Back to Journals » Cancer Management and Research » Volume 14
A Comparison of Clinical Prognostic Indices in Elderly Patients with Diffuse Large B-Cell Lymphoma Treated with a Pegylated Liposomal Doxorubicin Combination Regimen in China
Authors Gao H, Xu Y, Liu Y, Mi L, Wang X, Liu W, Zhu J, Song Y
Received 25 January 2022
Accepted for publication 1 September 2022
Published 14 September 2022 Volume 2022:14 Pages 2711—2721
DOI https://doi.org/10.2147/CMAR.S359956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Hongye Gao,* Yanfeng Xu,* Yanfei Liu, Lan Mi, Xiaopei Wang, Weiping Liu, Jun Zhu, Yuqin Song
Department of Lymphoma, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weiping Liu; Jun Zhu, Department of Lymphoma, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital & Institute, Beijing, People’s Republic of China, Tel +86 10 88196109, Fax +86 10 88196115, Email [email protected]; [email protected]
Background: There is no consensus regarding the risk stratification scores for elderly patients with diffuse large B-cell lymphoma (DLBCL). We aimed to compare the prognostic predictive ability of the current clinical scoring indices in DLBCL elderly patients treated with the R-CODP regimen (rituximab, cyclophosphamide, pegylated liposomal doxorubicin, vincristine, and prednisone).
Methods: We retrospectively collected the data of elderly DLBCL patients who received the R-CODP regimen as the first-line treatment. The efficacy of the regimen was evaluated. The Akaike information criteria (AIC), concordance index (C-index), and integrated discrimination improvement (IDI) were used to assess the fitness and prognostic performance of the current clinical prognostic indices.
Results: In the total of 158 patients enrolled, the median follow-up time was 6.7 years (95% CI: 6.3– 7.9), and the 5-year OS was 52.8% (95% CI: 45.5%– 61.2%). The International Prognostic Index (IPI), National Comprehensive Cancer Network-IPI (NCCN-IPI), and Elderly International Prognostic Index (E-IPI) were all significantly associated with OS (P < 0.001 for all). However, no significance was observed in 5-year OS in the low- vs low-intermediate-risk groups for IPI (P = 0.377), NCCN-IPI (P = 0.238), and E-IPI (P = 0.080). Compared with the IPI and NCCN-IPI, the E-IPI had the lowest AIC value of 747.5 and the highest C-index of 0.692. For predicting 5-year mortality, the E-IPI showed better performance (AUC: 0.715 for E-IPI vs 0.676 for IPI, P = 0.036), with the IDI of 6.29% (95% CI: 3.71%-8.88%, P < 0.001) and 4.80% (95% CI: 1.32%-8.28%, P = 0.007) compared to the IPI and NCCN-IPI, respectively.
Conclusion: The E-IPI might be a better prognostic prediction model in Chinese DLBCL generics treated with R-CODP for predicting 5-year mortality. However, the IPI, NCCN-IPI, and E-IPI did not seem to be able to distinguish patients with a favorable prognosis well.
Keywords: lymphoma, large B cell, diffuse, liposomal doxorubicin, survival analysis, prognosis
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of B-cell lymphoma and often occurs in elderly individuals.1,2 For these patients, the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) is recommended as the standard first-line treatment.3,4 However, the neurological and hematological side effects and cardiotoxicity of the treatment deserve attention, especially for elderly patients. Doxorubicin is the main agent that causes side effects, especially cardiotoxicity. Liposomal doxorubicin is a promising alternative drug to the current regimen, with a safety and efficacy profile through the enhanced permeability and retention effect and longer circulation.5–7 Regimens containing liposomal doxorubicin were reported to have less toxicity in treating patients with AIDS-related non-Hodgkin’s lymphoma,8 lymphoma with advanced age,9 and lymphoma with concurrent cardiac diseases.10 The liposomal doxorubicin combination regimen for patients with lymphoma has been evaluated in some research with special concerns11–14 and is the most commonly used among older adults.15
Identifying patients’ risk stratification is critical for treatment selection. There is no consensus regarding the risk stratification system for elderly patients with DLBCL. The International Prognostic Index (IPI) scoring index is widely accepted but has been questioned for elderly patients.16 The National Comprehensive Cancer Network-IPI (NCCN-IPI) refines the categorization of age and other clinical indicators to enhance discrimination for low- and high-risk patients. However, the discrimination declines for elderly patients of different risk categories. Most patients are classified into the high‐risk group due to the age.17 The elderly IPI (E-IPI) might have better prognostic performance for low- and low-intermediate-risk patients but still needs to be validated in the real world with larger samples.18
To the best of our knowledge, the comparison of the current clinical indices for generic DLBCL with the R-CODP in China has been less reported. Therefore, we aimed to compare the prognostic performance of the IPI, NCCN-IPI, and E-IPI for this population with multiple statistical methods.
Materials and Methods
Patients and Diagnosis
We retrospectively reviewed all consecutive patients at Peking University Cancer Hospital & Institute from September 2008 to February 2017. The inclusion criteria included patients with age equal to or over 60 years at diagnosis, histologically diagnosed with DLBCL, and receiving at least one cycle of the R-CODP regimen as the first-line treatment. Patients with primary mediastinal large B-cell lymphoma, primary central nervous system lymphoma, gray-zone lymphoma, and lymphoma associated with HIV were excluded.
The immunohistochemistry (IHC) panel included CD19, CD20, CD3, CD5, CD10, BCL-2, BCL-6, CD21, CD23, CyclinD1, CD79a, CD45RO, Ki-67, CD30, ALK, Mum-1, c-myc, EBV, etc. All patients enrolled in this study were pathologically reviewed by a panel of experts at our center. Germinal center B-cell-like (GCB) and non-GCB were subclassified using CD10, BCL-6, and MUM1 expression by IHC.19 FISH testing for C-MYC, BCL-2, and BCL-6 and next gene sequencing, if appropriate, are not needed. Clinical stages were classified according to the Ann Arbor staging system.20 All patients were staged by position emission tomography (PET)/CT or CT and bone marrow biopsy at baseline. The risk stratification, including the IPI,16 NCCN-IPI,17 and E-IPI,18 was calculated based on baseline information.
Treatments and Response Evaluation
Patients with the early-stage disease were generally recommended to complete 4–8 cycles of R-CODP ± radiotherapy (RT) in our center. If patients had poor tolerance, 4 cycles of the R-CODP regimen followed by RT were permitted. For patients aged 61–70 years old, we suggested 6–8 cycles of R-CODP or 6 cycles of R-CODP followed by 2 infusions of rituximab monotherapy. For patients aged 71–80 years, the dose was adjusted according to the modified comprehensive geriatric assessment described in the previous literature.21 R-miniCODP was administered for patients > 80 years old. All elderly patients were recommended to receive a granulocyte colony-stimulating factor agent against leukopenia or neutropenia.
An imaging examination was performed every 2 cycles during the treatment. The response (complete response [CR]; partial response [PR] and overall response rate [ORR]) was evaluated by the Lugano criteria accordingly.22
Endpoints
The primary endpoint was overall survival (OS), which was defined as the time from diagnosis to any cause of death or the last follow-up. Other endpoints were CR, PR, and ORR. Follow-ups for all-cause mortality were conducted until May 2022. Patient follow-up was performed by a combination of outpatient visits, medical records review, and telephone.
Model Comparison Parameters
Model fitness was compared by the Akaike information criteria (AIC).23 A smaller AIC represents a better fitting result. Generally, AIC comparisons between models generated a ΔAIC. ΔAIC ≥ 10 demonstrates a significant improvement in model fitness; ΔAIC between 2 and 10 shows an improvement in fitness; ΔAIC < 2 indicates no improvement in model fitness.24 We also calculated Harrell’s concordance index (C-index) to evaluate the prognostic performance of the models. A C-index of 1 indicates perfect discrimination, and a C-index of 0.5 reflects a random guess. The discriminant validity of mortality at fixed time points was assessed by the area under the curve (AUC) and compared by the correlated AUCs by DeLong et al’s method.25
We used the “PredictABEL” package for calculating the integrated discrimination improvement (IDI) index to compare models.26,27 Patients who were lost to follow-up before the landmark time were censored. We also presented the reclassification table to show clinical differences in shifts among risk categories.
Statistical Analysis
Kolmogorov–Smirnov tests were performed as the normality test. If appropriate, continuous variables were presented as the mean and standard deviations or medians and interquartile range (IQR) or range. Categorical variables were expressed as a number with percentages. Continuous variables were compared by Student’s t test or the Mann‒Whitney U-test according to whether the data fit a normal distribution. The comparison of qualitative variables was performed using the χ2 test or adjusted using Fisher’s exact test.
The survival rates were estimated by the Kaplan‒Meier method and compared using the Log rank test. Hazard ratios were calculated using the Cox proportional model. We reported confidence intervals (CIs) as 95% confidence intervals. All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant and conducted using R version 4.2.0 (https://www.r-project.org) and MedCal version 20.111 (https://www.medcalc.org).
Results
Baseline Characteristics
A total of 158 elderly patients with newly diagnosed DLBCL were enrolled in the present study. The median age was 72 years (range 60–86), with a male-to-female ratio of 1:1, and 93 (58.9%) patients were aged 70 years or older. More than half (56.3%) of the patients had advanced disease (Ann Arbor stage III–IV), and 30.4%, 19.6%, and 22.8% of the patients were categorized into the high-risk group according to the IPI (≥ 3), NCCN-IPI (≥ 4), and E-IPI (≥ 3), respectively (Table 1).
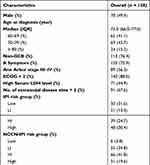 |
Table 1 Patient Characteristics at Baseline |
Treatment Delivery and Response Evaluation
A total of 853 cycles of chemotherapy (instead of 948 estimated) were given. A median of 6 cycles was completed. One hundred and seven (67.7%) patients received at least 4 cycles of treatment. The median doses of CTX and PLD were 638.8 mg/m2 (IQR: 463.5–727.5) and 21.0 mg/m2 (IQR: 12.6–23.6), respectively.
At the end of induction treatment, 150 patients were evaluable for response: the ORR was 74.7% (112/150), and CR was 50.7% (76/150). For 76 patients who achieved CR, only 3.9% were allocated to the low-risk NCCN-IPI group, whereas 55.3% and 42.1% of patients were classified in the low-risk E-IPI and IPI groups, respectively. For 38 non-responders (stable disease/progressive disease), 44.7%, 28.9%, and 34.2% were assigned to the high-risk group by IPI, NCCN-IPI, and E-IPI, respectively (Table 2).
 |
Table 2 Association Between Response and Clinical Prognostic Indices |
Overall Survival
The median follow-up time was 6.7 years (95% CI: 6.3-7.9) for the whole cohort, with a maximum follow-up time of 12.6 years. The median OS was 5.6 years (95% CI: 3.62-NA). The 1-, 3-, and 5-year OS rates were 79.1% (95% CI: 73.7%–85.7%), 60.0% (95% CI: 52.8%–68.2%) and 52.8% (95% CI: 45.5%–61.2%), respectively.
Three clinical risk scoring indices were significantly associated with OS (P < 0.0001 for all). NCCN-IPI had the greatest absolute difference estimates between the high- and low-risk groups in 1- and 3-year OS, with Δ48.4% and Δ57.5%, respectively. However, for the 5-year OS, the E-IPI had the greatest absolute difference (E-IPI: Δ52.8%; IPI: Δ45.4%; NCCN-IPI: Δ44.1%). Notably, the IPI, NCCN-IPI, and E-IPI all could not identify a subgroup of patients with favorable long-term survival (Figure 1). No significance was observed in 5-year OS between the low- and low-intermediate-risk categories for NCCN-IPI (66.7% vs 70.8%; P = 0.238), IPI (71.9% vs 71.4%; P = 0.377), and E-IPI (71.6% vs 73.3%, P = 0.080) (Supplementary Table 1).
Model Comparison
The fitness of the three models was compared using AIC. The AIC values were 763.1, 764.3, and 747.5 for the IPI, NCCN-IPI, and E-IPI, respectively. E-IPI with lower AIC values had the best fitness than IPI and NCCN-IPI with higher AIC values (Table 3). Although the difference was small in the evaluation of the C-index among the 3 clinical risk scoring indices, the E-IPI also had a higher discriminability than the IPI and NCCN-IPI (C-index: 0.692 for the E-IPI, 0.665 for the IPI, and 0.652 for the NCCN-IPI).
 |
Table 3 Stratification Models for OS |
Considering that the median follow-up time was 6.7 years (95% CI: 6.3–7.9), the risk prediction and model assessment horizon was chosen within 5 years. A significant improvement in predicting 5-year mortality was noted in the E-IPI with the IDI of 6.29% (95% CI: 3.71%-8.88%, P < 0.001) compared with the IPI and 4.80% (95% CI: 1.32%-8.28%, P = 0.007) and compared with the NCCN-IPI. However, NCCN-IPI failed to improve the predictive ability over IPI, with the IDI of 1.5% (95% CI: -1.72%-4.71%, P = 0.361). We also found that the E-IPI achieved the highest AUC value for predicting 1-, 3-, and 5-year mortality compared with the IPI and NCCN-IPI. Significance was observed between the IPI and E-IPI for predicting 5-year mortality (AUC: 0.676 vs 0.715, P = 0.036; Figure 2 and Table 4).
 |
Table 4 AUC for Predicting Mortality |
As shown in Figure 3, 2.7% (2/73) of patients with events incorrectly and 2.6% (2/78) without events correctly evaluated by E-IPI were reclassified in the lower (low/LI) or the higher (HI/high) risk groups compared to IPI. Similarly, 2.7% (2/73) of patients had events incorrectly, and 7.7% (6/78) did not have events correctly assessed by E-IPI compared to NCCN-IPI.
Discussion
The burden and mortality of lymphoma among elderly patients have increased in China.28,29 Treatments for elderly patients with DLBCL remain a challenge. The R-CODP regimen is an alternative treatment approach and is widely used for generics in China.15 To the best of our knowledge, our study is the first report to compare current clinical indices in Chinese generics treated with the R-CODP regimen. Apart from the commonly used AIC and C-index, we adopted the IDI as a novel prognostic parameter to support the finding: the E-IPI, as a simple and convenient prognostic index, seemed to have better performance in predicting 5-year mortality for this population.
The standard R-CHOP21 improved the survival of elderly patients, with a 5-year OS rate of nearly 60%.3,30 R-miniCHOP was recommended in patients aged over 80 years old.31 However, elderly patients who were considered unfit for doxorubicin were excluded from clinical trials. In addition, for patients with concurrent cardiac diseases or pretreated with anthracyclines, the replacement of doxorubicin with liposomal doxorubicin was reported to be safe and effective.10 R-CODP is the most common regimen for Chinese geriatric patients with DLBCL in clinical practice. Our previous study indicated that tolerance and identifying risk categories are critical for clinical decision-making in this population.15
However, there is no standard prognostic clinical model in elderly patients with DLBCL. Ruppert et al showed that NCCN-IPI outperformed IPI and revised-IPI in patients with DLBCL treated with the R-CHOP regimen for the whole age group.32 However, elderly patients have a greater possibility of being classified into higher-risk groups in NCCN-IPI because of the high weighted age. For the E-IPI model, the age of 70 years was used instead of 60 years as the cutoff point in the IPI. Our results showed that E-IPI might serve as a better prognostic prediction model compared to IPI and NCCN-IPI in elderly patients, similar to those from the study by Advani et al.18 A previous study found that in patients > 60 years old with DLBCL treated with R-CHOP, E-IPI provided additional prognostic discrimination for low- and low-intermediate-risk groups and ranked higher in model fit and discrimination measures than other indices. However, we did not observe that the E-IPI performed well in distinguishing the low- and low-intermediate-risk groups in our data, indicating that the results need to be validated further. A revised IPI (R-IPI) redistributed IPI factors into 3 distinct prognostic groups. Previous studies showed that R-IPI is a better predictor of outcome, but neither IPI nor R-IPI identified a risk group with less than a 50% chance of survival.33 Considering that all elderly patients enrolled had at least 1 factor of R-IPI (age ≥ 60 years old), no one could be identified into a very good category. Therefore, we did not compare the R-IPI with other indices in the present study.
Optimal approaches to assessing the predictivity of models are fundamental to clinical care. Recently, several novel indices, including net reclassification improvement34 and IDI, have been proposed to test the incremental value of one model over another, which are considered to be more sensitive than AUC in miscalibration.35–37 According to the definition, a reclassification table could illustrate how patients’ classification changes. IDI statistics showed an improved discrimination ability for 5-year mortality using E-IPI compared to IPI or NCCN-IPI. However, this was a single-center study, and the limited number of patients may affect the statistical significance of the results.
Notably, none of the 3 risk scoring indices seemed to be able to perfectly identify the patients in the lower-risk group. New prognostic models involving novel molecular prognostic biomarkers are urgently needed. Recently, four genetic subtypes termed MCD, BN2, N1, and EZB have been proposed for DLBCL.38 Chapuy et al identified 5 robust DLBCL subsets by integrating genetic drivers, which could independently predict outcomes of the IPI.39 A large population-based study identified 5 molecular DLBCL subtypes (MYD88, BCL2, SOCS1/SGK1, TET2/SGK1, and NOTCH2).40 These studies suggested that stratification according to molecular subtypes might help to evaluate the risk of patients with high heterogeneity. New prognostic indices containing novel predictors may assist in identifying high-risk patients in need of alternative therapies rather than R-CHOP or R-CODP.
Conclusions
Compared to the IPI and NCCN-IPI, the E-IPI had a good predictive ability to predict 5-year mortality in elderly patients with DLBCL treated with R-CODP. For better identification of patients with heterogeneity, a novel prognostic index with molecular techniques is needed.
Abbreviations
DLBCL, diffuse large B-cell lymphoma; IPI, International prognostic index; NCCN-IPI, National Comprehensive Cancer Network-International prognostic index; E-IPI, Elderly International prognostic index; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; R-CODP, rituximab, cyclophosphamide, pegylated liposomal doxorubicin, vincristine, and prednisone; OS, overall survival; PFS, progression-free survival; ORR, overall response rate; CR, complete response; AIC, Akaike information criteria; C-index, concordance index; AUC, area under the curve; NRI, net reclassification improvement; IDI, integrated discrimination improvement.
Data Sharing Statement
The corresponding authors are glad to share the anonymized dataset upon receiving a reasonable request.
Ethics Approval & Consent to Participate
This study was approved and judged to be exempt from written informed consent by the Ethics Committee of Peking University Cancer Hospital & Institute. All procedures abided by the principles of the Declaration of Helsinki and all patient data were anonymized in this study.
Acknowledgments
We thank the colleagues at Peking University Cancer Hospital & Institute for their support and assistance in this study.
Author Contributions
All authors made significant contributions to one or more aspects of the conception, study design, execution, acquisition, analysis, and interpretation of the data of this work. All authors participated in drafting, revising, or critically reviewing the manuscript; gave approval of the final version to be published; consented to the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81870154, 81972807, 81670187, 81970179, and 81700197), Beijing Natural Science Foundation (No. 7202025 and 7202026), and Beijing Municipal Science & Technology Commission (Z181100001918019); Clinical Research Fund For Distinguished Young Scholars of Beijing Cancer Hospital (QNJJ202106).
Disclosure
Dr Hongye Gao reports grants from National Natural Science Foundation of China, grants from Beijing Natural Science Foundation, grants from Beijing Municipal Science & Technology Commission, during the conduct of the study. The authors declare no competing financial interests.
References
1. SEER 21. Cancer stat facts: NHL — Diffuse Large B-Cell Lymphoma (DLBCL); 2019. Available from: https://seer.cancer.gov/statfacts/html/dlbcl.html.
2. Liu W, Liu J, Song Y, et al. Burden of lymphoma in China, 2006–2016: an analysis of the global burden of disease study 2016. J Hematol Oncol. 2019;12(1):115. doi:10.1186/s13045-019-0785-7
3. Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. doi:10.1056/NEJMoa011795
4. Pfreundschuh M, Schubert J, Ziepert M, et al. Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol. 2008;9(2):105–116. doi:10.1016/S1470-2045(08)70002-0
5. Gabizon A, Shmeeda H, Grenader T. Pharmacological basis of pegylated liposomal doxorubicin: impact on cancer therapy. Eur J Pharm Sci. 2012;45(4):388–398. doi:10.1016/j.ejps.2011.09.006
6. Hashizume H, Baluk P, Morikawa S, et al. Openings between defective endothelial cells explain tumor vessel leakiness. Am J Pathol. 2000;156(4):1363–1380. doi:10.1016/S0002-9440(10)65006-7
7. Sadzuka Y, Sugiyama I, Tsuruda T, Sonobe T. Characterization and cytotoxicity of mixed polyethyleneglycol modified liposomes containing doxorubicin. Int J Pharm. 2006;312(1–2):83–89. doi:10.1016/j.ijpharm.2005.12.043
8. Levine AM, Tulpule A, Espina B, et al. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS-related non-Hodgkin’s lymphoma: results of therapy and correlates of response. J Clin Oncol. 2004;22(13):2662–2670. doi:10.1200/JCO.2004.10.093
9. Ricciuti G, Finolezzi E, Luciani S, et al. Combination of rituximab and nonpegylated liposomal doxorubicin (R-NPLD) as front-line therapy for aggressive non-Hodgkin lymphoma (NHL) in patients 80 years of age or older: a single-center retrospective study. Hematol Oncol. 2018;36(1):44–48. doi:10.1002/hon.2386
10. Rigacci L, Mappa S, Nassi L, et al. Liposome-encapsulated doxorubicin in combination with cyclophosphamide, vincristine, prednisone and rituximab in patients with lymphoma and concurrent cardiac diseases or pre-treated with anthracyclines. Hematol Oncol. 2007;25(4):198–203. doi:10.1002/hon.827
11. Fridrik MA, Jaeger U, Petzer A, et al. Cardiotoxicity with rituximab, cyclophosphamide, non-pegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B-cell lymphoma: a randomised phase-III study from the Austrian cancer drug therapy working group [Arbeitsgemeinschaft Medikamentöse Tumortherapie AGMT](NHL-14). Eur J Cancer. 2016;58:112–121. doi:10.1016/j.ejca.2016.02.004
12. Rohlfing S, Aurich M, Schöning T, Ho AD, Witzens-Harig M. Nonpegylated liposomal doxorubicin as a component of R-CHOP is an effective and safe alternative to conventional doxorubicin in the treatment of patients with diffuse large B-cell lymphoma and preexisting cardiac diseases. Clin Lymphoma Myeloma Leuk. 2015;15(8):458–463. doi:10.1016/j.clml.2015.03.008
13. Oki Y, Ewer MS, Lenihan DJ, et al. Pegylated liposomal doxorubicin replacing conventional doxorubicin in standard R-CHOP chemotherapy for elderly patients with diffuse large B-cell lymphoma: an open label, single arm, Phase II trial. Clin Lymphoma Myeloma Leuk. 2015;15(3):152–158. doi:10.1016/j.clml.2014.09.001
14. Visani G, Ferrara F, Alesiani F, et al. R-COMP 21 for frail elderly patients with aggressive B-cell non-Hodgkin lymphoma: a pilot study. Leuk Lymphoma. 2008;49(6):1081–1086. doi:10.1080/10428190802043853
15. Gao H, Liu Y, Xu Y, et al. Impact of relative dose intensity of R-CCOP regimen in elderly patients with diffuse large B-cell lymphoma in China. J Formos Med Assoc. 2022. doi:10.1016/j.jfma.2022.06.003
16. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987–994. doi:10.1056/NEJM199309303291402
17. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced international prognostic index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014;123(6):837–842. doi:10.1182/blood-2013-09-524108
18. Advani RH, Chen H, Habermann TM, et al. Comparison of conventional prognostic indices in patients older than 60 years with diffuse large B-cell lymphoma treated with R-CHOP in the US Intergroup Study (ECOG 4494, CALGB 9793): consideration of age greater than 70 years in an elderly prognostic index (E-IPI). Br J Haematol. 2010;151(2):143–151. doi:10.1111/j.1365-2141.2010.08331.x
19. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103(1):275–282. doi:10.1182/blood-2003-05-1545
20. Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31(11):1860–1861.
21. Spina M, Balzarotti M, Uziel L, et al. Modulated chemotherapy according to modified comprehensive geriatric assessment in 100 consecutive elderly patients with diffuse large B-cell lymphoma. Oncologist. 2012;17(6):838–846. doi:10.1634/theoncologist.2011-0417
22. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-hodgkin lymphoma: the lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi:10.1200/JCO.2013.54.8800
23. Hirotugu A. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723.
24. Burnham KP. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. New York, NY: Springer Verlag New York; 2022.
25. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
26. Kundu S, Aulchenko YS, van Duijn CM, Janssens AC. PredictABEL: an R package for the assessment of risk prediction models. Eur J Epidemiol. 2011;26(4):261–264. doi:10.1007/s10654-011-9567-4
27. Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48(12):1703–1711. doi:10.1515/CCLM.2010.340
28. Liu W, Liu J, Song Y, et al. Mortality of lymphoma and myeloma in China, 2004–2017: an observational study. J Hematol Oncol. 2019;12(1):22. doi:10.1186/s13045-019-0706-9
29. Liu W, Qi J, Liu J, et al. Mortality Rate of Lymphoma in China, 2013–2020. Front Oncol. 2022;12:902643. doi:10.3389/fonc.2022.902643
30. Feugier P, Van Hoof A, Sebban C, et al. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2005;23(18):4117–4126. doi:10.1200/JCO.2005.09.131
31. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, Phase 2 trial. Lancet Oncol. 2011;12(5):460–468. doi:10.1016/S1470-2045(11)70069-9
32. Ruppert AS, Dixon JG, Salles G, et al. International prognostic indices in diffuse large B-cell lymphoma: a comparison of IPI, R-IPI, and NCCN-IPI. Blood. 2020;135(23):2041–2048. doi:10.1182/blood.2019002729
33. Sehn LH, Berry B, Chhanabhai M, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857–1861. doi:10.1182/blood-2006-08-038257
34. McKearnan SB, Wolfson J, Vock DM, Vazquez-Benitez G, O'Connor PJ. Performance of the Net Reclassification Improvement for Nonnested Models and a Novel Percentile-Based Alternative. Am J Epidemiol. 2018;187(6):1327-1335. doi:10.1093/aje/kwx374
35. Pencina MJ, Fine JP, D’Agostino RB Sr. Discrimination slope and integrated discrimination improvement - properties, relationships and impact of calibration. Stat Med. 2017;36(28):4482–4490. doi:10.1002/sim.7139
36. Braun SD, Kuhn M, Bergmann S, et al. Net reclassification improvement with serial biomarkers and bed-sided spirometry to early predict the need of organ support during the early post-transplantation in-hospital stay in allogeneic HCT recipients. Bone Marrow Transplant. 2019;54(2):265–274. doi:10.1038/s41409-018-0258-6
37. Pencina MJ, D’Agostino RB
38. Schmitz R, Wright GW, Huang DW, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med. 2018;378(15):1396–1407. doi:10.1056/NEJMoa1801445
39. Chapuy B, Stewart C, Dunford AJ, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med. 2018;24(5):679–690. doi:10.1038/s41591-018-0016-8
40. Lacy SE, Barrans SL, Beer PA, et al. Targeted sequencing in DLBCL, molecular subtypes, and outcomes: a haematological malignancy research network report. Blood. 2020;135(20):1759–1771. doi:10.1182/blood.2019003535
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



