Back to Journals » Infection and Drug Resistance » Volume 13
A Clinical Review and Critical Evaluation of Imipenem-Relebactam: Evidence to Date
Authors Campanella TA , Gallagher JC
Received 7 July 2020
Accepted for publication 3 November 2020
Published 25 November 2020 Volume 2020:13 Pages 4297—4308
DOI https://doi.org/10.2147/IDR.S224228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sahil Khanna
Toni A Campanella,1 Jason C Gallagher2
1Department of Pharmacy, Jefferson Health Northeast, Philadelphia, PA, USA; 2Department of Pharmacy Practice, Temple University, Philadelphia, PA, USA
Correspondence: Jason C Gallagher
Temple University School of Pharmacy, 3307 N. Broad Street, Philadelphia, PA 19140, USA
Tel +1 215-707-2573
Fax +1 215-707-8326
Email [email protected]
Abstract: Imipenem-relebactam (I-R) is a novel beta-lactam/beta-lactamase inhibitor combination given with cilastatin. It is indicated for the treatment of complicated urinary tract infections, complicated intra-abdominal infections, and hospital-acquired or ventilator-associated bacterial pneumonia. A literature search was completed to evaluate the evidence to date of I-R. I-R has in vitro activity against multidrug-resistant organisms including carbapenem-resistant Pseudomonas aeruginosa and extended-spectrum beta-lactamase and carbapenem-resistant Enterobacterales. It was granted FDA approval following the promising results of two phase II clinical trials in patients with complicated urinary tract infections and complicated intra-abdominal infections. The most common adverse drug events associated with I-R were nausea (6%), diarrhea (6%), and headache (4%). I-R is a new beta-lactam/beta-lactamase inhibitor combination that will be most likely used for patients with multidrug-resistant gram-negative infections in which there are limited or no available alternative treatment options.
Keywords: MK-7655, carbapenem-resistant Enterobacterales, extended-spectrum beta-lactamase, multidrug-resistant Pseudomonas
Introduction
The increasing rate of infections due to multidrug-resistant organisms is a critical public health issue and has led to serious therapeutic challenges. The Centers for Disease Control and Prevention has named carbapenem-resistant Acinetobacter and carbapenem-resistant Enterobacterales as urgent threats to public health and extended spectrum beta-lactamase-producing (ESBL) Enterobacterales and multidrug-resistant Pseudomonas aeruginosa as serious threats to public health.1 Infections due to multidrug-resistant bacteria has been associated with significant morbidity and mortality.2,3
Carbapenems are beta-lactam antibiotics that have maintained activity against pathogens many resistance mechanisms. Carbapenems are stable against many beta-lactamases including AmpC-producing beta-lactamases and ESBLs, but not carbapenemases.4 Unsurprisingly, there has been emerging resistance to carbapenems. These mechanisms of resistance include production of other beta-lactamases, decreased cell membrane permeability, and overexpression of efflux pumps.5
In order to overcome resistance among gram-negative bacteria, relebactam was combined with imipenem-cilastatin to create a novel beta-lactam/beta-lactamase inhibitor combination. Relebactam, a novel non-beta-lactam beta-lactamase inhibitor, maintains activity against a range of beta-lactamases. It is structurally related to the beta-lactamase inhibitor avibactam and has in vitro activity against class A and class B beta-lactamases.6–8 I-R was FDA-approved in July 2019 for the treatment of complicated intra-abdominal infections (cIAIs) and complicated urinary tract infections (cUTIs) in patients 18 years of older in which there are limited or no alternative treatment options available. I-R was also FDA-approved in June 2020 for the treatment of hospital-acquired and ventilator-associated bacterial pneumonia in patients 18 years of older in which there are limited or no alternative treatment options available. This article will review the evidence to date of I-R, including results from in vitro studies, clinical efficacy, and safety and tolerability.
Materials and Methods
A PubMed search was conducted for data using MeSH terms relebactam, MK-7655, and imipenem-relebactam. An internet search was conducted for unpublished clinical research. The literature search was limited to English-language studies that described clinical efficacy, safety, pharmacodynamics, and pharmacokinetics. There were 122 results identified and screened up until March 2020 and 60 of them were excluded (see Figure 1).
Results
History of Beta-Lactam/Beta-Lactamase Inhibitor Combinations
Beta-lactam antibiotics have been a mainstay of treatment for a multitude of infectious diseases. Beta-lactamase inhibitors (BLIs) were developed as a strategy to restore activity of beta-lactam antibiotics due to the production of beta-lactamases, a common mechanism of bacterial resistance. Beta-lactamase enzymes attack the amide bond of beta-lactams resulting in acylation at the carbonyl moiety rendering beta-lactam antibiotics ineffective. Beta-lactamases are most commonly organized by the Ambler classification, which is based on overall protein structure and amino acid sequences. The Ambler classification includes class A enzymes (eg, KPCs, ESBLs), class B metallo-beta-lactamases (eg, NDM, IMP), class C enzymes (AmpC-type), and class D enzymes (OXA type). Beta-lactamases have been reviewed extensively elsewhere in the literature.9–11
Similar to penicillins and cephalosporins, carbapenems possess a beta-lactam ring but also contain an alpha-hydroxyethyl side chain. Imipenem was the first available carbapenem antibiotic. In the kidney, the beta-lactam ring is hydrolyzed by the dehydropeptidase-1 (DHP-1) enzyme, causing necrosis of the proximal convoluted tubule of the kidney at high doses.12 The addition of cilastatin, a DHP-1 inhibitor, inhibits the renal metabolism of imipenem, increasing concentrations and decreasing toxicity. Imipenem has a broad spectrum of activity including against Gram-positive, Gram-negative, and anaerobic bacteria.13 Imipenem is inactive in vitro against Enterococcus faecium, Stenotrophomonas maltophilia, methicillin-resistant strains of Staphylococcus aureus (MRSA), and some isolates of Burkholderia cepacia.14 All carbapenems have MIC90 values ≤ 1 against most isolated Gram-positive aerobic bacteria, excluding Enterococcus spp. and MRSA.4 Imipenem demonstrates slightly lower MICs against Enterococcus faecalis.4 Although still susceptible, the MICs for imipenem are higher against Gram-negative bacteria including Enterobacterales and Haemophilus influenzae.4 Both demonstrate similar activity against anaerobic bacteria.
Resistance to carbapenems is due to either carbapenemase enzymes or a combination of mechanisms including the production of other beta-lactamases, decreased cell membrane permeability, and overexpression of efflux pumps. P. aeruginosa expresses resistance to imipenem via loss of outer membrane porin proteins (OprD) and increased AmpC production, whereas P. aeruginosa resistance to meropenem is a result of membrane impermeability and overexpression of efflux pumps.5
Most beta-lactamase inhibitors have minimal antibacterial activity alone, but when administered with a beta-lactam antibiotic they bind to beta-lactamases and protect the active antibiotic. In the United States, there are six available BLIs including clavulanate, sulbactam, tazobactam, avibactam, vaborbactam, and relebactam. Each differs in its level of inhibition against various enzymes. Relebactam, formerly known as MK-7655, is a non-beta-lactam, bicyclic diazabicyclooctane beta-lactamase inhibitor similar to avibactam with the addition of a piperidine ring at C2.1–13,15
In-vitro Activity
Enterobacterales
There are numerous studies in which I-R has been evaluated against Enterobacterales (Table 1).5–7,16–28 The FDA-approved and EUCAST breakpoints are represented in Table 2. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) are the two leading organizations that set standards for antimicrobial susceptibility testing. Major differences include their respective definitions for intermediate susceptible and susceptible dose dependent.30 Susceptibility rates for Klebsiella pneumoniae, Escherichia coli, Citrobacter spp., and Enterobacter spp. are above 95%. Serratia marcescens and Proteus mirabilis are less susceptible overall at 87% and 66%, respectively. Carbapenem-resistant Enterobacterales exhibit variable susceptibility. For KPC-producing K. pneumoniae, specifically KPC-2 and KPC-3 type, I-R is active against 100% of isolates in several studies.16,20,31 I-R demonstrated a lack of activity when evaluated against K. pneumoniae expressing OXA-48 type carbapenemases, therefore relebactam did not restore susceptibility of these K. pneumoniae strains.20,31,32 The MIC of imipenem for four isolates of K. pneumoniae expressing OXA-48, no ESBL, wild-type porins was 4 µg/mL compared to I-R which was 2 µg/mL.20 Of 20 isolates expressing OXA-48 type, 17 isolates (15%) were susceptible to I-R, compared to 90% susceptibility to ceftazidime-avibactam.31 Additionally, I-R lacks activity against class B metallo-beta-lactamases, including VIM, IMP, and NDM.22,23
Pseudomonas aeruginosa
There are several in vitro studies that evaluated activity of I-R against Pseudomonas aeruginosa (Table 1).5–8,17,21,22,33–37 Rates of susceptibility were approximately 90% for all P. aeruginosa isolates tested. Relebactam restored susceptibility to imipenem to 80% of imipenem-non-susceptible isolates of P. aeruginosa. P. aeruginosa demonstrates resistance to imipenem via downregulation of porin protein synthesis in combination with AmpC overproduction. Relebactam inhibits AmpC, therefore lowering the MIC and improving imipenem activity against P. aeruginosa.38 The MIC90 of I-R (2 mg/L) was decreased four-fold compared to imipenem alone in two studies (16 mg/L).6,7 Only 5% of P. aeruginosa isolates remained non-susceptible to I-R, which were found to contain MBL or GES carbapenemases.
Relebactam has the greatest impact on carbapenem-resistant P. aeruginosa that downregulate OprD porin expression and upregulate AmpC beta-lactamase production. Amikacin was the only agent that demonstrated similar in vitro activity to I-R with 95% active compared to the other agents tested which were less than 80% active. One unique study evaluated combination therapy of I-R with colistin and amikacin for 10 isolates of P. aeruginosa.35 By 24 hours, additivity or synergy was observed in 7 of the isolates with the combination of I-R and amikacin. The 3 isolates that did not demonstrate synergy were highly resistant to amikacin with MICs ≥64 µg/mL. The combination of I-R with colistin was synergistic at 24 hours in 8 of 10 isolates.
Acinetobacter Spp
Against Acinetobacter baumannii isolates, relebactam did not increase susceptibility to imipenem.7,17,21 Rates of resistance were more than 30% and imipenem MICs with and without relebactam were similar.
Anaerobes
Similar to imipenem alone, I-R is very active against anaerobic bacteria with a resistance rate of approximately 0.7% (Table 1).39,40 The addition of relebactam did not enhance activity of imipenem alone against anaerobic bacteria. In vitro studies of I-R demonstrated activity against the following Gram-negative anaerobic genera: Bacteroides, Prevotella, Fusobacterium, Parabacteroides, Porphyromonas, Bilophila, Desulfovibrio, and Veillonella and the following Gram-positive anaerobic genera: Eggerthelila, Actinomyces, Eubacterium, Flavonifractor, Mogibacterium, Slackia, Solobacterium, and Clostridioides.
Pharmacokinetics
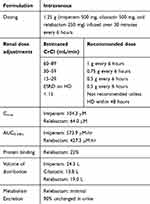
Characteristics of I-R are presented in Table 3. I-R is dosed intravenously (IV) 1.25 g, consisting of 500 mg of imipenem, 500 mg of cilastatin, and 250 mg of relebactam, administered over 30 minutes every 6 hours. Following a single-dose of 250 mg of relebactam, the concentration at the end of infusion is 45.4 ± 7.27 μM and the area under the concentration-time curve from zero hours to infinity (AUC0-∝) is 81.8 ± 9.07 μM*hr.41 The mean AUC0-∝ and maximum concentration of drug in serum increased at a dose-proportional relationship. The half-life of relebactam is similar to that of imipenem at 1.1 hours. Protein binding of relebactam is 22% and the apparent volume of distribution at terminal phase of relebactam is 20.8 ± 2.77 liters. The area under the concentration-time curve from zero to infinity in epithelial lining fluid relative to that in plasma for relebactam and imipenem is 54% and 55%, respectively.42
Relebactam is minimally metabolized and imipenem is metabolized in the kidneys by dehydropeptidase-1, which cilastatin inhibits. The mean percentage of a single dose of relebactam was excreted unchanged in the urine ranging from 94.7% to 100%.41 Utilizing population pharmacokinetics models, the most significant covariate identified for the clearance of both imipenem and relebactam is renal function by estimated creatinine clearance.43
Given that relebactam is predominately eliminated renally, Chan and colleagues sought out to identify the transporters involved in the renal elimination of relebactam and therefore identify possible drug-drug interactions (DDIs).44 Relebactam is shown to be a substrate of organic anion transporter (OAT) 3, OAT4, and multidrug and toxin extrusion (MATE) proteins MATE1 and MATE2K. Relebactam is not an inhibitor of major drug transporters, including OATP1B1, OATP1B3, OAT1, OAT3, organic cation transporter 2, MATE1, MATE2K, or breast cancer resistance protein. These in vitro data suggest that relebactam is safe to administer with medications that are substrates or inhibitors of major drug transporters. Bhagunde and colleagues administered I-R with the OAT inhibitor probenecid and found no clinically meaningful changes in plasma exposure or renal clearance of imipenem or relebactam identified, suggesting a lack of clinically relevant interactions with OAT inhibitors.43
Clinical Trials
Complicated Intra-Abdominal Infections
I-R received FDA approval for complicated intra-abdominal infections (cIAIs) following the favorable outcomes of a prospective, multicenter, double-blind, randomized controlled phase 2 trial (Table 4).45 The objective of the study was to evaluate the safety, tolerability, and efficacy of I-R compared to imipenem 500 mg alone in the treatment of cIAI. Patients were randomized 1:1:1 to receive relebactam 250 mg plus imipenem 500 mg, relebactam 125 mg plus imipenem 500 mg, or imipenem 500 mg alone intravenously over 30 minutes every 6 hours. The primary efficacy endpoint was the proportion of patients in the microbiologically evaluable (ME) population who achieved a favorable clinical response at discontinuation of IV therapy. Favorable clinical response was defined as the resolution of all or most presenting signs and symptoms of intra-abdominal infection without the need for additional antibiotic therapy. Patients included were 18 years of age or older with clinically suspected or bacteriologically documented cIAI requiring hospitalization and treatment with IV antibiotic therapy with a minimum duration of therapy for at least 96 hours.
There were 118 patients randomized into the 500–250 mg I-R group, 116 patients in the 500–125 mg I-R group, and 117 patients in the imipenem only group. The most common diagnosis was complicated appendicitis and the most common pathogens identified at baseline were E. coli (171 isolates), K. pneumoniae (38 isolates), and P. aeruginosa (37 isolates). There were 277 patients in the microbiological intention-to-treat (MITT) population and 255 patients in the ME population. Rates of favorable clinical response at the discontinuation of IV therapy in the ME population was 96.3%, 98.8%, and 95.2% in the 500–250 mg I-R group, 500–125 mg I-R group, and imipenem alone group, respectively (p < 0.001). Interestingly, there were 34 patients in the ME group at the discontinuation of IV therapy visit with imipenem-resistant isolates who demonstrated favorable clinical response. Though they met the primary endpoint, this outcome may be attributed to adequate surgical intervention as relebactam did not seem to restore susceptibility in vitro for most of the imipenem-resistant isolates. Rates of drug-related adverse events were similar among all treatment groups at 13.7%, 13.8%, and 9.6% in the 500–250 mg I-R group, 125 mg I-R group, and imipenem alone group, respectively. The most common adverse events in all three treatment groups were diarrhea, nausea, and vomiting. These results suggest both doses of I-R are well tolerated and noninferior to imipenem alone in the treatment of cIAI.
Urinary Tract Infections
I-R was also studied in a significant prospective, randomized, double-blind, multicenter, dose-ranging phase 2 study for the treatment of patients with complicated urinary tract infections (cUTIs) or acute pyelonephritis.46 The objective of this study was to assess the safety, tolerability, and efficacy of 500–250 mg of I-R, 500–125 mg of I-R, and imipenem 500 mg alone. The primary endpoint was favorable clinical response at discontinuation of IV therapy in the ME population. Patients 18 years of age and older were included if they were diagnosed with clinically suspected and/or bacteriologically documented cUTI or acute pyelonephritis, requiring hospitalization and IV antibiotic therapy.
Two hundred and thirty patients were included in the ME population at the discontinuation of IV therapy with 71 patients in the 500–250 mg I-R group, 79 patients in the 500–125 mg I-R group, and 80 patients in the imipenem alone group. Rates of favorable clinical response at the discontinuation of IV therapy was similar across all three treatment groups at 97.1%, 98.7%, and 98.8% in the 500–250 mg I-R group, 500–125 mg I-R group, and imipenem alone group, respectively. Drug-related adverse events occurred similarly among groups with nausea, headache, and diarrhea being the most common. These data suggest that both dosing regimens of I-R studied are noninferior to imipenem alone in the treatment of cUTI with a similar safety profile.
Infections Caused by Resistant Pathogens
The RESTORE-IMI 1 trial was a randomized, controlled, double-blind phase 3 trial that compared I-R to imipenem-cilastatin plus colistin (IMI+CST) in 47 adults with carbapenem-nonsusceptible cIAIs, cUTIs, HAP, and VAP.47 Patients were considered for inclusion if they required hospitalization and intravenous antibiotics. Important exclusion criteria included those with Acute Physiology and Chronic Health Evaluation II (APACHE II) score >30, creatine clearance (CrCl) <15 mL/min, requiring hemodialysis or peritoneal dialysis, prior colistin therapy, or concomitant systemic or inhaled agents active against Enterobacterales, Pseudomonas spp., and gram-negative anaerobic bacilli. Patients were randomized 2:1 to receive 500–250 mg I-R every 6 hours or 500 mg of imipenem every 6 hours plus 300 mg loading dose of colistin followed by 150 mg colistin every 12 hours for a duration of 5–21 days. The primary efficacy endpoint of favorable overall response was 71.4% and 70% in the modified microbiologic intent-to-treat I-R and IMI+CST groups, respectively. This consisted of patients who received at least one dose of the study drug and had a culture with at least one carbapenem-nonsusceptible organism. The most commonly isolated organism was P. aeruginosa. Day 28 all-cause mortality occurred in 2 of 21 patients in the I-R group compared to 3 of 10 patients in the IMI+CST group. Although this was a 20% lower mortality rate in the I-R group, it was not statistically significant. Serious adverse events occurred more frequently in the IMI+CST group at 31.3% compared to the I-R group at 9.7%. Treatment-emergent nephrotoxicity occurred at a rate of 10% in the I-R group and 56% in the IMI+CST group (p = 0.002). The results of RESTORE-IMI 1 support the conclusion that I-R is an effective and well-tolerated option for the treatment of carbapenem-nonsusceptible infections.
Hospital-Acquired Bacterial Pneumonia and Ventilator-Associated Bacterial Pneumonia (HABP/VABP)
RESTORE-IMI 2 (ClinicalTrials.gov identifier NCT02493764) was a randomized, double-blind, multicenter clinical trial that compared I-R to piperacillin-tazobactam (TZP) in patients with hospital-acquired bacterial pneumonia (HABP) and ventilator-associated bacterial pneumonia (VABP) in the intensive care unit. Preliminary data is available, but it is not yet published.48,49 Patients were considered for inclusion if they required intravenous antibiotics for HABP or VABP, had a lower respiratory tract specimen within 48 hours of screening, and fulfilled the following three diagnostic criteria with an onset of 48 hours after starting mechanical ventilation or hospitalization or within 7 days of hospital discharge: at least 1 clinical feature (eg, new-onset or worsening signs or symptoms, hypoxemia), at least 1 of the following signs including fever or hypothermia, white blood cell count ≥10,000 cells/mm3 or ≤4500 cells/mm3, or >15% immature neutrophils, and a chest radiograph showing ≥1 new or progressive infiltrate suggestive of bacterial pneumonia. Key exclusion criteria included patients with >24 hours of effective antibacterial therapy for the current HABP or VABP episode and CrCl <15 mL/min. Patients were randomized 1:1 to I-R 500 mg-250 mg or TZP 4.5 g, dose-adjusted based on renal function and given as 30 minutes intravenously every 6 hours for 7 to 14 days. Linezolid could be used empirically until confirmation of the presence or absence of methicillin-resistant Staphylococcus aureus. Other adjunctive antibacterial therapies were not permitted. The primary endpoint was day 28 all-cause mortality in the modified intent-to-treat (MITT) population, which included all randomized patients who received at least one dose of the study drug and a Gram stain that did not show only gram-positive cocci. The key secondary endpoint was favorable clinical response in the MITT population at early follow-up visit at 7 to 14 days after the last dose of study drug.
There were 535 patients that received at least one dose of the study drug and 531 patients were included in the MITT population; 264 patients in the I-R group and 267 in the TZP group. The most commonly isolated organism was Klebsiella spp. in the I-R group and polymicrobial in the TZP group. In a subgroup analysis of patients with VABP, the most commonly isolated organisms were Acinetobacter calcoaceticus-baumannii complex and polymicrobial. The majority of patients in the study population were ≥65 years old, in the intensive care unit, and APACHE II scores ≥15. I-R demonstrated noninferiority to TZP for day 28 all-cause mortality at 15.9% in the I-R group vs 21.3% with TZP. For the key secondary endpoint, 61% of patients in the I-R group compared to 55.8% of patients in the TZP group demonstrated favorable clinical response at early follow-up.
Safety
Warnings and precautions of I-R are similar to those associated with other carbapenem antibiotics. Although not observed in those receiving I-R, adverse reactions may include seizures, states of confusion, and myoclonus due to imipenem.13 It is unknown if relebactam alone exhibits specific adverse events. Concomitant use with valproic acid formulations can significantly decrease valproic acid concentrations, possibly leading to seizures.13,50 Ganciclovir should not be used concomitantly with I-R as generalized seizures have been reported with its use with imipenem. The most commonly reported adverse events the two phase 2 clinical trials that occurred at a rate of ≥3% were diarrhea, nausea, vomiting, headaches, elevated alanine aminotransferases, and elevated aspartate aminotransferases.45,46 In the RESTORE-IMI 1 trial, drug-related adverse events that were reported included decreased creatinine clearance, hyperglycemia, infusion site erythema, and pyrexia.47 In the RESTORE-IMI 2 trial, the most frequently reported drug-related adverse events were diarrhea, increased alanine aminotransferase, and increased aspartate aminotransferase.49
Place in Therapy
The novel beta-lactam/beta-lactamases inhibitor combinations have overlapping spectra and utility, but there are differences between them. Costs and prudent prescribing practices currently prohibit any of these drugs from “workhorse” roles, leaving them to serve niches of resistant bacteria. The role of I-R is primarily against carbapenem-resistant strains of Pseudomonas aeruginosa and Enterobacterales. It has a spectrum of activity for resistant Gram-negative organisms that is most similar to ceftazidime-avibactam, but may be active against some Klebsiella pneumoniae and Pseudomonas aeruginosa that are resistant to this drug. However, ceftazidime-avibactam is the only combination with activity against class D OXA-48 type producing organisms.31 Ceftolozane-tazobactam is a combination that is also active against multidrug-resistant Pseudomonas aeruginosa isolates and has been used to treat these infections successfully.51 Ceftolozane-tazobactam resistance occurs and it will be interesting to see how often I-R is active against these isolates.52
Meropenem-vaborbactam has attractive activity and data for CRE infections, but vaborbactam has only minor effects on the activity of meropenem in carbapenem-resistant P. aeruginosa.53,54 None of the available beta-lactam/beta-lactamase inhibitor combinations alone are active against MBL-producing Enterobacterales. Aztreonam is not hydrolyzed by MBLs, but aztreonam is hydrolyzed by the beta-lactamases that are often co-produced with MBLs.55 To overcome these mechanisms of resistance, a combination of aztreonam and beta-lactamase/beta-lactamase inhibitors has been explored. This combination of aztreonam and ceftazidime-avibactam has been shown to be highly synergistic against NDM-producing Enterobacterales.56,57 It is unknown whether I-R in combination with aztreonam would exhibit activity against MBL-producing Enterobacterales.
Compared to other beta-lactam/beta-lactamase inhibitor combinations, dosing frequency of I-R is more frequent than other antibiotics discussed such as meropenem-vaborbactam and ceftazidime-avibactam. Although dosing frequency is more often, the duration of infusion of I-R is only 30 minutes compared to two hours with meropenem-vaborbactam.13,58 The imipenem-backbone of I-R has an attendant risk of seizures compared to other beta-lactam/beta-lactamase inhibitor combinations.
There are multiple novel beta-lactamase inhibitors that are under development and have been described elsewhere in the literature. These include nacubactam (formerly RG6080 and OP05095), zidebactam (formerly WXK5107) taniborbactam (formerly VNRX-5133), enmetazobactam (formerly AAI-101), durlobactam (formerly ETX-2514), and QPX-7728.59–64 These may represent future alternative treatment options given the increase in resistant gram-negative infections.
Conclusion
The in vitro and clinical data of I-R support its role in the treatment of various multidrug-resistant gram-negative infections. It has activity against multidrug-resistant P. aeruginosa and carbapenem-resistant Enterobacterales that possess class A carbapenemases. I-R is not useful for infections due to pathogens with class B metallo-beta-lactamases or class D OXA-48 type carbapenemases. Clinical data suggests I-R is well tolerated. I-R is an alternative agent for the treatment of gram-negative resistant infections.
Disclosure
Toni A. Campanella reports no conflicts of interest in this work.
Jason C. Gallagher has been a member of the speaker’s bureau, advisory boards, and is a recipient of unrestricted educational grants from Merck, Inc, reports grants and personal fees from Merck, and personal fees from Achaogen, Melinta, Nabriva, Paratek, Qpex, Shionogi, Spero, and Tetraphase, outside the submitted work, and reports no other potential conflicts of interest for this work.
References
1. Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); 2019.
2. Bogan C, Kaye KS, Chopra T, et al. Outcomes of carbapenem-resistant Enterobacteriaceae isolation: matched analysis. Am J Infect Control. 2014;42(6):612–620. doi:10.1016/j.ajic.2014.02.013
3. Martin A, Fahrbach K, Zhao Q, Lodise T. Association between carbapenem resistance and mortality among adult, hospitalized patients with serious infections due to Enterobacteriaceae: results of a systematic literature review and meta-analysis. Open Forum Infect Dis. 2018;5(7). doi:10.1093/ofid/ofy150
4. Zhanel GG, Wiebe R, Dilay L, et al. Comparative review of the carbapenems. Drugs. 2007;67(7):1027–1052. doi:10.2165/00003495-200767070-00006
5. Hirsch EB, Ledesma KR, Chang K-T, Schwartz MS, Motyl MR, Tam VH. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant gram-negative bacteria. Antimicrob Agents Chemother. 2012;56(7):3753–3757. doi:10.1128/AAC.05927-11
6. Karlowsky JA, Lob SH, Kazmierczak KM, Young K, Motyl MR, Sahm DF. In vitro activity of imipenem-relebactam against clinical isolates of gram-negative bacilli isolated in hospital laboratories in the United States as part of the SMART 2016 program. Antimicrob Agents Chemother. 2018;62(7). doi:10.1128/AAC.00169-18
7. Karlowsky JA, Lob SH, Kazmierczak KM, et al. In vitro activity of imipenem/relebactam against gram-negative ESKAPE pathogens isolated in 17 European countries: 2015 SMART surveillance programme. J Antimicrob Chemother. 2018;73(7):1872–1879. doi:10.1093/jac/dky107
8. Lob SH, Hackel MA, Kazmierczak KM, et al. In vitro activity of imipenem-relebactam against gram-negative ESKAPE pathogens isolated by clinical laboratories in the United States in 2015 (Results from the SMART global surveillance program). Antimicrob Agents Chemother. 2017;61(6). doi:10.1128/AAC.02209-16
9. Bush K. Past and present perspectives on β-lactamases. Antimicrob Agents Chemother. 2018;62(10):e01076. doi:10.1128/AAC.01076-18
10. Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for beta-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39(6):1211–1233. doi:10.1128/AAC.39.6.1211
11. Crass RL, Pai MP. Pharmacokinetics and pharmacodynamics of β-lactamase inhibitors. Pharmacotherapy. 2019;39(2):182–195. doi:10.1002/phar.2210
12. McLeod DC, Lyon JA. Imipenem/cilastatin: the first carbapenem antibiotic. Drug Intell Clin Pharm. 1985;19(12):894–899. doi:10.1177/106002808501901202
13. Recarbrio (imipenem/cilastatin/relebactam) [package insert]. Whitehouse Station, NJ: Merck & Co Inc; July 2019.
14. Zhanel GG, Simor AE, Vercaigne L, Mandell L. Imipenem and meropenem: comparison of in vitro activity, pharmacokinetics, clinical trials and adverse effects. Can J Infect Dis. 1998;9(4):215–228.
15. Tooke CL, Hinchliffe P, Lang PA, et al. Molecular basis of class A β-lactamase inhibition by relebactam. Antimicrob Agents Chemother. 2019;63(10):e00564. doi:10.1128/AAC.00564-19
16. Papp-Wallace KM, Barnes MD, Alsop J, et al. Relebactam is a potent inhibitor of the KPC-2 β-lactamase and restores imipenem susceptibility in KPC-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(6). doi:10.1128/AAC.00174-18
17. Lob SH, Karlowsky JA, Young K, et al. In vitro activity of imipenem-relebactam against resistant phenotypes of Enterobacteriaceae and Pseudomonas aeruginosa isolated from intraabdominal and urinary tract infection samples - SMART surveillance Europe 2015–2017. J Med Microbiol. 2020;69(2):207–217. doi:10.1099/jmm.0.001142
18. El Hafi B, Rasheed SS, Abou Fayad AG, Araj GF, Matar GM. Evaluating the efficacies of carbapenem/β-lactamase inhibitors against carbapenem-resistant gram-negative bacteria in vitro and in vivo. Front Microbiol. 2019;10. doi:10.3389/fmicb.2019.00933.
19. Gomez-Simmonds A, Stump S, Giddins MJ, Annavajhala MK, Uhlemann A-C. Clonal background, resistance gene profile, and porin gene mutations modulate in vitro susceptibility to imipenem-relebactam in diverse Enterobacteriaceae. Antimicrob Agents Chemother. 2018;62(8). doi:10.1128/AAC.00573-18
20. Haidar G, Clancy CJ, Chen L, et al. Identifying spectra of activity and therapeutic niches for ceftazidime-avibactam and imipenem-relebactam against carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2017;61(9). doi:10.1128/AAC.00642-17
21. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of imipenem with relebactam against gram-negative pathogens from New York City. Antimicrob Agents Chemother. 2015;59(8):5029–5031. doi:10.1128/AAC.00830-15
22. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;dkt178. doi:10.1093/jac/dkt178
23. Schmidt-Malan SM, Mishra AJ, Mushtaq A, Brinkman CL, Patel R. In vitro activity of imipenem-relebactam and ceftolozane-tazobactam against resistant gram-negative bacilli. Antimicrob Agents Chemother. 2018;62(8). doi:10.1128/AAC.00533-18
24. Biagi M, Shajee A, Vialichka A, Jurkovic M, Tan X, Wenzler E. Activity of imipenem-relebactam and meropenem-vaborbactam against carbapenem-resistant, SME-producing serratia marcescens. Antimicrob Agents Chemother. 2020;64(4). doi:10.1128/AAC.02255-19
25. Johnston BD, Thuras P, Porter SB, et al. Activity of imipenem/relebactam against carbapenem-resistant Escherichia coli isolates from the United States in relation to clonal background, resistance genes, co-resistance, and region. Antimicrob Agents Chemother. 2020. doi:10.1128/AAC.02408-19
26. Kulengowski B, Burgess DS. Imipenem/relebactam activity compared to other antimicrobials against non-MBL-producing carbapenem-resistant Enterobacteriaceae from an academic medical center. Pathog Dis. 2019;77(4):ftz040. doi:10.1093/femspd/ftz040
27. Carpenter J, Neidig N, Campbell A, et al. Activity of imipenem/relebactam against carbapenemase-producing Enterobacteriaceae with high colistin resistance. J Antimicrob Chemother. 2019;74(11):3260–3263. doi:10.1093/jac/dkz354
28. Balabanian G, Rose M, Manning N, Landman D, Quale J. Effect of porins and blaKPC expression on activity of imipenem with relebactam in Klebsiella pneumoniae: can antibiotic combinations overcome resistance? Microb Drug Resist. 2018;24(7):877–881. doi:10.1089/mdr.2018.0065
29. Senchyna F, Gaur RL, Sandlund J, et al. Diversity of resistance mechanisms in carbapenem-resistant Enterobacteriaceae at a health care system in Northern California, from 2013 to 2016. Diagn Microbiol Infect Dis. 2019;93(3):250–257. doi:10.1016/j.diagmicrobio.2018.10.004
30. Kahlmeter G, Giske CG, Kirn TJ, Sharp SE. Point-counterpoint: differences between the European committee on antimicrobial susceptibility testing and clinical and laboratory standards institute recommendations for reporting antimicrobial susceptibility results. Caliendo AM ed. J Clin Microbiol. 2019;57(9):e01129. doi:10.1128/JCM.01129-19
31. Canver MC, Satlin MJ, Westblade LF, et al. Activity of imipenem-relebactam and comparator agents against genetically characterized isolates of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother. 2019;63(9). doi:10.1128/AAC.00672-19
32. Galani I, Nafplioti K, Adamou P, Karaiskos I, Giamarellou H, Antoniadou A. In vitro activity of imipenem-relebactam against non-MBL carbapenemase-producing Klebsiella pneumoniae isolated in Greek hospitals in 2015–2016. Eur J Clin Microbiol Infect Dis. 2019;38(6):1143–1150. doi:10.1007/s10096-019-03517-y
33. Barnes MD, Bethel CR, Alsop J, et al. Inactivation of the pseudomonas-derived cephalosporinase-3 (PDC-3) by relebactam. Antimicrob Agents Chemother. 2018;62(5):e02406–17. doi:10.1128/AAC.02406-17
34. Horner C, Mushtaq S, Livermore DM, et al. Potentiation of imipenem by relebactam for Pseudomonas aeruginosa from bacteraemia and respiratory infections. J Antimicrob Chemother. 2019;74(7):1940–1944. doi:10.1093/jac/dkz133
35. Asempa TE, Nicolau DP, Kuti JL. In vitro activity of imipenem-relebactam alone or in combination with amikacin or colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63(9). doi:10.1128/AAC.00997-19
36. Asempa TE, Nicolau DP, Kuti JL. Carbapenem-nonsusceptible Pseudomonas aeruginosa isolates from intensive care units in the United States: a potential role for new β-lactam combination agents. J Clin Microbiol. 2019;57(8). doi:10.1128/JCM.00535-19
37. Fraile-Ribot PA, Zamorano L, Orellana R, et al. Activity of imipenem-relebactam against a large collection of Pseudomonas aeruginosa clinical isolates and isogenic β-lactam-resistant mutants. Antimicrob Agents Chemother. 2020;64(2). doi:10.1128/AAC.02165-19
38. Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. CMR. 2010;23(1):160–201. doi:10.1128/CMR.00037-09
39. Snydman DR, Jacobus NV, McDermott LA. In vitro evaluation of the activity of imipenem-relebactam against 451 recent clinical isolates of bacteroides group and related species. Antimicrob Agents Chemother. 2016;60(10):6393–6397. doi:10.1128/AAC.01125-16
40. Goldstein EJC, Citron DM, Tyrrell KL, Leoncio E, Merriam CV. Comparative in vitro activities of relebactam, imipenem, the combination of the two, and six comparator antimicrobial agents against 432 strains of anaerobic organisms, including imipenem-resistant strains. Antimicrob Agents Chemother. 2018;62(2). doi:10.1128/AAC.01992-17
41. Rhee EG, Rizk ML, Calder N, et al. Pharmacokinetics, safety, and tolerability of single and multiple doses of relebactam, a-lactamase inhibitor, in combination with imipenem and cilastatin in healthy participants. Antimicrob Agents Chemother. 2018;62(9):16.
42. Rizk ML, Rhee EG, Jumes PA, et al. Intrapulmonary pharmacokinetics of relebactam, a novel β-lactamase inhibitor, dosed in combination with imipenem-cilastatin in healthy subjects. Antimicrob Agents Chemother. 2018;62(3). doi:10.1128/AAC.01411-17
43. Bhagunde P, Patel P, Lala M, et al. Population pharmacokinetic analysis for imipenem–relebactam in healthy volunteers and patients with bacterial infections. CPT Pharmacometrics Syst Pharmacol. 2019;8(10):748–758. doi:10.1002/psp4.12462
44. Chan G, Houle R, Lin M, et al. Role of transporters in the disposition of a novel β-lactamase inhibitor: relebactam (MK-7655). J Antimicrob Chemother. 2019;74(7):1894–1903. doi:10.1093/jac/dkz101
45. Lucasti C, Vasile L, Sandesc D, et al. Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother. 2016;60(10):6234–6243. doi:10.1128/AAC.00633-16
46. Sims M, Mariyanovski V, McLeroth P, et al. Prospective, randomized, double-blind, phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother. 2017;72(9):2616–2626. doi:10.1093/jac/dkx139
47. Motsch J, Murta de Oliveira C, Stus V, et al. RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis. 2019. doi:10.1093/cid/ciz530
48. Roquilly A, Titov I, Rodriguez Gonzalez D, et al. Outcomes in ventilated patients with hospital-acquired/ventilator-associated bacterial pneumonia (HABP/VABP) treated with imipenem/cilastatin/relebactam vs piperacillin/tazobactam: subgroup analysis of the RESTORE-IMI 2 randomized, controlled trial.
49. Titov I, Wunderink R, Roquilly A, et al. RESTORE-IMI 2: randomized, double-blind, phase 3 trial comparing efficacy and safety of imipenem/cilastatin/relebactam vs piperacillin/tazobactam in adult patients with hospital-acquired or ventilator-associated bacterial pneumonia (HABP/VABP).
50. Mori H, Takahashi K, Mizutani T. Interaction between valproic acid and carbapenem antibiotics. Drug Metab Rev. 2007;39(4):647–657. doi:10.1080/03602530701690341
51. Gallagher JC, Satlin MJ, Elabor A, et al. Ceftolozane-tazobactam for the treatment of multidrug-resistant Pseudomonas aeruginosa infections: a multicenter study. Open Forum Infect Dis. 2018;5(11):ofy280. doi:10.1093/ofid/ofy280
52. Tamma PD, Beisken S, Bergman Y, et al. Modifiable risk factors for the emergence of ceftolozane-tazobactam resistance. Clin Infect Dis. 2020. doi:10.1093/cid/ciaa1306
53. Zhanel GG, Lawrence CK, Adam H, et al. Imipenem–relebactam and meropenem–vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs. 2018;78(1):65–98. doi:10.1007/s40265-017-0851-9
54. Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against gram-negative clinical isolates in New York City. Antimicrob Agents Chemother. 2015;59(8):4856–4860. doi:10.1128/AAC.00843-15
55. Shields RK, Doi Y. Aztreonam combination therapy: an answer to metallo-β-lactamase–producing gram-negative bacteria? Clin Infect Dis. 2019;ciz1159. doi:10.1093/cid/ciz1159
56. Shaw E, Rombauts A, Tubau F, et al. Clinical outcomes after combination treatment with ceftazidime/avibactam and aztreonam for NDM-1/OXA-48/CTX-M-15-producing Klebsiella pneumoniae infection. J Antimicrob Chemother. 2018;73(4):1104–1106. doi:10.1093/jac/dkx496
57. Marshall S, Hujer AM, Rojas LJ, et al. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61(4):e02243–16. doi:10.1128/AAC.02243-16
58. Vabomere (meropenem and vaborbactam) [package insert]. Lincolnshire, IL: Melinta Therapeutics Inc; 2017.
59. Vena A, Castaldo N, Bassetti M. The role of new β-lactamase inhibitors in gram-negative infections. Curr Opin Infect Dis. 2019;32(6):638–646. doi:10.1097/QCO.0000000000000600
60. Wang X, Zhao C, Wang Q, et al. In vitro activity of the novel β-lactamase inhibitor taniborbactam (VNRX-5133), in combination with cefepime or meropenem, against MDR gram-negative bacterial isolates from China. J Antimicrob Chemother. 2020. doi:10.1093/jac/dkaa053
61. Hamrick JC, Docquier J-D, Uehara T, et al. VNRX-5133 (taniborbactam), a broad-spectrum inhibitor of serine- and metallo-β-lactamases, restores activity of cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020;64(3). doi:10.1128/AAC.01963-19
62. Tselepis L, Langley GW, Aboklaish AF, et al. In vitro efficacy of imipenem-relebactam and cefepime-AAI101 against a global collection of ESBL-positive and carbapenemase-producing Enterobacteriaceae. Int J Antimicrob Agents. 2020;56(1):105925. doi:10.1016/j.ijantimicag.2020.105925
63. Lomovskaya O, Nelson K, Rubio-Aparicio D, Tsivkovski R, Sun D, Dudley MN. Impact of intrinsic resistance mechanisms on potency of QPX7728, a new ultrabroad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases in Enterobacteriaceae, pseudomonas aeruginosa, and acinetobacter baumannii. Antimicrob Agents Chemother. 2020;64(6). doi:10.1128/AAC.00552-20
64. Tsivkovski R, Totrov M, Lomovskaya O. Biochemical characterization of QPX7728, a new ultra-broad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases. Antimicrob Agents Chemother. 2020;64(6). doi:10.1128/AAC.00130-20
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.