Back to Journals » Infection and Drug Resistance » Volume 17
A Case Report of Urinary Tract Infection and Fungemia Due to Pichia ohmeri Complicated with Pulmonary Thromboembolism
Authors Sun JJ, Shi R, Huang H
Received 27 September 2023
Accepted for publication 14 December 2023
Published 3 January 2024 Volume 2024:17 Pages 11—15
DOI https://doi.org/10.2147/IDR.S437788
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jiang-Jie Sun,1,2 Rui Shi,1,2 He Huang1,2
1Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Shenzhen University, Shenzhen, Guangdong Province, 518035, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Shenzhen Second People’s Hospital, Shenzhen, Guangdong Province, 518035, People’s Republic of China
Correspondence: He Huang, Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Shenzhen University, No. 3002 Sungang Road, Futian District, Shenzhen, Guangdong Province, 518035, People’s Republic of China, Email [email protected]
Abstract: Pichia ohmeri (P. ohmeri) is a rare human pathogen known to cause severe conditions such as sepsis, fungemia, endocarditis, and peritonitis, often resulting in high mortality rates. This report presents a case of a febrile 66-year-old male with a urinary tract infection and fungemia caused by P. ohmeri. The patient had additional complications of pulmonary thromboembolism (PTE) and deep venous thrombosis (DVT) in the left lower extremity. Notably, the pathogen was isolated in both urine and blood cultures, an uncommon finding in immunocompetent patients. Treatment with fluconazole was initiated based on the antifungal susceptibility testing (AFST) results. Following treatment, the patient experienced a gradual resolution of fever and urinary tract infection symptoms. Concurrently, anticoagulant therapy was administered for the management of PTE and DVT. The patient was eventually discharged in a stable condition.
Keywords: Pichia ohmeri, fungemia, urinary tract infection, pulmonary thromboembolism, deep venous thrombosis
Introduction
Pichia ohmeri (P. ohmeri) is an ascomycetous yeast that belongs to the Saccharomycetaceae family.1 It is a rare and emerging opportunistic pathogen. This pathogen was first isolated from pleural effusion in 1984 and was initially considered a contaminant. The first case of fungemia caused by P. ohmeri was reported in the United States in 1998.2 The number of reported cases of this pathogen has increased in recent years. Most infected patients have obvious risk factors, including diabetes, malignant tumors, and indwelling catheter. The mortality rate is high, but the appropriate treatment has not yet been determined.3 In this report, we present a case of urinary tract infection and fungemia caused by P. ohmeri, complicated with pulmonary thromboembolism (PTE) and left lower extremity deep venous thrombosis (DVT), successfully treated with fluconazole.
Case Report
A 66-year-old male patient was admitted to our hospital on June 23, 2021, with a complaint of fever lasting for 7 days. He experienced chills, sweating, and the highest recorded body temperature was 39 degrees Celsius. Additionally, he reported nausea and fatigue but no cough, dyspnea, or chest pain. The patient also presented with oliguria without frequent urination, urgency, or pain. Prior to admission, he had visited the emergency department of a local hospital, where he was diagnosed with a left upper ureteral calculus with left hydronephrosis and infection. Despite receiving imipenem/cilastatin, he continued to have recurrent fevers. A blood culture yielded positive results for fungal growth, and a chest CT scan showed multiple patchy shadows in both lungs, bilateral pleural effusion, and segmental atelectasis in the adjacent lung. Due to concerns about fungal infection leading to fungemia, the patient was transferred to our hospital for further diagnosis and treatment. The patient had a medical history of hypertension and type 2 diabetes mellitus, with poor blood glucose control while currently using insulin. Coarse breath sounds were detected in both lungs, with crackles in the left lung. No other significant findings were observed during the physical examination. An arterial blood gas analysis showed an oxygen partial pressure of 60.0 mmHg without hypercapnia or acidosis. Blood tests indicated an elevated white blood cell count and neutrophilia, along with high levels of inflammatory markers (Procalcitonin [PCT] level: 0.370 ng/mL [normal value <0.094ng/mL], hypersensitive C-reactive protein [hs-CRP]: 62.09 mg/L [normal value 0–5mg/L]). Urinalysis revealed positive leukocytes and glucose (4+). Glycated hemoglobin was 12.5% (normal value 4.2–6.2%), and D-dimer was 10.95 mg/L (normal value <0.55mg/L). Aspergillus galactomannan antigen was measured at 1.10 ug/L (normal value <0.65ug/L), and 1-3-β-D-glucan was 279.50 pg/mL (normal value <60pg/mL). Total IgE and cryptococcal antigen tests showed no abnormalities. Abdominal enhanced CT indicated the presence of multiple stones in both kidneys, a lower left ureteral calculus, and mild hydronephrosis of the upper left ureter and left kidney. Given the increased D-dimer and decreased oxygen partial pressure, pulmonary CT angiography was performed, revealing scattered multiple emboli in the right upper and lower pulmonary arteries (Figure 1), suggestive of pulmonary thromboembolism (PTE). A lower extremity venous ultrasound showed abnormal solid echoes in the left posterior tibial vein and one branch of the peroneal vein, indicating deep venous thrombosis (DVT).
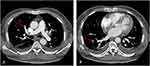 |
Figure 1 Pulmonary CT angiography of the patient. As the arrow showed scattered multiple emboli in the right upper pulmonary artery (A) and lower pulmonary artery (B). |
Initially, the patient received levofloxacin for empirical antibacterial therapy, enoxaparin for anticoagulation, as well as antihypertensive and antihyperglycemic treatments. However, he continued to experience intermittent low-grade fever, with body temperature fluctuating between 37.5 and 37.8 degrees Celsius. Then, laboratory reported isolation of P. ohmeri growth in both urine and blood cultures. The isolates were identified in laboratory via morphological (SabouraudDextrose agar) and biochemical methods (Vitek MS). The susceptibility to antifungal agents was tested using the ATB Fungus 3 system. The strain was found to be sensitive to fluconazole (MIC≤1mg/L) and voriconazole (MIC≤0.06mg/L) and itraconazole (MIC≤0.125 mg/L) and amphotericin B (MIC≤0.5mg/L). Therefore, a diagnosis of complicated upper urinary tract infection with fungemia was considered. Based on antifungal susceptibility testing (AFST) results, intravenous drip administration of fluconazole at a dose of 400 mg qd (600 mg qd on the first day) was initiated for antifungal treatment. The patient’s body temperature returned to normal two days later and did not relapse. There was no nausea, fatigue, dyspnea, or chest pain. His general condition became better, and his appetite gradually recovered. A repeat blood culture yielded no bacterial or fungal growth one week later, while blood leukocyte and neutrophil counts returned to normal levels. Result of PCT was 0.130 ng/mL, Hs-CRP 42.89 mg/L, D-dimer 1.16 mg/L, which were all decreased. A review of urine culture showed no bacterial or fungal growth after continuous fluconazole administration for 10 days. Two weeks after admission, the patient was discharged with a two-week oral fluconazole and rivaroxaban and continued antihypertensive and antihyperglycemic medications. Considering the rare pathogen infection and the presence of PTE, a screening test revealed mild abnormalities in ANA and anti-ScL-70 antibodies. The patient was advised to undergo regular follow-up visits with a rheumatologist. Subsequent follow-up treatment was conducted at the local hospital near the patient’s hometown.
Discussion
P. ohmeri is not a common clinical pathogen. In recent years, there has been a gradual increase in invasive fungal infections caused by P. ohmeri, including sepsis, catheter-related bloodstream infection, endocarditis, and peritonitis. Unfortunately, the mortality rate associated with these infections is high.3 In this particular case, the lower left ureteral calculus caused obstruction and infection in the patient, leading to further hematogenous dissemination. The microbiological analysis confirmed the presence of P. ohmeri as the causative pathogen, which is extremely rare. Generally, among fungi, yeasts other than Cryptococcus and Candida are considered to have weak pathogenicity. Infections caused by P. ohmeri are rare, and infection in immunocompetent individuals is even rarer.4 According to the literature, most patients infected by P. ohmeri have underlying conditions such as hematological diseases, solid malignant tumors, neutropenia after chemotherapy, immunosuppressive treatment, diabetes, or chronic renal failure.5 In this case, the patient had an upper urinary tract infection and hematogenous dissemination, which was considered to be related to poorly controlled long-term hyperglycemia in diabetics. Additionally, the patient had complications of PTE and DVT. There have been no previous reports of multi-site infections caused by P. ohmeri complicated with vascular thromboembolism (VTE). The formation of DVT is attributed to three major factors: venous endothelial injury, hypercoagulability, and venous stasis.6 Diabetes has serious adverse effects on various organ systems, inducing a transition from normal coagulation to hypercoagulability.7,8 An increasing body of evidence suggests that diabetic patients have an increased risk of arterial and venous diseases, including DVT and PTE.9,10 Infection has long been recognized as one of the risk factors for DVT, as established in the guidelines for the diagnosis and management of DVT and PTE.11 Therefore, it can be inferred that the DVT in this patient was related to diabetes and infection.
According to the literature, the prognosis of fluconazole treatment for P. ohmeri infection is usually poor, largely attributed to fluconazole resistance.3,12 In contrast, amphotericin B treatment has shown better response rates, while voriconazole, caspofungin, and micafungin have also demonstrated low minimum inhibitory concentration (MIC) values.5,13 However, in the case of our patient, based on the results of AFST, fluconazole was administered with a favorable outcome. Within 10 days, both blood and urine cultures became negative, and the white blood cell count, neutrophil count, PCT, hs-CRP, and D-dimers decreased to the normal range. The patient’s condition improved rapidly, attributed to the patient’s relatively mild illness and lack of previous exposure to triazole antifungal drugs.
In summary, P. ohmeri is a rare pathogen that often causes infections in individuals with high-risk factors and is prone to developing fungemia.3 However, due to the difficulty in defining the clinical breakpoints of antifungal drugs against P. ohmeri and the lack of prospective randomized controlled trials and evidence-based guidelines for treatment,14 it remains challenging to determine the most effective treatment approach.
Conclusion
This report described a rare invasive fungal infection case caused by P. ohmeri in an immunocompetent individual with the complications of PTE and DVT. In patients with infectious diseases of unknown origin and unidentified causative pathogens, early diagnosis is of great importance to guide individualized anti-infective treatment, control the infection, and improve overall outcomes. Identification of host factors and suspicious sites of infection through detailed medical history collection, immediate pathogen analysis, preferably before using anti-infective agents, can effectively improve early diagnosis. Meanwhile, attaching importance to the diagnostic significance of Aspergillus galactomannan antigen and 1-3-β-D-glucan, as well as the reasonable application of rapid molecular biology detection methods, such as metagenomic next-generation sequencing (mNGS), target next-generation sequencing (tNGS), are also of great help to early diagnosis. Furthermore, it is important to consider systematic invasive fungal infection and diabetes as significant risk factors for DVT and PTE, and these conditions should be screened cautiously, especially when a patient presents with unexplained hypoxemia.
Abbreviations
PTE, pulmonary thromboembolism; DVT, deep venous thrombosis; PCT, procalcitonin; hs-CRP, hypersensitive C-reactive protein; VTE, vascular thromboembolism; MIC, minimum inhibitory concentration.
Data Sharing Statement
All data generated or analysed during this study are included in this published article.
Ethics and Consent
This study was approved by the Ethics Committee at the Shenzhen Second People’s Hospital and complied with the principles of the Declaration of Helsinki (2023-075-01PJ). The patient provided written informed consent for the case details to be published.
Acknowledgments
We thank all the medical staff members involved in treating the patient.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Shenzhen Second People’s Hospital Clinical Research Fund of Shenzhen High-level Hospital Construction Project (20193357024).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chakrabarti A, Rudramurthy SM, Kale P, et al. Epidemiological study of a large cluster of fungaemia cases due to Kodamaea ohmeri in an Indian tertiary care centre. Clin Microbiol Infect. 2014;20(2):O83–O89. doi:10.1111/1469-0691.12337
2. Bergman MM, Gagnon D, Doern GV. Pichia ohmeri fungemia. Diagn Microbiol Infect Dis. 1998;30(3):229–231. doi:10.1016/S0732-8893(97)00233-2
3. Ioannou P, Papakitsou I. Kodamaea ohmeri infections in humans: a systematic review. Mycoses. 2020;63(7):636–643. doi:10.1111/myc.13094
4. Shaaban H, Choo HF, Boghossian J, Perez G. Kodamaea ohmeri fungemia in an immunocompetent patient treated with micafungin: case report and review of the literature. Mycopathologia. 2010;170(4):223–228. doi:10.1007/s11046-010-9315-4
5. Shang ST, Lin JC, Ho SJ, et al. The emerging life-threatening opportunistic fungal pathogen Kodamaea ohmeri: optimal treatment and literature review. J Microbiol Immunol Infect. 2010;43(3):200–206. doi:10.1016/S1684-1182(10)60032-1
6. Hemon F, Fouchard F, Tromeur C, et al. Association between hospitalization for acute medical illness and VTE risk: a lower efficacy of thromboprophylaxis in elderly patients? Results from the EDITH case-control study. Eur J Internal Med. 2017;44:39–43. doi:10.1016/j.ejim.2017.05.029
7. Tripodi A, Branchi A, Chantarangkul V, et al. Hypercoagulability in patients with type 2 diabetes mellitus detected by a thrombin generation assay. J Thromb Thrombolysis. 2011;31(2):165–172. doi:10.1007/s11239-010-0506-0
8. Beijers HJBH, Ferreira I, Spronk HMH, et al. Impaired glucose metabolism and type 2 diabetes are associated with hypercoagulability: potential role of central adiposity and low-grade inflammation–the Hoorn Study. Thromb Res. 2012;129(5):557–562. doi:10.1016/j.thromres.2011.07.033
9. Chung WS, Lin CL, Kao CH. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost. 2015;114(10):812–818. doi:10.1160/TH14-10-0868
10. Ageno W, Becattini C, Brighton T, et al. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation. 2008;117(1):93–102. doi:10.1161/CIRCULATIONAHA.107.709204
11. Konstantinides SV, Meyer G, Becattini C, et al.; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543–603. doi:10.1093/eurheartj/ehz405
12. Lee JS, Shin JH, Kim MN, et al. Kodamaea ohmeri isolates from patients in a University Hospital: identification, antifungal susceptibility, and pulsed-field gel electrophoresis analysis. J Clin Microbiol. 2007;45(3):1005–1010. doi:10.1128/JCM.02264-06
13. García-Martos P, Domínguez I, Marín P, García-Agudo R, Aoufi S, Mira J. Antifungal susceptibility of emerging yeast pathogens. Enferm Infecc Microbiol Clin. 2001;19(6):249–256. In Spanish. doi:10.1016/S0213-005X(01)72630-4
14. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; EuropeanConfederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76–98. doi:10.1111/1469-0691.12360
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
