucage SL. Solid-phase synthesis of siRNA oligonucleotides. Curr Opin Drug Discov Devel. 2008;11(2):203–216.
34. Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2(12):711–719. doi:10.1038/nchembio839
35. Dong Y, Siegwart DJ, Anderson DG. Strategies, design, and chemistry in siRNA delivery systems. Adv Drug Deliv Rev. 2019;144:133–147. doi:10.1016/j.addr.2019.05.004
36. Garber K. Alnylam launches era of RNAi drugs. Nat Biotechnol. 2018;36(9):777–778. doi:10.1038/nbt0918-777
37. Adams D, Gonzalez-Duarte A, O’Riordan WD, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi:10.1056/NEJMoa1716153
38. Idris A, Davis A, Supramaniam A, et al. A SARS-CoV-2 targeted siRNA-nanoparticle therapy for COVID-19. Mol Ther. 2021;29(7):2219–2226. doi:10.1016/j.ymthe.2021.05.004
39. Wong SC, Klein JJ, Hamilton HL, et al. Co-injection of a targeted, reversibly masked endosomolytic polymer dramatically improves the efficacy of cholesterol-conjugated small interfering RNAs in vivo. Nucleic Acid Ther. 2012;22(6):380–390. doi:10.1089/nat.2012.0389
40. Zhang L, Wu T, Shan Y, et al. Therapeutic reversal of Huntington’s disease by in vivo self-assembled siRNAs. Brain. 2021;144(11):3421–3435. doi:10.1093/brain/awab354
41. Pasi KJ, Lissitchkov T, Mamonov V, et al. Targeting of antithrombin in hemophilia A or B with investigational siRNA therapeutic fitusiran-Results of the Phase 1 inhibitor cohort. J Thromb Haemost. 2021;19(6):1436–1446. doi:10.1111/jth.15270
42. Engelbeen S, Pasteuning-Vuhman S, Boertje-van der Meulen J, et al. Efficient downregulation of Alk4 in skeletal muscle after systemic treatment with conjugated siRNAs in a mouse model for Duchenne muscular dystrophy. Nucleic Acid Ther. 2023;33(1):26–34. doi:10.1089/nat.2022.0021
43. Kosmas CE, Munoz Estrella A, Sourlas A, et al. Inclisiran: a new promising agent in the management of hypercholesterolemia. Diseases. 2018;6(3):63. doi:10.3390/diseases6030063
44. Fitzgerald K, White S, Borodovsky A, et al. A highly durable RNAi therapeutic inhibitor of PCSK9. N Engl J Med. 2017;376(1):41–51. doi:10.1056/NEJMoa1609243
45. Subhan MA, Torchilin VP. Efficient nanocarriers of siRNA therapeutics for cancer treatment. Transl Res. 2019;214:62–91. doi:10.1016/j.trsl.2019.07.006
46. Mirzaei S, Gholami MH, Ang HL, et al. Pre-clinical and clinical applications of small interfering RNAs (siRNA) and co-delivery systems for pancreatic cancer therapy. Cells. 2021;10(12):3348. doi:10.3390/cells10123348
47. Sahin B, Fife J, Parmar MB, et al. siRNA therapy in cutaneous T-cell lymphoma cells using polymeric carriers. Biomaterials. 2014;35(34):9382–9394. doi:10.1016/j.biomaterials.2014.07.029
48. Zogg H, Singh R, Ro S. Current advances in RNA therapeutics for human diseases. Int J Mol Sci. 2022;23(5):2736. doi:10.3390/ijms23052736
49. Sajid MI, Moazzam M, Kato S, Yeseom Cho K, Tiwari RK. Overcoming Barriers for siRNA Therapeutics: from Bench to Bedside. Pharmaceuticals. 2020;13(10):294. doi:10.3390/ph13100294
50. Tieu T, Wei Y, Cifuentes‐Rius A, Voelcker NH. Overcoming barriers: clinical translation of siRNA nanomedicines. Advanced Therapeutics. 2021;4(9). doi:10.1002/adtp.202100108
51. Shegokar R, Al Shaal L, Mishra PR. SiRNA delivery: challenges and role of carrier systems. Pharmazie. 2011;66(5):313–318.
52. Kim SS, Garg H, Joshi A, Manjunath N. Strategies for targeted nonviral delivery of siRNAs in vivo. Trends Mol Med. 2009;15(11):491–500. doi:10.1016/j.molmed.2009.09.001
53. Judge A, MacLachlan I. Overcoming the innate immune response to small interfering RNA. Hum Gene Ther. 2008;19(2):111–124. doi:10.1089/hum.2007.179
54. Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23(4):457–462. doi:10.1038/nbt1081
55. Owens DE 3rd, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi:10.1016/j.ijpharm.2005.10.010
56. Zhou Y, Zhang C, Liang W. Development of RNAi technology for targeted therapy--a track of siRNA based agents to RNAi therapeutics. J Control Release. 2014;193:270–281. doi:10.1016/j.jconrel.2014.04.044
57. Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi:10.1038/nrd2742
58. Musacchio T, Torchilin VP. siRNA delivery: from basics to therapeutic applications. Front Biosci (Landmark Ed). 2013;18(1):58–79. doi:10.2741/4087
59. Subhan MA, Torchilin VP. siRNA based drug design, quality, delivery and clinical translation. Nanomedicine. 2020;29:102239. doi:10.1016/j.nano.2020.102239
60. Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151(3):220–228. doi:10.1016/j.jconrel.2010.11.004
61. Fedorov Y, Anderson EM, Birmingham A, et al. Off-target effects by siRNA can induce toxic phenotype. RNA. 2006;12(7):1188–1196. doi:10.1261/rna.28106
62. Cho WG, Albuquerque RJ, Kleinman ME, et al. Small interfering RNA-induced TLR3 activation inhibits blood and lymphatic vessel growth. Proc Natl Acad Sci U S A. 2009;106(17):7137–7142. doi:10.1073/pnas.0812317106
63. Corey DR. Chemical modification: the key to clinical application of RNA interference? J Clin Invest. 2007;117(12):3615–3622. doi:10.1172/JCI33483
64. Khvorova A, Watts JK. The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol. 2017;35(3):238–248. doi:10.1038/nbt.3765
65. Crooke ST, Wang S, Vickers TA, Shen W, Liang X-H. Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol. 2017;35(3):230–237. doi:10.1038/nbt.3779
66. Sioud M, Furset G, Cekaite L. Suppression of immunostimulatory siRNA-driven innate immune activation by 2’-modified RNAs. Biochem Biophys Res Commun. 2007;361(1):122–126. doi:10.1016/j.bbrc.2007.06.177
67. Song X, Wang X, Ma Y, Liang Z, Yang Z, Cao H. Site-specific modification using the 2’-methoxyethyl group improves the specificity and activity of siRNAs. Mol Ther Nucleic Acids. 2017;9:242–250. doi:10.1016/j.omtn.2017.10.003
68. Kariko K, Muramatsu H, Welsh FA, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi:10.1038/mt.2008.200
69. Anderson BR, Muramatsu H, Jha BK, Silverman RH, Weissman D, Kariko K. Nucleoside modifications in RNA limit activation of 2’-5’-oligoadenylate synthetase and increase resistance to cleavage by RNase L. Nucleic Acids Res. 2011;39(21):9329–9338. doi:10.1093/nar/gkr586
70. Winkler J. Therapeutic oligonucleotides with polyethylene glycol modifications. Future Med Chem. 2015;7(13):1721–1731. doi:10.4155/fmc.15.94
71. Bramsen JB, Kjems J. Engineering small interfering RNAs by strategic chemical modification. Methods Mol Biol. 2013;942:87–109 doi:10.1007/978-1-62703-119-6_5.
72. Kim D, Reyes-Ordonez A, Chen J. Lentivirus-mediated RNAi in skeletal myogenesis. Methods Mol Biol. 2019;1889:95–110 doi:10.1007/978-1-4939-8897-6_7.
73. Brandt MR, Kirste AG, Pozzuto T, et al. Adenovirus vector-mediated RNA interference for the inhibition of human parvovirus B19 replication. Virus Res. 2013;176(1–2):155–160. doi:10.1016/j.virusres.2013.05.020
74. Borel F, Kay MA, Mueller C. Recombinant AAV as a platform for translating the therapeutic potential of RNA interference. Mol Ther. 2014;22(4):692–701. doi:10.1038/mt.2013.285
75. Neuberg P, Kichler A. Recent developments in nucleic acid delivery with polyethylenimines. Adv Genet. 2014;88:263–288 doi:10.1016/B978-0-12-800148-6.00009-2.
76. Videira M, Arranja A, Rafael D, Gaspar R. Preclinical development of siRNA therapeutics: towards the match between fundamental science and engineered systems. Nanomedicine. 2014;10(4):689–702. doi:10.1016/j.nano.2013.11.018
77. Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6(3):659–668. doi:10.1021/mp900015y
78. Song F, Sakurai N, Okamoto A, et al. Design of a novel PEGylated liposomal vector for systemic delivery of siRNA to solid tumors. Biol Pharm Bull. 2019;42(6):996–1003. doi:10.1248/bpb.b19-00032
79. Wan C, Allen TM, Cullis PR. Lipid nanoparticle delivery systems for siRNA-based therapeutics. Drug Deliv Transl Res. 2014;4(1):74–83. doi:10.1007/s13346-013-0161-z
80. Zhou M, Zou X, Cheng K, et al. The role of cell-penetrating peptides in potential anti-cancer therapy. Clin Transl Med. 2022;12(5):e822. doi:10.1002/ctm2.822
81. Thangamani L, Balasubramanian B, Easwaran M, et al. GalNAc-siRNA conjugates: prospective tools on the frontier of anti-viral therapeutics. Pharmacol Res. 2021;173:105864. doi:10.1016/j.phrs.2021.105864
82. Kwon YJ. Before and after endosomal escape: roles of stimuli-converting siRNA/polymer interactions in determining gene silencing efficiency. Acc Chem Res. 2012;45(7):1077–1088. doi:10.1021/ar200241v
83. Sun M, Wang K, Oupicky D. Advances in stimulus-responsive polymeric materials for systemic delivery of nucleic acids. Adv Healthc Mater. 2018;7(4):56 doi:10.1002/adhm.201701070.
84. Zhu L, Torchilin VP. Stimulus-responsive nanopreparations for tumor targeting. Integr Biol. 2013;5(1):96–107. doi:10.1039/c2ib20135f
85. El Dika I, Lim HY, Yong WP, et al. An open‐label, multicenter, Phase I, dose escalation study with Phase II expansion cohort to determine the safety, pharmacokinetics, and preliminary antitumor activity of intravenous TKM‐080301 in subjects with advanced hepatocellular carcinoma. Oncologist. 2019;24(6):747–e218. doi:10.1634/theoncologist.2018-0838
86. Russo S, Saif MW. Gastrointestinal cancers symposium: update on pancreatic cancer. Ann Gastroenterol. 2016;29(2):238–240. doi:10.20524/aog.2016.0024
87. Triozzi P, Kooshki M, Alistar A, et al. Phase I clinical trial of adoptive cellular immunotherapy with APN401 in patients with solid tumors. J Immuno Therapy Cancer. 2015;3(S2). doi:10.1186/2051-1426-3-S2-P175.
88. Steegmaier M, Hoffmann M, Baum A, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17(4):316–322. doi:10.1016/j.cub.2006.12.037
89. O’Brien Z, Wang L, Majeti B, et al. Abstract 5917: a novel lipid nanoparticle (NBF-006) encapsulating glutathione S-transferase P (GSTP) siRNA for the treatment of KRAS-driven non-small cell lung cancer. Cancer Res. 2018;78:5917–5917. doi:10.1158/1538-7445.AM2018-5917
90. Golan T, Khvalevsky EZ, Hubert A, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6(27):24560–24570. doi:10.18632/oncotarget.4183
91. Zorde Khvalevsky E, Gabai R, Rachmut IH, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(51):20723–20728. doi:10.1073/pnas.1314307110
92. Mendt M, Kamerkar S, Sugimoto H, et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight. 2018;3(8). doi:10.1172/jci.insight.99263
93. Kamerkar S, LeBleu VS, Sugimoto H, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503. doi:10.1038/nature22341
94. Schultheis B, Strumberg D, Kuhlmann J, et al. Safety, efficacy and pharcacokinetics of targeted therapy with the liposomal RNA interference therapeutic Atu027 combined with gemcitabine in patients with pancreatic adenocarcinoma. A randomized phase Ib/IIa study. Cancers. 2020;12(11):3130. doi:10.3390/cancers12113130
95. Cho H, Kaelin WG. Targeting HIF2 in clear cell renal cell carcinoma. Cold Spring Harb Symp Quant Biol. 2016;81:113–121. doi:10.1101/sqb.2016.81.030833
96. Tolcher AW, Rodrigueza WV, Rasco DW, et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2014;73(2):363–371. doi:10.1007/s00280-013-2361-0
97. Goroshchuk O, Kolosenko I, Vidarsdottir L, Azimi A, Palm-Apergi C. Polo-like kinases and acute leukemia. Oncogene. 2019;38(1):1–16. doi:10.1038/s41388-018-0443-5
98. Crooke ST, Witztum JL, Bennett CF, Baker BF. RNA-targeted therapeutics. Cell Metab. 2018;27(4):714–739. doi:10.1016/j.cmet.2018.03.004
99. Jensen SA, Day ES, Ko CH, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):209ra152. doi:10.1126/scitranslmed.3006839
100. Dannull J, Haley NR, Archer G, et al. Melanoma immunotherapy using mature DCs expressing the constitutive proteasome. J Clin Invest. 2013;123(7):3135–3145. doi:10.1172/JCI67544
101. Durig J, Duhrsen U, Klein-Hitpass L, et al. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia. 2011;25(4):638–647. doi:10.1038/leu.2010.322
102. Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843–856. doi:10.1038/nrd4685
103. Curreri A, Sankholkar D, Mitragotri S, Zhao Z. RNA therapeutics in the clinic. Bioeng Transl Med. 2022;8(1). doi:10.1002/btm2.10374
104. Harb W, Lakhani NJ, Messmann R, Klencke B, Al-Katib AM. A Phase 2 study of PNT2258 for treatment of relapsed or refractory B-cell malignancies. Clin Lymphoma Myeloma Leuk. 2021;21(12):823–830. doi:10.1016/j.clml.2021.07.016
105. Tabernero J, Shapiro GI, LoRusso PM, et al. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 2013;3(4):406–417. doi:10.1158/2159-8290.CD-12-0429
106. Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi:10.1038/nature08956
107. Schultheis B, Strumberg D, Santel A, et al. First-in-human phase I study of the liposomal RNA interference therapeutic Atu027 in patients with advanced solid tumors. J Clin Oncol. 2014;32(36):4141–4148. doi:10.1200/JCO.2013.55.0376
108. Wagner MJ, Mitra R, McArthur MJ, et al. Preclinical mammalian safety studies of EPHARNA (DOPC nanoliposomal EphA2-targeted siRNA). Mol Cancer Ther. 2017;16(6):1114–1123. doi:10.1158/1535-7163.MCT-16-0541
109. Landen CN Jr, Chavez-Reyes A, Bucana C, et al. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 2005;65(15):6910–6918. doi:10.1158/0008-5472.CAN-05-0530
110. Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. EphA2: a determinant of malignant cellular behavior and a potential therapeutic target in pancreatic adenocarcinoma. Oncogene. 2004;23(7):1448–1456. doi:10.1038/sj.onc.1207247
111. Downward J. RNA interference. BMJ. 2004;328(7450):1245–1248. doi:10.1136/bmj.328.7450.1245
112. Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18(6):421–446. doi:10.1038/s41573-019-0017-4
113. Lim SA, Cox A, Tung M, Chung EJ. Clinical progress of nanomedicine-based RNA therapies. Bioact Mater. 2022;12:203–213 doi:10.1016/j.bioactmat.2021.10.018.
114. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12(11):967–977. doi:10.1038/nmat3765
115. Saw PE, Song EW. siRNA therapeutics: a clinical reality. Sci China Life Sci. 2020;63(4):485–500. doi:10.1007/s11427-018-9438-y
116. Singh A, Trivedi P, Jain NK. Advances in siRNA delivery in cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46(2):274–283. doi:10.1080/21691401.2017.1307210
117. Hanahan D, Weinberg R. The Hallmarks of cancer. Cell. 2000;100:57–70. doi:10.1016/S0092-8674(00)81683-9
118. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12(1):31–46. doi:10.1158/2159-8290.CD-21-1059
119. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. doi:10.1038/35077213
120. Shakil S, Baig MH, Tabrez S, et al. Molecular and enzoinformatics perspectives of targeting Polo-like kinase 1 in cancer therapy. Semin Cancer Biol. 2019;56:47–55. doi:10.1016/j.semcancer.2017.11.004
121. Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J Biochem. 2003;133(1):17–27. doi:10.1093/jb/mvg019
122. Aleku M, Schulz P, Keil O, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68(23):9788–9798. doi:10.1158/0008-5472.CAN-08-2428
123. Pasquale EB. Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat Rev Cancer. 2010;10(3):165–180. doi:10.1038/nrc2806
124. Sasaki T, Nakashiro K, Tanaka H, et al. Knockdown of Akt isoforms by RNA silencing suppresses the growth of human prostate cancer cells in vitro and in vivo. Biochem Biophys Res Commun. 2010;399(1):79–83. doi:10.1016/j.bbrc.2010.07.045
125. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi:10.1016/j.cell.2011.09.024
126. Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450–1464. doi:10.1038/nm.3391
127. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. doi:10.1007/s00018-019-03351-7
128. Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3(1):46–54. doi:10.1038/nrc968
129. Dietlein F, Thelen L, Reinhardt HC. Cancer-specific defects in DNA repair pathways as targets for personalized therapeutic approaches. Trends Genet. 2014;30(8):326–339. doi:10.1016/j.tig.2014.06.003
130. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. doi:10.1016/j.cell.2015.03.030
131. Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14(1):11–31. doi:10.1038/nrclinonc.2016.60
132. Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599. doi:10.1038/nature09454
133. Zuo Z, Liu J, Sun Z, et al. ERK and c-Myc signaling in host-derived tumor endothelial cells is essential for solid tumor growth. Proc Natl Acad Sci U S A. 2023;120(1):e2211927120. doi:10.1073/pnas.2211927120
134. Samatar AA, Poulikakos PI. Targeting RAS-ERK signalling in cancer: promises and challenges. Nat Rev Drug Discov. 2014;13(12):928–942. doi:10.1038/nrd4281
135. Assaraf YG, Brozovic A, Goncalves AC, et al. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resistance Updates. 2019;46:100645 doi:10.1016/j.drup.2019.100645.
136. Bukowski K, Kciuk M, Kontek R. Mechanisms of multidrug resistance in cancer chemotherapy. Int J Mol Sci. 2020;21(9):3233. doi:10.3390/ijms21093233
137. Reddy A, Kaelin WG Jr. Using cancer genetics to guide the selection of anticancer drug targets. Curr Opin Pharmacol. 2002;2(4):366–373. doi:10.1016/S1471-4892(02)00178-9
138. Kaelin WG Jr. The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5(9):689–698. doi:10.1038/nrc1691
139. Layzer JM, McCaffrey AP, Tanner AK, Huang Z, Kay MA, Sullenger BA. In vivo activity of nuclease-resistant siRNAs. RNA. 2004;10(5):766–771. doi:10.1261/rna.5239604
140. Van de Water FM, Boerman OC, Wouterse AC, Peters JGP, Russel FGM, Masereeuw R. Intravenously administered short interfering RNA accumulates in the kidney and selectively suppresses gene function in renal proximal tubules. Drug Metab Dispos. 2006;34(8):1393–1397. doi:10.1124/dmd.106.009555
141. Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123(8):1183–1189. doi:10.1242/jcs.066399
142. Marques JT, Williams BRG. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23(11):1399–1405. doi:10.1038/nbt1161
143. Jackson AL, Linsley PS. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov. 2010;9(1):57–67. doi:10.1038/nrd3010
144. Czech MP, Aouadi M, Tesz GJ. RNAi-based therapeutic strategies for metabolic disease. Nat Rev Endocrinol. 2011;7(8):473–484. doi:10.1038/nrendo.2011.57
145. Ku SH, Jo SD, Lee YK, Kim K, Kim SH. Chemical and structural modifications of RNAi therapeutics. Adv Drug Deliv Rev. 2016;104:16–28. doi:10.1016/j.addr.2015.10.015
146. Chen XL, Shen L, Wang JH. Poly-2 ‘-DNP-RNAs with enhanced efficacy for inhibiting cancer cell growth. Oligonucleotides. 2004;14(2):90–99. doi:10.1089/1545457041526326
147. Hall AHS, Wan J, Shaughnessy EE, Shaw BR, Alexander KA. RNA interference using boranophosphate siRNAs: structure-activity relationships. Nucleic Acids Res. 2004;32(20):5991–6000. doi:10.1093/nar/gkh936
148. David RM, Doherty AT. Viral vectors: the road to reducing genotoxicity. Toxicol Sci. 2017;155(2):315–325. doi:10.1093/toxsci/kfw220
149. Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15(7):445–451. doi:10.1038/nrg3742
150. Buyens K, De Smedt SC, Braeckmans K, et al. Liposome based systems for systemic siRNA delivery: stability in blood sets the requirements for optimal carrier design. J Control Release. 2012;158(3):362–370. doi:10.1016/j.jconrel.2011.10.009
151. Sahu R, Jha S, Pattanayak SP. Therapeutic silencing of mTOR by systemically administered siRNA-loaded neutral liposomal nanoparticles inhibits DMBA-induced mammary carcinogenesis. Br J Cancer. 2022;127(12):2207–2219. doi:10.1038/s41416-022-02011-1
152. Ramos-Gonzalez MR, Vazquez-Garza E, Garcia-Rivas G, Rodriguez-Aguayo C, Chavez-Reyes A. Therapeutic effects of WT1 silencing via respiratory administration of neutral DOPC liposomal-siRNA in a lung metastasis melanoma murine model. Noncoding RNA. 2023;9(2). doi:10.3390/ncrna9020021
153. Mainini F, Eccles MR. Lipid and polymer-based nanoparticle siRNA delivery systems for cancer therapy. Molecules. 2020;25(11):2692. doi:10.3390/molecules25112692
154. Sorensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327(4):761–766. doi:10.1016/S0022-2836(03)00181-5
155. Balgobind A, Daniels A, Ariatti M, Singh M. HER2/neu oncogene silencing in a breast cancer cell model using cationic lipid-based delivery systems. Pharmaceutics. 2023;15(4):1190. doi:10.3390/pharmaceutics15041190
156. Jarallah SJ, Aldossary AM, Tawfik EA, et al. GL67 lipid-based liposomal formulation for efficient siRNA delivery into human lung cancer cells. Saudi Pharm J. 2023;31(7):1139–1148. doi:10.1016/j.jsps.2023.05.017
157. Santel A, Aleku M, Keil O, et al. A novel siRNA-lipoplex technology for RNA interference in the mouse vascular endothelium. Gene Ther. 2006;13(16):1222–1234. doi:10.1038/sj.gt.3302777
158. Bao YJ, Jin Y, Chivukula P, et al. Effect of PEGylation on biodistribution and gene silencing of siRNA/Lipid nanoparticle complexes. Pharm Res. 2013;30(2):342–351. doi:10.1007/s11095-012-0874-6
159. Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–221. doi:10.1038/nrd1033
160. Kolli S, Wong SP, Harbottle R, Johnston B, Thanou M, Miller AD. pH-triggered nanoparticle mediated delivery of siRNA to liver cells in vitro and in vivo. Bioconjugate Chem. 2013;24(3):314–332. doi:10.1021/bc3004099
161. Lin SY, Zhao WY, Tsai HC, Hsu WH, Lo CL, Hsiue GH. Sterically polymer-based liposomal complexes with dual-shell structure for enhancing the siRNA delivery. Biomacromolecules. 2012;13(3):664–675. doi:10.1021/bm201746t
162. Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23(8):1002–1007. doi:10.1038/nbt1122
163. Ozpolat B, Sood AK, Lopez-Berestein G. Liposomal siRNA nanocarriers for cancer therapy. Adv Drug Deliv Rev. 2014;66:110–116. doi:10.1016/j.addr.2013.12.008
164. Judge AD, Robbins M, Tavakoli I, et al. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119(3):661–673. doi:10.1172/JCI37515
165. Abdel-Bar HM, Walters AA, Lim Y, et al. An “eat me” combinatory nano-formulation for systemic immunotherapy of solid tumors. Theranostics. 2021;11(18):8738–8754. doi:10.7150/thno.56936
166. Akinc A, Zumbuehl A, Goldberg M, et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi:10.1038/nbt1402
167. Klochkov SG, Neganova ME, Nikolenko VN, et al. Implications of nanotechnology for the treatment of cancer: recent advances. Semin Cancer Biol. 2021;69:190–199. doi:10.1016/j.semcancer.2019.08.028
168. Bartlett DW, Davis ME. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 2006;34(1):322–333. doi:10.1093/nar/gkj439
169. Bartlett DW, Su H, Hildebrandt IJ, Weber WA, Davis ME. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc Natl Acad Sci U S A. 2007;104(39):15549–15554. doi:10.1073/pnas.0707461104
170. Wu Y, Wang W, Chen Y, et al. The investigation of polymer-siRNA nanoparticle for gene therapy of gastric cancer in vitro. Int J Nanomedicine. 2010;5:129–136. doi:10.2147/IJN.S8503
171. Sun TM, Du JZ, Yan LF, Mao HQ, Wang J. Self-assembled biodegradable micellar nanoparticles of amphiphilic and cationic block copolymer for siRNA delivery. Biomaterials. 2008;29(32):4348–4355. doi:10.1016/j.biomaterials.2008.07.036
172. Mao CQ, Du JZ, Sun TM, et al. A biodegradable amphiphilic and cationic triblock copolymer for the delivery of siRNA targeting the acid ceramidase gene for cancer therapy. Biomaterials. 2011;32(11):3124–3133. doi:10.1016/j.biomaterials.2011.01.006
173. Charbe NB, Amnerkar ND, Ramesh B, et al. Small interfering RNA for cancer treatment: overcoming hurdles in delivery. Acta Pharm Sin B. 2020;10(11):2075–2109. doi:10.1016/j.apsb.2020.10.005
174. Ma Z, Wong SW, Forgham H, et al. Aerosol delivery of star polymer-siRNA nanoparticles as a therapeutic strategy to inhibit lung tumor growth. Biomaterials. 2022;285:121539. doi:10.1016/j.biomaterials.2022.121539
175. Yang XZ, Dou S, Sun TM, Mao CQ, Wang HX, Wang J. Systemic delivery of siRNA with cationic lipid assisted PEG-PLA nanoparticles for cancer therapy. J Control Release. 2011;156(2):203–211. doi:10.1016/j.jconrel.2011.07.035
176. Ghareghomi S, Ahmadian S, Zarghami N, Hemmati S. hTERT-molecular targeted therapy of ovarian cancer cells via folate-functionalized PLGA nanoparticles co-loaded with MNPs/siRNA/wortmannin. Life Sci. 2021;277:119621. doi:10.1016/j.lfs.2021.119621
177. Tomalia DA, Baker H, Dewald J, et al. A new class of polymers: starburst-dendritic macromolecules. Polym J. 1985;17:117–132. doi:10.1295/polymj.17.117
178. Wang Y, Sun H. Polymeric nanomaterials for efficient delivery of antimicrobial agents. Pharmaceutics. 2021;13(12):2108. doi:10.3390/pharmaceutics13122108
179. Bober Z, Bartusik-Aebisher D, Aebisher D. Application of dendrimers in anticancer diagnostics and therapy. Molecules. 2022;27(10):3237. doi:10.3390/molecules27103237
180. Cheng Y, Wang J, Rao T, He X, Xu T. Pharmaceutical applications of dendrimers: promising nanocarriers for drug delivery. Front Biosci. 2008;13:1447–1471. doi:10.2741/2774
181. Gao Y, Gao G, He Y, Liu T, Qi R. Recent advances of dendrimers in delivery of genes and drugs. Mini Rev Med Chem. 2008;8(9):889–900. doi:10.2174/138955708785132729
182. Gorzkiewicz M, Kopec O, Janaszewska A, et al. Poly(lysine) dendrimers form complexes with siRNA and provide its efficient uptake by myeloid cells: model studies for therapeutic nucleic acid delivery. Int J Mol Sci. 2020;21(9):3138. doi:10.3390/ijms21093138
183. Del Olmo Sanz N, Holota M, Michlewska S, et al. Copper (II) metallodendrimers combined with pro-apoptotic siRNAs as a promising strategy against breast cancer cells. Pharmaceutics. 2020;12(8):727. doi:10.3390/pharmaceutics12080727
184. Pan J, Mendes LP, Yao M, et al. Polyamidoamine dendrimers-based nanomedicine for combination therapy with siRNA and chemotherapeutics to overcome multidrug resistance. Eur J Pharm Biopharm. 2019;136:18–28. doi:10.1016/j.ejpb.2019.01.006
185. Chen J, Zhu D, Lian B, et al. Cargo-selective and adaptive delivery of nucleic acid therapeutics by bola-amphiphilic dendrimers. Proc Natl Acad Sci U S A. 2023;120(21):e2220787120 doi:10.1073/pnas.2220787120.
186. Paunovska K, Loughrey D, Dahlman JE. Drug delivery systems for RNA therapeutics. Nat Rev Genet. 2022;23(5):265–280. doi:10.1038/s41576-021-00439-4
187. Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432(7014):173–178. doi:10.1038/nature03121
188. Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25(10):1149–1157. doi:10.1038/nbt1339
189. Masimov R, Buyukkoroglu G. HDL-chitosan nanoparticles for siRNA delivery as an SR-B1 receptor targeted system. Comb Chem High Throughput Screen. 2023;26(14):2541–2553. doi:10.2174/1386207326666230406124524
190. Xia CF, Zhang Y, Zhang Y, Boado RJ, Pardridge WM. Intravenous siRNA of brain cancer with receptor targeting and avidin-biotin technology. Pharm Res. 2007;24(12):2309–2316. doi:10.1007/s11095-007-9460-8
191. Wang X, Xiao X, Feng Y, Li J, Zhang Y. A photoresponsive antibody-siRNA conjugate for activatable immunogene therapy of cancer. Chem Sci. 2022;13(18):5345–5352. doi:10.1039/D2SC01672A
192. Chu TC, Twu KY, Ellington AD, Levy M. Aptamer mediated siRNA delivery. Nucleic Acids Res. 2006;34(10):e73. doi:10.1093/nar/gkl388
193. Yang L, Li Z, Binzel DW, Guo P, Williams TM. Targeting oncogenic KRAS in non-small cell lung cancer with EGFR aptamer-conjugated multifunctional RNA nanoparticles. Mol Ther Nucleic Acids. 2023;33:559–571. doi:10.1016/j.omtn.2023.07.027
194. Chiu YL, Ali A, Chu CY, Cao H, Rana TM. Visualizing a correlation between siRNA localization, cellular uptake, and RNAi in living cells. Chem Bio. 2004;11(8):1165–1175. doi:10.1016/j.chembiol.2004.06.006
195. Yang YY, Zhang W, Liu H, Jiang JJ, Wang WJ, Jia ZY. Cell-penetrating peptide-modified graphene oxide nanoparticles loaded with Rictor siRNA for the treatment of triple-negative breast cancer. Drug Des Devel Ther. 2021;15:4961–4972. doi:10.2147/DDDT.S330059
196. Cai M, Yao Y, Yin D, et al. Enhanced lysosomal escape of cell penetrating peptide-functionalized metal-organic frameworks for co-delivery of survivin siRNA and oridonin. J Colloid Interface Sci. 2023;646:370–380. doi:10.1016/j.jcis.2023.04.126
197. Moschos SA, Jones SW, Perry MM, et al. Lung delivery studies using siRNA conjugated to TAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem. 2007;18(5):1450–1459. doi:10.1021/bc070077d
198. Ma R, Nai J, Zhang J, Li Z, Xu F, Gao C. Co-delivery of CPP decorated doxorubicin and CPP decorated siRNA by NGR-modified nanobubbles for improving anticancer therapy. Pharm Dev Technol. 2021;26(6):634–646. doi:10.1080/10837450.2021.1912090
199. Biessen EAL, Beuting DM, Roelen HCPF, Vandemarel GA, Vanboom JH, Vanberkel TJC. Synthesis of cluster galactosides with high-affinity for the hepatic asialoglycoprotein receptor. J Med Chem. 1995;38(9):1538–1546. doi:10.1021/jm00009a014
200. Khan AA, Alanazi AM, Jabeen M, Chauhan A, Ansari MA. Therapeutic potential of functionalized siRNA nanoparticles on regression of liver cancer in experimental mice. Sci Rep. 2019;9(1):15825. doi:10.1038/s41598-019-52142-4
201. Li XA, Wang X, Liu N, Wang QY, Hu J. Inhibition of metastatic hepatocarcinoma by combined chemotherapy with silencing VEGF/VEGFR2 genes through a GalNAc-modified integrated therapeutic system. Molecules. 2022;27(7):2082 doi:10.3390/molecules27072082.
202. Porter JB, Scrimgeour A, Martinez A, et al. SLN124, a GalNAc conjugated 19-mer siRNA targeting tmprss6, reduces plasma iron and increases hepcidin levels of healthy volunteers. Am J Hematol. 2023;98(9):1425–1435. doi:10.1002/ajh.27015
203. Yi Y, Kim HJ, Zheng M, et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J Control Release. 2019;295:268–277. doi:10.1016/j.jconrel.2019.01.006
204. Baghani L, Noroozi Heris N, Khonsari F, Dinarvand S, Dinarvand M, Atyabi F. Trimethyl-chitosan coated gold nanoparticles enhance delivery, cellular uptake and gene silencing effect of EGFR-siRNA in breast cancer cells. Front Mol Biosci. 2022;9:871541. doi:10.3389/fmolb.2022.871541
205. Mora-Raimundo P, Lozano D, Benito M, Mulero F, Manzano M, Vallet-Regi M. Osteoporosis remission and new bone formation with mesoporous silica nanoparticles. Adv Sci. 2021;8(16):e2101107. doi:10.1002/advs.202101107
206. Kumar P, Salve R, Paknikar KM, Gajbhiye V. Nucleolin aptamer conjugated MSNPs-PLR-PEG multifunctional nanoconstructs for targeted co-delivery of anticancer drug and siRNA to counter drug resistance in TNBC. Int J Biol Macromol. 2023;229:600–614. doi:10.1016/j.ijbiomac.2022.12.266
207. Sun J, Zhou Y, Jin G, Jin Y, Quan J. Preparation and preliminary evaluation of dual-functional nanoparticles for MRI and siRNA delivery. Iran J Pharm Res. 2021;20(4):265–277. doi:10.22037/ijpr.2021.115099.15219
208. Wang R, Degirmenci V, Xin H, et al. PEI-coated Fe3O4 nanoparticles enable efficient delivery of therapeutic siRNA targeting REST into glioblastoma cells. Int J Mol Sci. 2018;19(8):2230 doi:10.3390/ijms19082230.
209. Panday R, Abdalla AME, Yu M, Li X, Ouyang C, Yang G. Functionally modified magnetic nanoparticles for effective siRNA delivery to prostate cancer cells in vitro. J Biomater Appl. 2020;34(7):952–964. doi:10.1177/0885328219886953
210. Yu Q, Xiong X, Zhao L, Xu T, Wang Q. Antifibrotic effects of specific siRNA targeting connective tissue growth factor delivered by polyethyleneimine‑functionalized magnetic iron oxide nanoparticles on LX‑2 cells. Mol Med Rep. 2020;21(1):181–190. doi:10.3892/mmr.2019.10834
211. Li J, Xue S, Mao ZW. Nanoparticle delivery systems for siRNA-based therapeutics. J Mater Chem B. 2016;4(41):6620–6639. doi:10.1039/C6TB01462C
212. Chung S, Sugimoto Y, Huang J, Zhang M. Iron oxide nanoparticles decorated with functional peptides for a targeted siRNA delivery to glioma cells. ACS Appl Mater Interfaces. 2023;15(1):106–119. doi:10.1021/acsami.2c17802
213. Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158(1):108–114. doi:10.1016/j.jconrel.2011.10.020
214. Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142(3):416–421. doi:10.1016/j.jconrel.2009.11.008
215. Kara G, Parlar A, Cakmak MC, Cokol M, Denkbas EB, Bakan F. Silencing of survivin and cyclin B1 through siRNA-loaded arginine modified calcium phosphate nanoparticles for non-small-cell lung cancer therapy. Colloids Surf B Biointerfaces. 2020;196:111340. doi:10.1016/j.colsurfb.2020.111340
216. Hsieh HT, Huang HC, Chung CW, et al. CXCR4-targeted nitric oxide nanoparticles deliver PD-L1 siRNA for immunotherapy against glioblastoma. J Control Release. 2022;352:920–930. doi:10.1016/j.jconrel.2022.10.047
217. Siu KS, Zheng X, Liu Y, et al. Single-walled carbon nanotubes noncovalently functionalized with lipid modified polyethylenimine for siRNA delivery in vitro and in vivo. Bioconjug Chem. 2014;25(10):1744–1751. doi:10.1021/bc500280q
218. Zhao Y, Zhao T, Cao Y, et al. Temperature-sensitive lipid-coated carbon nanotubes for synergistic photothermal therapy and gene therapy. ACS Nano. 2021;15(4):6517–6529. doi:10.1021/acsnano.0c08790
219. Wen Z, Feng Y, Hu Y, et al. Multiwalled carbon nanotubes co-delivering sorafenib and epidermal growth factor receptor siRNA enhanced tumor-suppressing effect on liver cancer. Aging. 2021;13(2):1872–1882. doi:10.18632/aging.103905
220. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–U672. doi:10.1038/ncb1596
221. El-Andaloussi S, Lee Y, Lakhal-Littleton S, et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat Protoc. 2012;7(12):2112–2126. doi:10.1038/nprot.2012.131
222. Alvarez-Erviti L, Seow YQ, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–U179. doi:10.1038/nbt.1807
223. Huang C, Zhou Y, Feng X, Wang J, Li Y, Yao X. Delivery of engineered primary tumor-derived exosomes effectively suppressed the colorectal cancer chemoresistance and liver metastasis. ACS Nano. 2023;17(11):10313–10326. doi:10.1021/acsnano.3c00668
224. Ponnuswamy N, Bastings MMC, Nathwani B, et al. Oligolysine-based coating protects DNA nanostructures from low-salt denaturation and nuclease degradation. Nat Commun. 2017;8:15654. doi:10.1038/ncomms15654
225. Hong CA, Eltoukhy AA, Lee H, Langer R, Anderson DG, Nam YS. Dendrimeric siRNA for efficient gene silencing. Angew Chem Int Ed Engl. 2015;54(23):6740–6744. doi:10.1002/anie.201412493
226. Lee H, Lytton-Jean AKR, Chen Y, et al. Molecularly self-assembled nucleic acid nanoparticles for targeted in vivo siRNA delivery. Nat Nanotechnol. 2012;7(6):389–393. doi:10.1038/nnano.2012.73
227. Xu T, Yu S, Sun Y, et al. DNA origami frameworks enabled self-protective siRNA delivery for dual enhancement of chemo-photothermal combination therapy. Small. 2021;17(46):e2101780. doi:10.1002/smll.202101780
228. Ding F, Mou Q, Ma Y, et al. A crosslinked nucleic acid nanogel for effective siRNA delivery and antitumor therapy. Angew Chem Int Ed Engl. 2018;57(12):3064–3068. doi:10.1002/anie.201711242
229. Lee JB, Hong J, Bonner DK, Poon Z, Hammond PT. Self-assembled RNA interference microsponges for efficient siRNA delivery. Nat Mater. 2012;11(4):316–322. doi:10.1038/nmat3253
230. Roh YH, Deng JZ, Dreaden EC, et al. A multi-RNAi microsponge platform for simultaneous controlled delivery of multiple small interfering RNAs. Angew Chem Int Ed Engl. 2016;55(10):3347–3351. doi:10.1002/anie.201508978
231. Yarmush ML, Golberg A, Sersa G, Kotnik T, Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi:10.1146/annurev-bioeng-071813-104622
232. Yang TR, Huang D, Li CH, et al. Rolling microneedle electrode array (RoMEA) empowered nucleic acid delivery and cancer immunotherapy. Nano Today. 2021;36:101017. doi:10.1016/j.nantod.2020.101017
233. Shokouhi AR, Chen Y, Yoh HZ, et al. Electroactive nanoinjection platform for intracellular delivery and gene silencing. J Nanobiotechnology. 2023;21(1):273. doi:10.1186/s12951-023-02056-1
234. Wayteck L, Xiong RH, Braeckmans K, De Smedt SC, Raemdonck K. Comparing photoporation and nucleofection for delivery of small interfering RNA to cytotoxic T cells. J Control Release. 2017;267:154–162. doi:10.1016/j.jconrel.2017.08.002
235. Xiong R, Hua D, Van Hoeck J, et al. Photothermal nanofibres enable safe engineering of therapeutic cells. Nat Nanotechnol. 2021;16(11):1281–1291. doi:10.1038/s41565-021-00976-3
236. Zhang XY, Qin B, Wang M, et al. Dual pH-responsive and tumor-targeted nanoparticle-mediated anti-angiogenesis siRNA delivery for tumor treatment. Int J Nanomedicine. 2022;17:953–967. doi:10.2147/IJN.S340926
237. Mutlu Agardan NB, Sarisozen C, Torchilin VP. Redox-triggered intracellular siRNA delivery. Chem Commun (Camb). 2018;54(49):6368–6371. doi:10.1039/C8CC01376D
238. Hollstein S, Ali LMA, Coste M, et al. A triazolium-anchored self-immolative linker enables self-assembly-driven siRNA binding and esterase-induced release. Chemistry. 2023;29(8):e202203311. doi:10.1002/chem.202203311
239. Perche F, Biswas S, Wang T, Zhu L, Torchilin VP. Hypoxia-targeted siRNA delivery. Angew Chem Int Ed Engl. 2014;53(13):3362–3366. doi:10.1002/anie.201308368
240. Jiang C, Qi Z, Jia H, et al. ATP-responsive low-molecular-weight polyethylenimine-based supramolecular assembly via host-guest interaction for gene delivery. Biomacromolecules. 2019;20(1):478–489. doi:10.1021/acs.biomac.8b01395
241. Koetting MC, Guido JF, Gupta M, Zhang A, Peppas NA. pH-responsive and enzymatically-responsive hydrogel microparticles for the oral delivery of therapeutic proteins: effects of protein size, crosslinking density, and hydrogel degradation on protein delivery. J Control Release. 2016;221:18–25. doi:10.1016/j.jconrel.2015.11.023
242. Ling D, Park W, Park SJ, et al. Multifunctional tumor pH-sensitive self-assembled nanoparticles for bimodal imaging and treatment of resistant heterogeneous tumors. J Am Chem Soc. 2014;136(15):5647–5655. doi:10.1021/ja4108287
243. Mi P, Kokuryo D, Cabral H, et al. A pH-activatable nanoparticle with signal-amplification capabilities for non-invasive imaging of tumour malignancy. Nat Nanotechnol. 2016;11(8):724–730. doi:10.1038/nnano.2016.72
244. Xu X, Wu J, Liu Y, et al. Multifunctional envelope-type siRNA delivery nanoparticle platform for prostate cancer therapy. ACS Nano. 2017;11(3):2618–2627. doi:10.1021/acsnano.6b07195
245. Parrott MC, Luft JC, Byrne JD, Fain JH, Napier ME, Desimone JM. Tunable bifunctional silyl ether cross-linkers for the design of acid-sensitive biomaterials. J Am Chem Soc. 2010;132(50):17928–17932. doi:10.1021/ja108568g
246. Wang Z, Zhang X, Han M, et al. An ultra pH-responsive peptide nanocarrier for cancer gene therapy. J Mater Chem B. 2023;11(37):8974–8984. doi:10.1039/D3TB01311A
247. Li S, Saw PE, Lin C, et al. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials. 2020;234:119760. doi:10.1016/j.biomaterials.2020.119760
248. Xu X, Saw PE, Tao W, et al. ROS-responsive polyprodrug nanoparticles for triggered drug delivery and effective cancer therapy. Adv Mater. 2017;29(33). doi:10.1002/adma.201700141
249. Shim MS, Xia Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew Chem Int Ed Engl. 2013;52(27):6926–6929. doi:10.1002/anie.201209633
250. Wang M, Sun S, Neufeld CI, Perez-Ramirez B, Xu Q. Reactive oxygen species-responsive protein modification and its intracellular delivery for targeted cancer therapy. Angew Chem Int Ed Engl. 2014;53(49):13444–13448. doi:10.1002/anie.201407234
251. Liu Y, Ji X, Tong WWL, et al. Engineering multifunctional RNAi nanomedicine to concurrently target cancer hallmarks for combinatorial therapy. Angew Chem Int Ed Engl. 2018;57(6):1510–1513. doi:10.1002/anie.201710144
252. Chen D, Zhang P, Li M, et al. Hyaluronic acid-modified redox-sensitive hybrid nanocomplex loading with siRNA for non-small-cell lung carcinoma therapy. Drug Deliv. 2022;29(1):574–587. doi:10.1080/10717544.2022.2032874
253. Liu X, Zhou XQ, Shang XW, et al. Inhibition of chemotherapy-related breast tumor EMT by application of redox-sensitive siRNA delivery system CSO-ss-SA/siRNA along with doxorubicin treatment. J Zhejiang Univ Sci B. 2020;21(3):218–233. doi:10.1631/jzus.B1900468
254. Wen LJ, Wen CL, Zhang FT, Wang K, Yuan H, Hu FQ. siRNA and chemotherapeutic molecules entrapped into a redox-responsive platform for targeted synergistic combination therapy of glioma. Nanomedicine. 2020;28:102218 doi:10.1016/j.nano.2020.102218.
255. Chen GJ, Wang YY, Xie RS, Gong SQ. Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. J ControlRelease. 2017;259:105–114 doi:10.1016/j.jconrel.2017.01.042.
256. Zheng N, Song ZY, Liu Y, et al. Redox-responsive, reversibly-crosslinked thiolated cationic helical polypeptides for efficient siRNA encapsulation and delivery. J ControlRelease. 2015;205:231–239 doi:10.1016/j.jconrel.2015.02.014.
257. Shi Z, Yang Y, Guo Z, Feng S, Wan Y. A cathepsin B/GSH dual-responsive fluorinated peptide for effective siRNA delivery to cancer cells. Bioorg Chem. 2023;135:106485. doi:10.1016/j.bioorg.2023.106485
258. Huo D, Liu S, Zhang C, et al. Hypoxia-targeting, tumor microenvironment responsive nanocluster bomb for radical-enhanced radiotherapy. ACS Nano. 2017;11(10):10159–10174. doi:10.1021/acsnano.7b04737
259. Yu J, Zhang Y, Hu X, Wright G, Gu Z. Hypoxia-sensitive materials for biomedical applications. Ann Biomed Eng. 2016;44(6):1931–1945. doi:10.1007/s10439-016-1578-6
260. Son S, Rao NV, Ko H, et al. Carboxymethyl dextran-based hypoxia-responsive nanoparticles for doxorubicin delivery. Int J Biol Macromol. 2018;110:399–405. doi:10.1016/j.ijbiomac.2017.11.048
261. Cheng Y, Hao J, Lee LA, Biewer MC, Wang Q, Stefan MC. Thermally controlled release of anticancer drug from self-assembled gamma-substituted amphiphilic poly(epsilon-caprolactone) micellar nanoparticles. Biomacromolecules. 2012;13(7):2163–2173. doi:10.1021/bm300823y
262. Honda Y, Onodera S, Takemoto H, et al. Thermo-responsive polymer-siRNA conjugates enabling artificial control of gene silencing around body temperature. Pharm Res. 2023;40(1):157–165. doi:10.1007/s11095-022-03414-8
263. Mo R, Jiang T, Gu Z. Enhanced anticancer efficacy by ATP-mediated liposomal drug delivery. Angew Chem Int Ed Engl. 2014;53(23):5815–5820. doi:10.1002/anie.201400268
264. Mo R, Jiang T, Sun W, Gu Z. ATP-responsive DNA-graphene hybrid nanoaggregates for anticancer drug delivery. Biomaterials. 2015;50:67–74. doi:10.1016/j.biomaterials.2015.01.053
265. Naito M, Ishii T, Matsumoto A, Miyata K, Miyahara Y, Kataoka K. A phenylboronate-functionalized polyion complex micelle for ATP-triggered release of siRNA. Angew Chem Int Ed Engl. 2012;51(43):10751–10755. doi:10.1002/anie.201203360
266. Mo R, Jiang T, DiSanto R, Tai W, Gu Z. ATP-triggered anticancer drug delivery. Nat Commun. 2014;5:3364. doi:10.1038/ncomms4364
267. Biswas S, Kinbara K, Niwa T, et al. Biomolecular robotics for chemomechanically driven guest delivery fuelled by intracellular ATP. Nat Chem. 2013;5(7):613–620. doi:10.1038/nchem.1681
268. Miele E, Spinelli GP, Miele E, et al. Nanoparticle-based delivery of small interfering RNA: challenges for cancer therapy. Int J Nanomedicine. 2012;7:3637–3657. doi:10.2147/IJN.S23696
269. Gao Y, Zhu J, Lu H. Single domain antibody-based vectors in the delivery of biologics across the blood-brain barrier: a review. Drug Deliv Transl Res. 2021;11(5):1818–1828. doi:10.1007/s13346-020-00873-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.