Back to Journals » Journal of Inflammation Research » Volume 15
A Comparative Review of Pyroptosis in Mammals and Fish
Authors Song Z, Zou J, Wang M, Chen Z, Wang Q
Received 5 February 2022
Accepted for publication 30 March 2022
Published 11 April 2022 Volume 2022:15 Pages 2323—2331
DOI https://doi.org/10.2147/JIR.S361266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Adam D Bachstetter
Zixi Song,* Jiahong Zou,* Mengya Wang, Zhenwei Chen, Qingchao Wang
Engineering Research Center of Green Development for Conventional Aquatic Biological Industry in the Yangtze River Economic Belt, Ministry of Education, College of Fisheries, Huazhong Agricultural University, Wuhan, 430070, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingchao Wang, Engineering Research Center of Green Development for Conventional Aquatic Biological Industry in the Yangtze River Economic Belt, Ministry of Education, College of Fisheries, Huazhong Agricultural University, Shizishan Street 1st, Hongshan District, Wuhan, Hubei, People’s Republic of China, Tel +86-138 71499065, Fax +86-27 87282113, Email [email protected]
Abstract: Pyroptosis is a form of programmed cell death, which is executed by gasdermin family proteins. Under the stimulation of pathogen- and/or damage-associated molecular patterns, pattern recognition receptors (PRRs) such as Nod like receptors could recruit apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspases to form inflammasomes and then activate caspases through various pathways. The activated caspases then cleave gasdermin family proteins, and N-terminal (NT) domains of gasdermins were released to form oligomeric pores, resulting in the increased membrane permeability, cell swelling, and final pyroptosis. During this process, caspases also promote the maturation and release of inflammatory cytokines such as IL-1β and IL-18, thus pyroptosis is also named inflammatory cell death. Unlike numerous gasdermin family proteins in mammals, only gasdermin E (GSDME) has been identified in fish. GSDME in fish can be cleaved by caspase-a/-b to release its NT domain and induce pyroptosis. Studies indicated that pyroptosis in fish mainly depends on NLR family pyrin domain-containing 3 (NLRP3) inflammasome. ASC and different caspase proteins also were identified in different fish species. The influences of pathogenic microorganism infection and environmental pollutants on fish pyroptosis were studied in recent years. Considering that fish living environment is affected by multiple factors such as water salinity, temperature, oxygen supply, and highly fluctuating food supply, the in-depth research about fish pyroptosis will contribute to revealing the mechanism of pyroptosis during evolution.
Keywords: pyroptosis, fish, gasdermin E, caspases
Graphical Abstract:
Introduction
Pyroptosis was first discovered by Zychlinsky et al in 1992, when macrophages exhibited suicidal behavior after Shigella flexneri infection.1 Due to its features similar to apoptosis, such as caspase-dependence, DNA damage, and nuclear pyknosis, pyroptosis was considered to be apoptosis in early stage.2 Subsequent studies indicated that apoptosis and pyroptosis are different, and pro-inflammatory programmed cell death represented by the latter was officially named pyroptosis in 2001.3 Like apoptosis, necroptosis, and autophagy, pyroptosis is also a form of programmed cell death, and plays an important role in the innate immune system of vertebrates.4 Recent studies confirmed its role in neurological diseases, infectious diseases, cardiovascular diseases, and tumors.5,6 Pyroptosis is executed by the cleaved N-terminal (NT) domain of the gasdermin family member proteins via oligomerization in the cell membrane, resulting in cell perforation formation, ion homeostasis destruction, and inflammatory mediator release.7 Under toxin challenge or pathogen infection, multiple inflammasome complexes are assembled to activate diverse caspases including caspase-1, −4, −5, −11, and −12. The activated caspases cleave gasdermin family proteins, resulting in the release of their NT domains which cause the perforation of cell membrane, and these caspases also promote the maturation of IL-1β and IL-18.8 Therefore, the process of pyroptosis is accompanied by the release of a large number of pro-inflammatory factors, which distinguishes pyroptosis from other forms of programmed cell death.4,8 Pyroptosis cells lose their ability to regulate the ingress and egress of substances due to the formation of micropores in cell membrane, thus osmotic balance on both sides of cell membrane is destructed, resulting in the efflux of K+ and the release of intracellular enzymes.8 In addition, a large number of liquid enters the cell, and the plasma membrane is separated from cytoskeleton, thereby causing cell swelling and rupture, finally ending up with cell lysis. The release of inflammatory mediators can further transduce these signals to their neighboring cells and more inflammatory cells are recruited to cause obvious inflammatory response.7 Therefore, the typical morphological changes of pyroptosis can be detected using scanning electron microscope (SEM). Moreover, the level of pyroptosis is comprehensively evaluated by detecting the enzyme activities of lactate dehydrogenase (LDH) and succinate dehydrogenase, mRNA level and protein levels of related factors, and enzyme activities of caspases and gasdermin members, as well as via calcein AM/propidium iodide (PI) double staining.9
As the early bony vertebrates, teleosts have extremely diverse species, live in diverse water environments (ocean and freshwater), and possess various life history strategies, morphological characteristics, and migration behaviors.10 Unlike the land where mammals live, the water environment in which fish live is more conducive to the survival of bacteria and other microorganisms and the exposure to diverse environmental pollutants.11,12 Therefore, pyroptosis induced by pathogenic infection is more common in fish. Under environmental selection pressure, fish evolved some characteristics to adapt to various, even extreme conditions, including pyroptosis characteristics that are not easy to study in mammals.13 Currently, in addition to zebrafish (Danio rerio),14 the studies on pyroptosis in fish are performed on tongue sole (Cynoglossus semilaevis),15 carp (Cyprinus carpio),16 Japanese flounder (Paralichthys olivaceus),17 and turbot (Scophthalmus maximus).18 Several key regulatory proteins of pyroptosis were identified in these fish species. In this article, we summarized current research progress of pyroptosis in fish by analogizing that in mammals.
Pyroptosis in Mammals
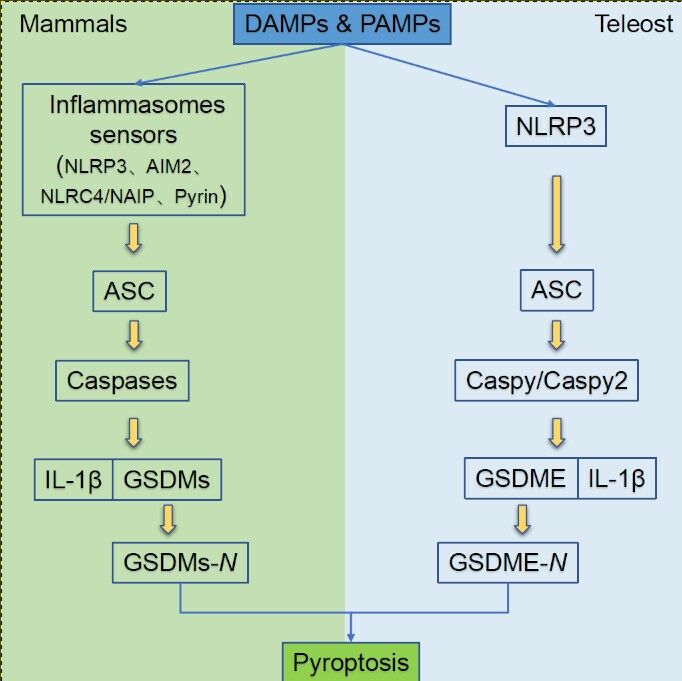
Gasdermin family proteins are the main executors of pyroptosis. Gasdermin A (GSDMA) was firstly named for its specific expression in the gastrointestinal tract and skin epithelium of mice,19 and the N-terminal domain of gasdermin proteins shares a high similarity with that of DFNA5, an earlier identified human hearing loss-related protein.20 According to gene structure similarity, 6 gasdermin family members have been identified in humans, namely, gasdermin A (GSDMA), gasdermin B (GSDMB), gasdermin C (GSDMC), gasdermin D (GSDMD), gasdermin E (GSDME, DFNA5), and PJVK (DFNB59).21 GSDMB is not expressed in mice, but mice have 3 GSDMA homologs (GSDMA1–3) and 4 GSDMC homologs (GSDMC1–4).22 Excepting DFNB59, all gasdermins contain a highly conserved N-terminal (NT) domain and a C-terminal (CT) domain,8 which are also known as pore-forming domain (PFD) and solubilizing inhibitory domain (SID), respectively.23 GSDMD and GSDME are the two most important proteins in the gasdermin family related to pyroptosis, and the PFD produced by cleaved GSDMD is responsible for pyroptosis, while SID inhibits the cytotoxicity of PFD under the binding state. In mammals, the activation of pyroptosis pyroptosis can occur in four manners, including classical pathway, non-classical pathway, caspase-3/8-mediated pathway, and granzyme-mediated pathway (Figure 1A).
 |
Figure 1 The pyroptosis pathway in mammals (A) and teleost (B). (A) Pyroptosis in mammals can generally be divided into four pathways.21,32,36 In the classical pathway, PAMPs and DAMPs can be recognized by the PRRs in the cell membrane to induce NLRP3 which further activate ASC and pro-caspase-1 through PYD-PYD and CARD-CARD interaction. Activated caspase-1 can cleave GSDMD to release its NT-domain which can oligomerize to induce perforation in the cell membrane and also activate IL-1β and IL-18 which can release via the cell pore. In the non-classical pathway, caspase-4/5 (human) and −11 (mouse) can be activated after directly binding to cytoplasmic LPS. The activated caspase-4/5 and −11 can cleave GSDMD to release its NT-domain and form the micropores in cell membrane. Caspase-4/5 do not have the same ability to activate IL-1β and IL-18 as caspase-1, but caspase-11 can promote the mature of pro-IL-1β and IL-18 by NLRP3. In the caspase-3/8-mediated pathway, caspase-8 can be activated by TNF-α and other apoptotic signals to activate caspase-3, which can cleave GSDMD to release its NT-domain to form pores in cell membrane and induce pyroptosis. In the Granzyme-mediated pathway, GzmA derived from lymphocytes can cleave the Lys229/Lys244 site of GSDMB to induce cell perforation, meanwhile, GzmB released by CAR T cells can activate the caspase-3/GSDME pathway to induce pyroptosis.(B) Pyroptosis in teleost are executed by GSDME and can be divided into two ways according to the ASC-dependence or not.15,37 In the ASC-dependent pathway, the activated NLRP3 inflammasome by DAMPs or PAMPs can recruit ASC through the PYD-PYD interaction, which can further recruit caspy and then caspy2 through the CARD-CARD interaction. The activated caspy/caspy2 is responsible for the cleavage of GSDMEa/b and the mature of IL-1β. In the ASC-independent pathway, the activated NLRP3 can directly bind with caspy2 through PYD-PYD interaction, and the activated caspy2 can cleave GSDMEa/b to release its NT-domain and form pores in the cell membrane. |
Classical Pathway
In the classical pathway, pyroptosis is executed by the cleavage of GSDMD, regulated by caspase-1 and multiple inflammasomes. Pathogen-/damage-related molecular patterns can be recognized by pattern recognition receptors (PRRs) on the cell surface,24 which are then assembled with pro-caspase-1 and ASC into different inflammasomes such as nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome, nod-like receptor family containing a caspase activation and recruitment domain 4 (NLRC4) inflammasome, absent in melanoma 2 (AIM2) inflammasome, and pyrin inflammasome.25 NLRCs contain the leucine-rich repeat (LRR), NACHT, and caspase activating and recruitment domain (CARD), whereas the LRR, NACHT and pyrin domain (PYD) domain exist in NLRPs.26 ASC also contains PYD and CARD domain, and thus it can be recruited by NLRPs via PYD-PYD domain interaction, and ASC can further recruit and activate pro-caspase-1 through CARD domain.27 NLRCs contain CARD domain, and thus they can directly recruit and activate pro-caspase-1 independent of ASC. After being activated, caspase-1 is hydrolyzed into two fragments to form a dimer, namely, the mature cleaved caspase-1. Mature caspase-1 can cleave GSDMD at Asp275 site to form a CT domain (22kDa) and a NT domain (31kDa).28 The GSDMD-NT domain can oligomerize to form micropores with an inner diameter of ~10–14 nm in the cell membrane, causing cell swelling and final pyroptosis. The activated caspase-1 can also cleave the precursors of IL-1β and IL-18 into active forms, which are released to the outside of cells through the micropores to recruit more inflammatory cells, thus expanding inflammatory response.8
Non-Classical Pathway
Caspase-4/5 in humans and caspase-11 in mice are responsible for the non-classical pyroptosis pathway, which directly bind to cytoplasmic LPS through the CARD domain,24 thus triggering their oligomerization and activation. Like caspase-1, the activated caspase-4/5 and −11 also have the activity to cleave GSDMD, and GSDMD-NT domain can oligomerize to induce perforation in the cell membrane.22 Caspase-4/5 and −11 cannot cleave pro-IL-1β or pro-IL-18, but one previous study has revealed that the activated caspase-11 can induce low-level secretion of IL-1β in a NLRP3/caspase-1-dependent manner.29 In addition, the micropores formed by GSDMD-NT domain after the cleveage of caspase-4/5 and −11 lead to the efflux of K+, thus inducing the assembly of NLRP3 inflammasomes, finally resulting in pyroptosis.30 Therefore, the inflammatory response in the non-classical pyroptosis pathway is NLRP3-dependent. Taken together, the non-canonical pyroptosis is the main response of caspase-4/5/11 after recognizing cytoplasmic LPS, and such recognition can induce NLRP3-mediated IL-1β/18 secretion in some cell subpopulations expressing NLRP3.
Caspase-3/8-Mediated Pathway
Early studies suggested that apoptosis initiator caspases such as caspase-8 and apoptosis executor caspases such as caspase-3 cannot cleave gasdermin members or induce pyroptosis. However, recent studies have found that some chemo-therapeutics can cleave GSDME via caspase-3 to induce the pyroptosis of tumor cells.31 Caspase-3 can cleave GSDME and release its complete NT domain which can oligomerize to form cell pores. One study reported that apoptosis caused by the activated caspase-3 during TNF-α induction could be converted into pyroptosis when the inflammatory caspase cleavage site of GSDMD was mutated to a special site of GSDME.31 This indicates that GSDME can be specifically cleaved by caspase-3, causing pyroptosis which is faster than apoptosis. In mouse macrophage infected by Yersinia pestis, YopJ protein has been found to inhibit TAK1 and induce caspase-8-mediated GSDMD cleavage.32 In addition, antibiotic chemotherapy drugs can trigger caspase-8/GSDMC-mediated pyroptosis in breast cancer cells.33
Granzyme-Mediated Pathway
In 2020, Liu et al firstly reported that chimeric antigen receptor (CAR) T cells could rapidly activate caspase-3 in target cells through release of granzyme B. The latter cleaves gasdermin E (GSDME), a pore-forming protein highly expressed in B leukemic and other target cells, which results in extensive pyroptosis.34 GzmB is also reported to directly cleave GSDME and induce cell death, thereby activating the anti-tumor immune responses to inhibit tumor growth.35 GSDMB is highly expressed in certain tissues, especially in the epithelium of the digestive tract, including epithelium-derived tumors. Natural killer cells and cytotoxic T lymphocytes (CTL) can kill virus-infected or transformed cells or grafted tissues through pyroptosis pathway, in which GzmA derived from lymphocytes mediates the cleavage of GSDMB at Lys229/Lys244 site. Therefore, GzmA can hydrolyze GSDMB protein at non-aspartic acid sites and induce cell perforations, indicating that pyroptosis can not only be activated by caspases.36
Pyroptosis in Fish
Pyroptosis research in fish has gradually increased in recent years. Similar to that in mammals, pyroptosis in fish is initiated with the activation of inflammasomes, which can further recruit and activate caspases. The activated inflammatory caspases will cleave gasdermin E (GSDME), which is the sole gasdermin family protein in fish, to induce pyroptosis (Figure 1B).
Inflammasomes in Fish
Several inflammasomes have been identified in different fish species, including the NLRP3 inflammasomes in zebrafish,37 tongue sole,15 carp,16 turbot,18 and Japanese flounder (Figure 2A),17 and different NLRCs inflammasomes in zebrafish,38,39 goldfish,40 rainbow trout,41 Japanese flounder,42,43 and channel catfish (Ictalurus punctatus).44,45 The existing studies on fish pyroptosis mostly focused on the NLRP3-dependent pathway. The analogs of mammalian AIM2 and pyrin inflammasomes have not been identified in fish yet, and genomic studies have reported that genes encoding these two proteins appear after the differentiation of tetrapod from fish, thus these two inflammasomes may not exist in fish.46 Importantly, ASC, one important component of inflammasomes, has also been identified in several fish species (Figure 2B). ASC spot complexes can be detected in zebrafish keratinocytes and macrophages during the occurrence of pyroptosis.47,48 In addition to zebrafish, the gene encoding ASC has also been reported in other fish species, such as mandarin fish (Siniperca chuatsi),49 Japanese flounder,17 and turbot.18 According to the ASC-dependence or not, fish pyroptosis can be divided into ASC-dependent NLRP3-activated pyroptosis and ASC-independent one. The ASC-dependent NLRP3-activated fish pyroptosis is similar to that in mammals, since both recruit caspases through PYD-PYD interaction between NLRP3 and ASC. NLRP3 and ASC could firstly recruit and activate caspase-a (89.92% structural similarity to ASC), and then recruit and activate caspase-b (55.46% structural similarity to ASC) in zebrafish, to execute GSDME cleavage.37 Meanwhile, caspases can promote the maturation of IL-1β in an ASC-dependent manner, and mature IL-1β are released via the cell micropores to induce inflammatory response. The ASC-independent NLRP3-activated fish pyroptosis relies on the direct binding of NLRP3 with caspase-b through the PYD-PYD interaction, thus cleaving GSDMEa/b to induce pyroptosis.37
 |
Figure 2 Phylogenetic trees of genes involved in pyroptosis including GSDMD and GSDME (A), caspases (B), NLRP3 (C), ASC (D) in representative teleost and mammals. |
Caspases in Fish
The activated inflammasomes can further recruit and activate caspases in fish, and gene sequences of multiple caspases (caspase-1, −2, −3, −8, −9, −10, −20, and others) were identified in various teleost species, such as zebrafish,50,51 Atlantic salmon (Salmo salar),52 sea bass (Dicentrarchus labrax),53,54 medaka (Oryzias latipes),55 large yellow croaker (Pseudosciaena crocea),56 rock bream (Oplegnathus fasciatus),57 tongue sole,58 murrel (Channa striatus),59 and rainbow trout (Oncorhynchus mykiss) (Figure 2C).60 Moreover, duplicated isoforms are identified in several fish caspases such as caspase-3a/3b, caspase-8a/8b, and caspase-6a/6b/6c in zebrafish,61 caspase-2a/2b, caspase-3/3a1/3a2/3b, caspase-9a/9b, caspase-10a/10b, and caspase-20a/20b in rainbow trout.60 Caspases in fish can generally be divided into three subgroups: apoptosis initiator caspases (caspase-2, −8, −9, −10, and −20), apoptosis executor caspases (caspase-3, −6 and −7), and inflammatory caspases (caspase-a (caspase-1, caspy) and caspase-b (caspase-19a, caspy2)). Caspase-a/caspase-b in zebrafish are reported to be homologous analogues of caspase-1 and caspase-4/5/11 in mammals,14,62 which could be activated by NLRP3/ASC inflammasome to cleave GSDME.37 The constructed turbot caspase plasmid exhibits proteolytic enzyme activity similar to mammalian caspase-5, and this plasmid can cleave turbot GSDME-b.18 Caspase-a can also promote pyroptosis of Japanese flounder after activation.17 Recently, new inflammatory caspases have been identified, including caspase-19b and caspase-23 in zebrafish,61 and caspase-1a and caspase-1b in rainbow trout.60 With the discovery of more functions of caspases, the previous subdivision of caspases has become obsolete. For example, the caspase-3 cleavage site is found in zebrafish GSDMEa rather than in GSDMEb.63 In tongue sole, GSDME is mainly cleaved by caspase-a, but lowly cleaved by caspase-3 and caspase-7, which is different from the complete cleavage of human GSDME by caspase-3.15
Gasdermin E in Fish
Pyroptosis in fish is also executed by gasdermin family protein, which can be cleaved by the above-mentioned inflammatory caspases. Till now, only one gasdermin family protein, GSDME, has been identified in teleosts including zebrafish, tongue sole, turbot, and Japanese flounder (Figure 2D); however, there could be subtypes of GSDME in teleosts, for example, two homologous genes (GSDME-a and GSDME-b) exist in zebrafish.31 Similar to gasdermins in mammals, GSDME in fish also contains a highly conserved NT domain and a CT domain and its cleaved GSDME-NT domain also has pore-forming function to induce pyroptosis.15 In humans, GSDME has been identified to be associated with non-syndromic deafness,20 which defect has been further related to the pyroptosis.31 In zebrafish, GSDMEb deletion also causes semicircular canal malformations in its inner ear,64 suggesting that fish GSDME shares functions similar to mammalian GSDME. Evolutionarily, the origin of GSDME can be traced back to coelenterata phylum such as corals, sea anemones, and jellyfish.65 Interestingly, coelenterata GSDME shares similar gene structure to vertebrate GSDME and DFNB59, and even exhibits more intimate relationship with vertebrate DFNB59.66 Therefore, GSDME is highly conserved during evolution and has similar functions in executing pyroptosis in both invertebrates (such as coelenterates) and vertebrates (such as fish and mammals).
Currently, fish pyroptosis is investigated mainly under pathogenic microorganism infection and environmental pollutants exposure. A typical NLRP3/ASC inflammasome complex has been detected in zebrafish embryos, and this inflammasome triggers caspase-a and caspase-b activation under bacterial infection and promotes the maturation of IL-1β, finally leading to pyroptosis.37 The NLRP3 inflammasome in the head kidney macrophages of turbot was activated after Edwardsiella piscicida infection to recruit a large amount of caspases, thus promoting the release of IL-1β into the blood.18 The NLRP3 inflammasome was also detected in the head kidney phagocytic cells of Japanese flounder after Edwardsiella piscicida infection, and a model of NLRP3-mediated caspase-a activation and IL-1β maturation was successfully established.17 In addition to pathogenic microorganism infection, diverse environmental pollutants have been also reported to induce fish pyroptosis.16,67 The studies on the lymphocytes in head kidneys and spleens of carp under cadmium treatment showed that cadmium could activate NLRP3 inflammasome to induce pyroptosis of these lymphocytes, but the inhibition of NLRP3 activity reduced the cadmium-induced lymphocyte pyroptosis.16 Moreover, in vitro study confirmed the role of chlorpyrifos in triggering epithelioma papulosum cyprini cell pyroptosis via miR-124-3p/CAPN1 axis.67
Conclusion
Similar to that in mammals, pyroptosis in fish is also activated by caspases through NLRP3 inflammasome to cleave GSDME and release its NT domain, and GSDME-NT domain can oligomerize to cause perforation in the cell membrane, in accompany with a large number of pro-inflammatory mediator release. Current research on the mechanism of fish pyroptosis is far from enough, and most studies only focus on the induction by pathogenic microorganism infection. However, the water environment where fish lives is affected by multiple factors, and few studies about the regulation of fish pyroptosis under these different environmental conditions have been reported. Additionally, the identified pyroptosis pathways in fish are mainly all dependent on NLRP3 inflammasomes. Future studies are suggested to reveal the role of other identified inflammasomes such as NLRCs in fish pyroptosis. The effects of caspases on GSDMEa and GSDMEb proteins are reported to differentiate in different fish species, but the specific activation mechanism remains to be further investigated. Finally, granzyme was recently found to hydrolyze GSDMD and induce pyroptosis in mammals. Whether granzyme can induce pyroptosis by activating GSDME in fish and if yes, whether such pyroptosis induction is caspase-dependent or caspase-independent will be interesting topics. The exploration of these questions is of great significance for revealing the characteristics of pyroptosis from evolutionary perspective.
Acknowledgments
This article was funded by National Natural Science Foundation of China (Grant No. 32172996, 31802317).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zychlinsky A, Prevost MC, Sansonetti PJ. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358(6382):167–169. doi:10.1038/358167a0
2. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther. 2021;6(1):128. doi:10.1038/s41392-021-00507-5
3. D’Souza CA, Heitman J. Dismantling the Cryptococcus coat. Trends Microbiol. 2001;9(3):112–113. doi:10.1016/s0966-842x(00)01945-4
4. Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265(1):130–142. doi:10.1111/imr.12287
5. Song L, Pei L, Yao S, Wu Y, Shang Y. NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci. 2017;11:63. doi:10.3389/fncel.2017.00063
6. Pezuk JA. Pyroptosis in combinatorial treatment to improve cancer patients’ outcome, is that what we want? EBioMedicine. 2019;41:17–18. doi:10.1016/j.ebiom.2019.03.007
7. Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: toward identification of the effector molecules. Science. 2016;352(6281):aaf2154. doi:10.1126/science.aaf2154
8. Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–665. doi:10.1038/nature15514
9. Zheng Z, Bian Y, Zhang Y, Ren G, Li G. Metformin activates AMPK/SIRT1/NF-κB pathway and induces mitochondrial dysfunction to drive caspase3/GSDME-mediated cancer cell pyroptosis. Cell Cycle. 2020;19(10):1089–1104. doi:10.1080/15384101.2020.1743911
10. Fricke R, Eschmeyer W, Laan R. Eschmeyer’s catalog of fishes: genera, species, references. 2019.
11. Cossins AR, Crawford DL. Opinion - fish as models for environmental genomics. Nat Rev Genet. 2005;6(4):324–333. doi:10.1038/nrg1590
12. Li Z, Ali Shah SW, Zhou Q, Yin X, Teng X. The contributions of miR-25-3p, oxidative stress, and heat shock protein in a complex mechanism of autophagy caused by pollutant cadmium in common carp (Cyprinus carpio L.) hepatopancreas. Environ Pollut. 2021;287:117554. doi:10.1016/j.envpol.2021.117554
13. Krumschnabel G, Podrabsky JE. Fish as model systems for the study of vertebrate apoptosis. Apoptosis. 2009;14(1):1–21. doi:10.1007/s10495-008-0281-y
14. Wen Y, Chen S, Jiang Z, et al. Dysregulated haemolysin promotes bacterial outer membrane vesicles-induced pyroptotic-like cell death in zebrafish. Cell Microbiol. 2019;21(6):e13010. doi:10.1111/cmi.13010
15. Jiang S, Gu H, Zhao Y, Sun L. Teleost gasdermin e is cleaved by caspase 1, 3, and 7 and induces pyroptosis. J Immunol. 2019;203(5):1369–1382. doi:10.4049/jimmunol.1900383
16. Zhang Y, Liu Q, Yin H, Li S. Cadmium exposure induces pyroptosis of lymphocytes in carp pronephros and spleens by activating NLRP3. Ecotoxicol Environ Saf. 2020;202:110903. doi:10.1016/j.ecoenv.2020.110903
17. Chen H, Ding S, Tan J, Yang D, Zhang Y, Liu Q. Characterization of the Japanese flounder NLRP3 inflammasome in restricting Edwardsiella piscicida colonization in vivo. Fish Shellfish Immunol. 2020;103:169–180. doi:10.1016/j.fsi.2020.04.063
18. Chen S, Jin P, Chen H, et al. Dual function of a turbot inflammatory caspase in mediating both canonical and non-canonical inflammasome activation. Dev Comp Immunol. 2021;121:104078. doi:10.1016/j.dci.2021.104078
19. Saeki N, Kuwahara Y, Sasaki H, Satoh H, Shiroishi T. Gasdermin (GSDM) localizing to mouse Chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm Genome. 2000;11(9):718–724. doi:10.1007/s003350010138
20. Van Laer L, Huizing EH, Verstreken M, et al. Nonsyndromic hearing impairment is associated with a mutation in DFNA5. Nat Genet. 1998;20(2):194–197. doi:10.1038/2503
21. Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157. doi:10.1038/s41577-019-0228-2
22. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
23. Sborgi L, Rühl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35(16):1766–1778. doi:10.15252/embj.201694696
24. Shi J, Zhao Y, Wang Y, et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514(7521):187–192. doi:10.1038/nature13683
25. Zhao Y, Shao F. Diverse mechanisms for inflammasome sensing of cytosolic bacteria and bacterial virulence. Curr Opin Microbiol. 2016;29:37–42. doi:10.1016/j.mib.2015.10.003
26. Ye Z, Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol. 2008;20(1):3–9. doi:10.1016/j.coi.2008.01.003
27. Dick MS, Sborgi L, Ruhl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2016;7(1):11929. doi:10.1038/ncomms11929
28. Miao EA, Leaf IA, Treuting PM, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11(12):1136–1142. doi:10.1038/ni.1960
29. Li P, Allen H, Banerjee S, et al. Mice deficient in IL-1 beta-converting enzyme are defective in production of mature IL-1 beta and resistant to endotoxic shock. Cell. 1995;80(3):401–411. doi:10.1016/0092-8674(95)90490-5
30. Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K+ efflux is the common trigger of nlrp3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi:10.1016/j.immuni.2013.05.016
31. Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547(7661):99–103. doi:10.1038/nature22393
32. Orning P, Weng D, Starheim K, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362(6418):1064–1069. doi:10.1126/science.aau2818
33. Hou J, Zhao R, Xia W, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol. 2020;22(10):1264–1275. doi:10.1038/s41556-020-0575-z
34. Liu Y, Fang Y, Chen X, et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol. 2020;5(43). doi:10.1126/sciimmunol.aax7969
35. Zhang Z, Zhang Y, Lieberman J. Lighting a fire: can we harness pyroptosis to ignite antitumor immunity? Cancer Immunol Res. 2021;9(1):2–7. doi:10.1158/2326-6066.Cir-20-0525
36. Zhou Z, He H, Wang K, et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science. 2020;368:6494. doi:10.1126/science.aaz7548
37. Li JY, Wang YY, Shao T, et al. The zebrafish NLRP3 inflammasome has functional roles in ASC-dependent interleukin-1β maturation and gasdermin E-mediated pyroptosis. J Biol Chem. 2020;295(4):1120–1141. doi:10.1074/jbc.RA119.011751
38. Fang H, Wu XM, Hu YW, Song YJ, Zhang J, Chang MX. NLRC3-like 1 inhibits NOD1-RIPK2 pathway via targeting RIPK2. Dev Comp Immunol. 2020;112:103769. doi:10.1016/j.dci.2020.103769
39. Hu YW, Yu ZL, Xue NN, Nie P, Chang MX. Expression and protective role of two novel NACHT-containing proteins in pathogen infection. Dev Comp Immunol. 2014;46(2):323–332. doi:10.1016/j.dci.2014.05.007
40. Xie J, Hodgkinson JW, Katzenback BA, Kovacevic N, Belosevic M. Characterization of three Nod-like receptors and their role in antimicrobial responses of goldfish (Carassius auratus L.) macrophages to Aeromonas salmonicida and Mycobacterium marinum. Dev Comp Immunol. 2013;39(3):180–187. doi:10.1016/j.dci.2012.11.005
41. Chang M, Wang T, Nie P, Zou J, Secombes CJ. Cloning of two rainbow trout nucleotide-binding oligomerization domain containing 2 (NOD2) splice variants and functional characterization of the NOD2 effector domains. Fish Shellfish Immunol. 2011;30(1):118–127. doi:10.1016/j.fsi.2010.09.014
42. Unajak S, Santos MD, Hikima J, et al. Molecular characterization, expression and functional analysis of a nuclear oligomerization domain proteins subfamily C (NLRC) in Japanese flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2011;31(2):202–211. doi:10.1016/j.fsi.2011.05.007
43. Park SB, Hikima J, Suzuki Y, et al. Molecular cloning and functional analysis of nucleotide-binding oligomerization domain 1 (NOD1) in olive flounder, Paralichthys olivaceus. Dev Comp Immunol. 2012;36(4):680–687. doi:10.1016/j.dci.2011.11.007
44. Rajendran KV, Zhang J, Liu S, et al. Pathogen recognition receptors in channel catfish: I. Identification, phylogeny and expression of NOD-like receptors. Dev Comp Immunol. 2012;37(1):77–86. doi:10.1016/j.dci.2011.12.005
45. Li M, Wang QL, Lu Y, Chen SL, Li Q, Sha ZX. Expression profiles of NODs in channel catfish (Ictalurus punctatus) after infection with Edwardsiella tarda, Aeromonas hydrophila, Streptococcus iniae and channel catfish hemorrhage reovirus. Fish Shellfish Immunol. 2012;33(4):1033–1041. doi:10.1016/j.fsi.2012.06.033
46. Howe K, Schiffer PH, Zielinski J, et al. Structure and evolutionary history of a large family of NLR proteins in the zebrafish. Open Biol. 2016;6(4):160009. doi:10.1098/rsob.160009
47. Kuri P, Schieber NL, Thumberger T, Wittbrodt J, Schwab Y, Leptin M. Dynamics of in vivo ASC speck formation. J Cell Biol. 2017;216(9):2891–2909. doi:10.1083/jcb.201703103
48. Vincent WJ, Freisinger CM, Lam PY, Huttenlocher A, Sauer JD. Macrophages mediate flagellin induced inflammasome activation and host defense in zebrafish. Cell Microbiol. 2016;18(4):591–604. doi:10.1111/cmi.12536
49. Sun Y, Wang J, Lao H, et al. Molecular cloning and expression analysis of the ASC gene from mandarin fish and its regulation of NF-kappaB activation. Dev Comp Immunol. 2008;32(4):391–399. doi:10.1016/j.dci.2007.07.006
50. Masumoto J, Zhou W, Chen FF, et al. Caspy, a zebrafish caspase, activated by ASC oligomerization is required for pharyngeal arch development. J Biol Chem. 2003;278(6):4268–4276. doi:10.1074/jbc.M203944200
51. Negron JF, Lockshin RA. Activation of apoptosis and caspase-3 in zebrafish early gastrulae. Dev Dyn. 2004;231(1):161–170. doi:10.1002/dvdy.20124
52. Takle H, McLeod A, Andersen O. Cloning and characterization of the executioner caspases 3, 6, 7 and Hsp70 in hyperthermic Atlantic salmon (Salmo salar) embryos. Comp Biochem Physiol B Biochem Mol Biol. 2006;144(2):188–198. doi:10.1016/j.cbpb.2006.02.006
53. Reis MI, Do Vale A, Pereira PJ, Azevedo JE, Dos Santos NM. Caspase-1 and IL-1beta processing in a teleost fish. PLoS One. 2012;7(11):e50450. doi:10.1371/journal.pone.0050450
54. Reis MI, Nascimento DS, Do Vale A, Silva MT, Dos Santos NM. Molecular cloning and characterisation of sea bass (Dicentrarchus labrax L.) caspase-3 gene. Mol Immunol. 2007;44(5):774–783. doi:10.1016/j.molimm.2006.04.028
55. Iijima N, Yokoyama T. Apoptosis in the medaka embryo in the early developmental stage. Acta Histochem Cytochem. 2007;40(1):1–7. doi:10.1267/ahc.06013
56. Li M, Ding Y, Mu Y, Ao J, Chen X. Molecular cloning and characterization of caspase-3 in large yellow croaker (Pseudosciaena crocea). Fish Shellfish Immunol. 2011;30(3):910–916. doi:10.1016/j.fsi.2011.01.018
57. Elvitigala DA, Whang I, Premachandra HK, et al. Caspase 3 from rock bream (Oplegnathus fasciatus): genomic characterization and transcriptional profiling upon bacterial and viral inductions. Fish Shellfish Immunol. 2012;33(1):99–110. doi:10.1016/j.fsi.2012.04.008
58. Long H, Sun L. Molecular characterization reveals involvement of four caspases in the antibacterial immunity of tongue sole (Cynoglossus semilaevis). Fish Shellfish Immunol. 2016;57:340–349. doi:10.1016/j.fsi.2016.08.047
59. Kumaresan V, Ravichandran G, Nizam F, et al. Multifunctional murrel caspase 1, 2, 3, 8 and 9: conservation, uniqueness and their pathogen-induced expression pattern. Fish Shellfish Immunol. 2016;49:493–504. doi:10.1016/j.fsi.2016.01.008
60. Zeng C, Hou ZS, Zhao HK, et al. Identification and characterization of caspases genes in rainbow trout (Oncorhynchus mykiss) and their expression profiles after Aeromonas salmonicida and Vibrio anguillarum infection. Dev Comp Immunol. 2021;118:103987. doi:10.1016/j.dci.2020.103987
61. Spead O, Verreet T, Donelson CJ, Poulain FE. Characterization of the caspase family in zebrafish. PLoS One. 2018;13(5):e0197966. doi:10.1371/journal.pone.0197966
62. Li Y, Li Y, Cao X, Jin X, Jin T. Pattern recognition receptors in zebrafish provide functional and evolutionary insight into innate immune signaling pathways. Cell Mol Immunol. 2017;14(1):80–89. doi:10.1038/cmi.2016.50
63. Rogers C, Fernandes-Alnemri T, Mayes L, Alnemri D, Cingolani G, Alnemri ES. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat Commun. 2017;8:14128. doi:10.1038/ncomms14128
64. Busch-Nentwich E, Sollner C, Roehl H, Nicolson T. The deafness gene dfna5 is crucial for ugdh expression and HA production in the developing ear in zebrafish. Development. 2004;131(4):943–951. doi:10.1242/dev.00961
65. Zhang Z, Lieberman J. Lighting a fire on the reef. Sci Immunol. 2020;5(54). doi:10.1126/sciimmunol.abf0905
66. Jiang S, Zhou Z, Sun Y, Zhang T, Sun L. Coral gasdermin triggers pyroptosis. Sci Immunol. 2020;5(54). doi:10.1126/sciimmunol.abd2591
67. Miao Z, Miao Z, Teng X, Xu S. Chlorpyrifos triggers epithelioma papulosum cyprini cell pyroptosis via miR-124-3p/CAPN1 axis. J Hazard Mater. 2022;424(PtA):127318. doi:10.1016/j.jhazmat.2021.127318
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
