Back to Journals » Infection and Drug Resistance » Volume 13
A Case Report of Cerebral Meningitis Caused by Penicillin-Non-Susceptible Group B Streptococcus in an Immunocompromised Adult Patient
Authors Hirai J, Kinjo T, Haranaga S, Fujita J
Received 19 March 2020
Accepted for publication 24 June 2020
Published 6 July 2020 Volume 2020:13 Pages 2155—2160
DOI https://doi.org/10.2147/IDR.S251250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jun Hirai, Takeshi Kinjo, Shusaku Haranaga, Jiro Fujita
Department of Infectious, Respiratory and Digestive Medicine, Graduate School of Medicine, University of the Ryukyus, Okinawa, Japan
Correspondence: Jun Hirai Email [email protected]
Abstract: Streptococcus agalactiae, also known as Group B Streptococcus (GBS), is the leading cause of bacteremia and meningitis in neonates; however, it also causes meningitis in adults, although much less frequently. After the detection of penicillin-non-susceptible GBS (PRGBS) for the first time in 2008 by Japanese researchers, clinical PRGBS isolates have been reported worldwide. These isolates need to be given due attention for being non-susceptible to multiple drugs. Herein, we present the first clinical report of meningitis caused by PRGBS. A 41-year-old Japanese male receiving an immunosuppressant visited hospital complaining of fever. Although he did not have meningitis-related symptoms or physical findings, determination of the cause of fever by Gram-staining of the spinal fluid revealed gram-positive cocci in pairs and chains. Initially, he was hospitalized on the diagnosis of cerebral meningitis caused by Streptococcus pneumoniae. However, culture of the spinal fluid revealed the β-hemolytic colonies on blood agar. Biochemical testing and mass spectrometry revealed the isolated organism as GBS (serotype Ib). The minimum inhibitory concentration (MIC) of penicillin G for the isolated strain was 0.5 μg/mL, which is greater than the MIC criteria for “susceptibility” to penicillin G for beta-hemolytic streptococci according to Clinical and Laboratory Standards Institute standards. The isolated strain was also resistant to macrolide (MIC ≥ 8 μg/mL) and fluoroquinolone (MIC ≥ 8 μg/mL). The patient recovered without neurologic sequelae upon treatment with ceftriaxone, vancomycin, and corticosteroids for 4 days, and subsequently with ampicillin for 17 days. The rate of isolation of PRGBS in the clinics has gradually increased, particularly in Japan. Although PRGBS isolated in the present case was susceptible to ampicillin and cephalosporins, strains not susceptible to ampicillin, cefotaxime, and ceftriaxone have already been isolated, indicating the prospects for limited range of effective antibiotics against PRGBS infections, including meningitis, in the near future.
Keywords: adult, meningitis, Streptococcus agalactiae, PRGBS, serotype Ib, corticosteroids
Introduction
Streptococcus agalactiae, also known as Group B Streptococcus (GBS), was first differentiated from other streptococci by Rebecca Lancefield in the 1930s.1 It is a gram-positive coccus that causes beta-hemolysis on blood agar. GBS colonizes the human gastrointestinal tract, urinary tract, skin, and genital tract as a harmless commensal bacterium. However, it also causes lethally invasive infections, such as necrotizing fasciitis, bacteremia, peritonitis, pneumonia, and arthritis and is also associated with high mortality and morbidity among the elderly (20.6%).2 Among the GBS infections, meningitis and sepsis are the most common in neonates, whereas primary bacteremia occurs most frequently in adults (24%).3,4 In adults, GBS meningitis is very rare. In fact, a prospective 9-year cohort study revealed a frequency of only 1% (21 of 1412 community-acquired cases); other cohort studies on adults also showed that the frequency of GBS meningitis was 0.4%–7.4% of all the cases of meningitis.5,6 However, GBS was not isolated as a causative pathogen in any of the 71 adult patients in a 3-year multicenter survey of bacterial meningitis in Japan.7
GBS is considered to be universally susceptible to beta-lactams, with penicillin being the first-line drug for the treatment and prevention of GBS infections. However, the emergence of penicillin-non-susceptible GBS (PRGBS) mainly due to amino acid substitutions, such as V405A and/or Q557E, in the penicillin-binding protein 2X has been reported in many countries, including USA, Canada, Scotland, and Germany, for over a decade since it was first reported in Japan in 2008.8–12
The rate of isolation of PRGBS from various clinical specimens, such as sputum, urine, and vaginal discharge, has gradually increased from 2.3% (between 2005 and 2006) to 14.7% (between 2012 and 2013) in geographically separate hospitals, and particularly in Japan.13 In addition, PRGBS isolates often tend to be non-susceptible to multiple drugs. In fact, Kitamura et al reported that the rates of occurrence of non-susceptibility to ampicillin (ABPC), cefotaxime (CTX), and ceftriaxone (CTRX) were 15%, 28%, and 36%, respectively, for the 74 PRGBS isolates in Japan.14 In another investigation the ratio of resistance to both erythromycin and levofloxacin in 45 PRGBS strains isolated from different places in Japan from January 2012 to July 2013 was found to be 71.1% and 95.6%, respectively.13 These findings indicate that the range of antibiotics likely to be effective against PRGBS infections in Japan is limited, highlighting the need for careful drug selection for treatment of GBS infections in the clinical setting.
Although PRGBS was isolated in a case with bacteremia and joint infection,15,16 a case of meningitis caused by PRGBS has not been reported, thus far. To the best of our knowledge, this case is the first documented clinical report of meningitis caused by PRGBS. This strain needs to be carefully observed, considering the prospects of drug penetration into the cerebrospinal fluid (CSF) in addition to antimicrobial susceptibility when treating meningitis patients.
Written informed consent was obtained from the patient, and the patient also provided written informed consent for publishing the case details and all accompanying images. The ethics committee of our hospital approved the waiver in this case report, based on the Japanese ethical guidelines for clinical research.
Case Report
On January 21, 2016, a 41-year-old Japanese male, with Langerhans cell histiocytosis, on immunosuppressive therapy with cyclophosphamide, was admitted to our hospital complaining of a 4-h history of fever and chills. He had a medical history of a methicillin-susceptible Staphylococcus aureus cervical spine infection that occurred postoperatively after a compression fracture, 3 years ago. On arrival, the score on Glasgow Coma Scale was 15, and the patient’s vital signs were as follows: blood pressure was 125/74 mmHg, cardiac rate was 135 beats/minute, body temperature was 38.6 °C, and oxygenation level was 98% while breathing room air. No significant physical findings were noted. There were no Kernig or Brudzinski signs or any other neurological abnormalities. Laboratory data indicated that the leukocyte count was 15,700 cells/mm3 (neutrophils, 97%; lymphocytes, 2%; and monocytes, 1%), and the level of C-reactive protein was 19.37 mg/dL (normal range: < 0.03 mg/dL). These results indicated the presence of a bacterial infection.
Although the patient did not have meningitis-related symptoms, such as headache and vomiting, considering the past medical history of a surgical site infection on the neck, we conducted a lumbar puncture and observed a purulent CSF at an elevated pressure with a neutrophil count of 5954 cells/mm,3 a protein level of 1245 mg/dL, and a glucose level of 1 mg/dL. Gram-staining of the spinal fluid revealed gram-positive cocci in pairs and chains indicating that the infective organism was probably S. pneumoniae (Figure 1). The computed tomography scan of the head was normal. After obtaining a CSF culture and two sets of blood culture, initial treatment was started with intravenous ceftriaxone (4 g/day), vancomycin (1.2 g/day), and dexamethasone (39.6 mg/day) following a diagnosis of bacterial meningitis attributable to S. pneumoniae. However, we observed the growth of a β-hemolytic organism in the CSF culture on blood agar. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis promptly identified this microorganism as GBS. Finally, the isolate was confirmed as GBS by biochemical testing (RAISUS automated method; Nissui Pharmaceuticals Co., Ltd., Tokyo). The serotype was Ib based on an agglutination test. The MIC for antimicrobial susceptibility was determined by the broth microdilution method with the RAISUS automated system. Consequently, MIC of penicillin for the isolated strain was determined to be 0.5 μg/mL, which is greater than the MIC criteria for “susceptibility” to penicillin G for beta-hemolytic streptococci according to Clinical and Laboratory Standards Institute standards, 2016 (Table 1). For further confirmation of penicillin sensitivity of the isolate, we conducted additional E-testing, and found that the penicillin sensitivity of the isolate was 0.5 μg/mL, which also indicated that the isolate was “penicillin-non-susceptible.” Blood cultures drawn at the time of hospitalization were sterile.
 |
Table 1 Antimicrobial Susceptibility of PRGBS Isolated in the Present Case. Drug Susceptibility Was Based on Clinical and Laboratory Standards Institute, 2016 |
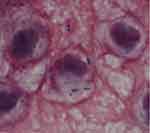 |
Figure 1 Spinal fluid smear for the case patient. Note: Photomicrograph of a Gram-stained spinal fluid showed gram-positive cocci (magnification; 1000x). |
The patient rapidly showed satisfactory progress and became afebrile on the third day after admission. We continued ceftriaxone, vancomycin, and dexamethasone for 4 days. Thereafter, the treatment regimen was changed to ampicillin (12 g/day) as a definitive therapy on hospital day 5 and was continued until day 21. Inflammatory markers, such as white blood cells and C-reactive protein, were almost normal on hospital day 10. Also, the subsequent lumbar puncture revealed a decreased neutrophil count of 57 cells/mm,3 and a sterile CSF culture. The patient recovered without neurologic sequelae and completed a 3-week course of medication.
Discussion
The number of GBS invasive infections among adults has increased significantly by 2.8-fold during the periods, 1998–2002 and 2003–2007, in Japan.17 Other countries have also reported increasing number of invasive GBS cases, such as bacteremia and endocarditis, in non-pregnant adults, during the last decade.18,19 However, due to the relatively low prevalence of GBS meningitis in adults, there are limited data for this life-threatening infection. The findings from a recent review article describing community-acquired GBS meningitis in 141 adult patients suggest that GBS meningitis in adults most frequently develops between 41 and 66 years of age, and is equally frequent in men and women (52% vs 48%).20 Autoimmune disease and/or immunosuppressive therapy, in addition to diabetes or hepatic disorders, was indicated as one of the important predisposing factors for adult GBS meningitis.20 These findings were consistent with those of the present case. Forty-four (31%) of the 141 patients died within five days of admission, and deaths were related with advanced age and an immunocompromised condition.20 Therefore, we propose that physicians should consider the possibility of GBS in addition to S. pneumoniae when gram-positive cocci are observed in the smears of CSF from patients with a similar background and risk factors.
Regardless of PRGBS isolation in the present case, we treated the patient with a high dose of ampicillin (12 g/day) as a definitive therapy for a total of 3 weeks as the antimicrobial susceptibility testing for isolation revealed the MIC of ampicillin to be ≤ 0.25 μg/mL (Table 1). The reason as to why the susceptibility of PRGBS to penicillin G and ampicillin is different is not known. However, based on a previous investigation,8 we assumed that the reduced binding ability of penicillin G or ampicillin to mutated PBP2X of PRGBS could be the main cause for such a difference. Additional professional research is needed to resolve this discrepancy because, in general, microbiological laboratories do not routinely test the level of mutation of PBP in a clinical setting. In addition, it is still not clear if we can treat a PRGBS meningitis patient using ampicillin safely and effectively because based on the MIC of ampicillin some PRGBS strains show ampicillin-insusceptible properties (MICs, 0.25–0.5 μg/mL).8,14 This means that the failure of treatment with ampicillin in a patient with PRGBS meningitis would occur because of inadequate ampicillin concentration in the CSF, and the above mentioned MIC cannot be maintained over time even when a high dose of ampicillin (12 g/day) is administered. Although the recommended duration for treatment of GBS meningitis is 14–21 days,21 the appropriate treatment duration of PRGBS meningitis is not known. More cumulative evidence is needed to clarify these problems as there have been no reports on this topic to date.
Furthermore, we used corticosteroid in addition to antimicrobial agents for 3 days, as the presented case was initially diagnosed with meningitis caused by S. pneumoniae. Although it is known that corticosteroids reduce the mortality in patients with S. pneumoniae meningitis (relative risk [RR], 0.84; 95% confidence interval [Cl], 0.72 to 0.98) and in children with with Haemophilus influenzae meningitis (RR, 0.34; 95%Cl, 0.20 to 0.59) having severe auditory disturbance, the effect of corticosteroids among GBS meningitis patients is still not mentioned in the Cochrane database systematic review.22 Additional investigations are, thus, needed to assess the role of corticosteroid treatment in reducing the overall mortality and sequelae in GBS meningitis patients.
There is limited information regarding the epidemiology of GBS serotypes that cause meningitis in adults. S. agalactiae is sub-classified into 10 different capsular serotypes (Ia, Ib, and II–IX) depending on the immunologic reactivity of the polysaccharide capsule. According to several studies on adult GBS meningitis,23–26 the most common capsular serotype is Ib (39.5%), which was seen in our case, followed by serotypes III (28.9%), Ia (15.8%), and V (7.9%). It is not known as to which serotype of PRGBS is more likely to cause meningitis and this remain a challenging topic for investigation in the future.
This study has several limitations. First, we should have submitted the isolated strain to a public health laboratory for further confirmation of the isolated strain as GBS although we re-identified/re-tested it and checked the penicillin sensitivity using several different methods, including biochemical tests, MALDI-TOF MS, and E-testing, in the clinical laboratory at our hospital. Lartigue et al reported that MALDI-TOF MS is 100% accurate for the identification of GBS,27 and other previous studies have also demonstrated it to be a valuable tool for the identification of GBS with a high level of accuracy.28–31 Therefore, we strongly believe that the isolated strain was GBS. Furthermore, the serotype of the isolated GBS in the present case was conformed as serotype Ib, using the anti-GBS serotype-specific serum. It has been reported in previous investigations that serotype Ib GBS is the predominant strain associated with invasive diseases, such as meningitis, and that it is highly resistant to antimicrobial agents, such as erythromycin and clindamycin.32,33 As mentioned earlier, PRGBS also tends to show resistance to fluoroquinolone and/or macrolides.13,34 These serotype characteristics and drug susceptibility were consistent with those of the isolated strain in the present case, which indicates that our identification of the isolate and the results of antimicrobial susceptibility testing were correct. Second, we did not confirm that the present strain harbors the relevant amino acid substitutions in PBP, such as Q557E and/or V405A, which induce non-susceptibility to penicillin G in GBS.8 However, we conducted additional E-testing to check the penicillin sensitivity of the isolate and confirmed that the isolated GBS was a penicillin-non-susceptible strain. Further, we could not identify why the present patient had a PRGBS infection and could not unravel the relationship between the strain PRGBS and meningitis because studies including the risk factors and epidemiology of invasive infections caused by PRGBS strains have been scarce till date. Further investigation is needed to clarify the clinical features in PRGBS infections by accumulating infectious cases, and researching the microbiological characteristics, including serotype, sequence type, clonal complex, and whole genome sequencing.
In conclusion, we present the first identified case of meningitis caused by PRGBS. Although only a small number of infections due to PRGBS have been reported, this strain requires particular attention as it has a tendency to be multidrug non-susceptible and the number of clinical isolates is increasing. We should, thus, monitor it carefully to clarify the clinical characteristics, predisposing factors, and prognosis of infection caused by this pathogen, in view of an increasing number of infected cases.
Acknowledgments
We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lancefield RC. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933;57(4):571–595. doi:10.1084/jem.57.4.571
2. Crespo-Ortiz Mdel P, Castañeda-Ramirez CR, Recalde-Bolaños M, Vélez-Londoño JD. Emerging trends in invasive and noninvasive isolates of Streptococcus agalactiae in a Latin American hospital: a 17-year study. BMC Infect Dis. 2014;14:428. doi:10.1186/1471-2334-14-428
3. Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33(4):556–561. doi:10.1086/322696
4. Sendi P, Johansson L, Norrby-Teglund A. Invasive group B Streptococcal disease in non-pregnant adults: a review with emphasis on skin and soft-tissue infections. Infection. 2008;36(2):100–111. doi:10.1007/s15010-007-7251-0
5. Bijlsma MW, Brouwer MC, Kasanmoentalib ES. Community-acquired bacterial meningitis in adults in the Netherlands:2006-14: a prospective cohort study. Lancet Infect Dis. 2016;16(3):339–347. doi:10.1016/S1473-3099(15)00430-2
6. van Kassel MN, van Haeringen KJ, Brouwer MC, Bijlsma MW, Diedrick VDB. Community-acquired group B streptococcal meningitis in adults: 33 cases from prospective cohort studies. J Infect. 2020;80(3):255–260. doi:10.1016/j.jinf.2019.12.002
7. Sakata H, Sato Y, Nonoyama M, et al. Results of a multicenter survey of diagnosis and treatment for bacterial meningitis in Japan. J Infect Chemother. 2010;16(6):396–406. doi:10.1007/s10156-010-0064-6
8. Kimura K, Suzuki S, Wachino J, et al. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob Agents Chemother. 2008;52(8):2890–2897. doi:10.1128/AAC.00185-08
9. Dahesh S, Hensler ME, Van Sorge NM, et al. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob Agents Chemother. 2008;52(8):2915–2918. doi:10.1128/AAC.00461-08
10. Longtin J, Vermeiren C, Shahinas D, et al. Novel mutations in a patient isolate of Streptococcus agalactiae with reduced penicillin susceptibility emerging after long-term oral suppressive therapy. Antimicrob Agents Chemother. 2011;55(6):2983–2985. doi:10.1128/AAC.01243-10
11. Cooper K, Abbott F, Gould IM. Reduced penicillin susceptibility of group B Streptococcus: an assessment of emergence in Grampian, Scotland. Br J Biomed Sci. 2016;73:25–27. doi:10.1080/09674845.2016.1144550
12. van der Linden M, Mamede R, Levina N, et al. Heterogeneity of penicillin-non-susceptible group B streptococci isolated from a single patient in Germany. J Antimicrob Chemother. 2020;75(2):296–299. doi:10.1093/jac/dkz465
13. Seki T, Kimura K, Reid ME, et al. High isolation rate of MDR group B streptococci with reduced penicillin susceptibility in Japan. J Antimicrob Chemother. 2015;70(10):2725–2728. doi:10.1093/jac/dkv203
14. Kitamura M, Kimura K, Ido A, et al. Relatively high rates of cefotaxime- and ceftriaxone-non-susceptible isolates among group B streptococci with reduced penicillin susceptibility (PRGBS) in Japan. J Antimicrob Chemother. 2019;74(4):931–934. doi:10.1093/jac/dky542
15. Chu YW, Tse C, Tsang GK, So DK, Fung JT, Lo JY. Invasive group B Streptococcus isolates showing reduced susceptibility to penicillin in Hong Kong. J Antimicrob Chemother. 2007;60(6):1407–1409. doi:10.1093/jac/dkm390
16. Gaudreau C, Lecours R, Ismaïl J, Gagnon S, Jetté L, Roger M. Prosthetic hip joint infection with a Streptococcus agalactiae isolate not susceptible to penicillin G and ceftriaxone. J Antimicrob Chemother. 2010;65(3):594–595. doi:10.1093/jac/dkp458
17. Matsubara K, Yamamoto G. Invasive group B streptococcal infections in a tertiary care hospital between 1998 and 2007 in Japan. Int J Infect Dis. 2009;13(6):679–684. doi:10.1016/j.ijid.2008.10.007
18. Pimentel BA, Martins CA, Mendonça JC, et al. Streptococcus agalactiae infection in cancer patients: a five-year study. Eur J Clin Microbiol Infect Dis. 2016;35(6):927–933. doi:10.1007/s10096-016-2617-9
19. Smith EM, Khan MA, Reingold A, Watt JP. Group B streptococcus infections of soft tissue and bone in California adults:1995-2012. Epidemiol Infect. 2015;143(15):3343–3450. doi:10.1017/S0950268815000606
20. van Kassel MN, Bijlsma MW, Brouwer MC, van der Ende A, van de Beek D. Community-acquired group B streptococcal meningitis in adults: 33 cases from prospective cohort studies. J Infect. 2019;78(1):54–57. doi:10.1016/j.jinf.2018.07.009
21. Rodrigo MD, ed. Treatment of Bacterial Meningitis Caused by Specific Pathogens in Adults. Waltham, MA: UpToDate Inc. Available from: https://www.uptodate.com.
22. Brouwer MC, McIntyre P, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev. 2015;6:CD004405.
23. Morozumi M, Wajima T, Takata M, Iwata S, Ubukata K. Molecular characteristics of group B streptococci isolated from adults with invasive infections in Japan. J Clin Microbiol. 2016;54(11):2695–2700. doi:10.1128/JCM.01183-16
24. Gudjónsdóttir MJ, Hentz E, Berg S, et al. Serotypes of group B streptococci in western Sweden and comparison with serotypes in two previous studies starting from 1988. BMC Infect Dis. 2015;15(1):507. doi:10.1186/s12879-015-1266-4
25. Salloum M, van der Mee-marquet N, Valentin-Domelier AS, Quentin R. Diversity of prophage DNA regions of Streptococcus agalactiae clonal lineages from adults and neonates with invasive infectious disease. PLoS One. 2011;6(5):e20256. doi:10.1371/journal.pone.0020256
26. Wong SS, Tsui K, Liu QD, et al. Serotypes, surface proteins, and clinical syndromes of invasive Group B streptococcal infections in northern Taiwan:1998-2009. J Microbiol Immunol Infect. 2011;44(1):8–14. doi:10.1016/j.jmii.2011.01.003
27. Lartigue MF, Héry-Arnaud G, Haguenoer E, et al. Identification of Streptococcus agalactiae isolates from various phylogenetic lineages by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2019;47(7):2284–2287. doi:10.1128/JCM.00175-09
28. Ka-Ning T, Cornwell E, Daniel R, et al. Evaluation of Matrix-Assisted Laser Desorption Ionisation Time-Of-Flight Mass Spectrometry (MALDI-TOF MS) for the identification of Group B Streptococcus. BMC Res Notes. 2019;12(1):85. doi:10.1186/s13104-019-4119-1
29. Binghuai L, Yanli S, Shuchen Z, Fengxia Z, Dong L, Yanchao C. Use of MALDI-TOF Mass Spectrometry for Rapid Identification of Group B Streptococcus on chromID Strepto B Agar. Int J Infect Dis. 2014;27:44–48. doi:10.1016/j.ijid.2014.06.023
30. Lartigue MF, Kostrzewa M, Salloum M, et al. Rapid detection of “highly virulent” Group B Streptococcus ST-17 and Emerging ST-1 Clones by MALDI-TOF mass spectrometry. J Microbial Methods. 2011;86(2):262–265. doi:10.1016/j.mimet.2011.05.017
31. Alatoom AA, Cunningham SA, Ihde SM, Mandrekar J, Patel R. Comparison of direct colony method versus extraction method for identification of gram-positive cocci by use of bruker biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Mirobiol. 2011;49(8):2868–2873. doi:10.1128/JCM.00506-11
32. Metcalf BJ, Chochua S, RE G
33. Tsai MH, Hsu JF, Lai MY, et al. Molecular characteristics and antimicrobial resistance of Group B streptococcus strains causing invasive disease in neonates and adults. Front Microbiol. 2019;18:264. doi:10.3389/fmicb.2019.00264
34. Kimura K, Nagano N, Nagano Y, et al. High frequency of fluoroquinolone- and macrolide-resistant streptococci among clinically isolated group B streptococci with reduced penicillin susceptibility. J Antimicrob Chemother. 2013;68(3):539–542. doi:10.1093/jac/dks423
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
