Back to Journals » Journal of Inflammation Research » Volume 16
A Case of Ulcerative Colitis Induced by Paraneoplastic Syndrome?
Authors Zhang T, Pan ZB, Tong WJ, Zhou YL, Cheng Y, Jin DQ, Qi SQ, Zhang ZQ
Received 25 April 2023
Accepted for publication 1 August 2023
Published 8 August 2023 Volume 2023:16 Pages 3319—3327
DOI https://doi.org/10.2147/JIR.S418733
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Tao Zhang,1 Zhu-Bin Pan,1 Wen-Jia Tong,2 Yu-Liang Zhou,1 Yuan Cheng,1 Dan-Qun Jin,2 Shi-Qin Qi,1 Zhen-Qiang Zhang1
1Department of General Surgery, Anhui Provincial Children’s Hospital, Children’s Hospital of Fudan University Anhui Hospital, Children’s Hospital of Anhui Medical University, Hefei, 230000, People’s Republic of China; 2Department of Pediatric Intensive Care Unit, Anhui Provincial Children’s Hospital, Children’s Hospital of Fudan University Anhui Hospital, Children’s Hospital of Anhui Medical University, Hefei, 230000, People’s Republic of China
Correspondence: Zhu-Bin Pan, Department of General Surgery, Anhui Provincial Children’s Hospital, Children’s Hospital of Fudan University Anhui Hospital, Children’s Hospital of Anhui Medical University, No. 39 of Wang Jiang Dong Street, Bao He District, Hefei, 230000, People’s Republic of China, Tel +86 13349207050, Fax +86 551 62237461, Email [email protected]
Background: Paraneoplastic syndromes often cause endocrine, neurological, cutaneous, and hematologic pathologies, and cases with digestive symptoms as prominent cases are rare.
Case Description: A 1-year-old child admitted to the emergency department with severe abdominal distension was later diagnosed with sacrococcygeal yolk cystoma with ulcerative colitis. After symptomatic management, surgical removal of the tumor, and JEB chemotherapy, the symptoms of ulcerative colitis disappeared completely. After 7 years of follow-up, the child grew and developed well, and there was no recurrence of tumor and ulcerative colitis.
Conclusion: Yolk sac tumor with ulcerative colitis is a rare paraneoplastic syndrome with complex clinical manifestations.
Keywords: children, paraneoplastic syndrome, toxic megacolon, ulcerative colitis, yolk sac tumor
Introduction
Paraneoplastic syndrome is a clinical syndrome associated with malignant tumors. It does not appear from a direct invasion or compression of the tumor and instead is caused by distant tissues or organs that have been affected by tumor products, abnormal immune responses, or other unknown causes.1 Up to 40% of malignant tumor patients have paraneoplastic syndrome. The paraneoplastic syndrome can involve multiple systems including Cardiovascular, neuromuscular, and other systems. Patients usually present with one or more clinical manifestations.2 Yolk sac tumor (YST) is a rare and severe malignant germ cell tumor that mainly occurs in the testis or ovary, with extragonadal yolk sac tumor being extremely rare. One of the common sites for extragonadal germ cell tumors is the sacrococcygeal area. While sacrococcygeal YST and primary gonadal YST have the same histomorphology, they differ in terms of clinical manifestations and prognosis.3 No literature on pediatric ulcerative colitis (UC) caused by paraneoplastic syndrome was found after retrieval. In this paper, we describe the treatment course of a rare case of a child who had sacrococcygeal YST-inducing UC and toxic megacolon (TM) to help with the clinical diagnosis and treatment of these diseases.
Case Report
General Data
A 1-year-old boy was admitted to our hospital for “abdominal distension for 1 week and anus blockage, and no flatulence and defecation for 3 days”. One week prior to being admitted, the child developed abdominal distension without obvious inducement, no fever, no nausea and vomiting, no diarrhea, and he received no special treatment. His symptom of abdominal distension persisted, and there was no flatulence or defecation for 3 days ago. So the child was admitted to our emergency department. Manifestations of the child at admission: shock; low blood pressure (55/30 mmHg); rapid heart rate (200 beats/min); significantly prolonged capillary refilling time (4 s); elevated lactic acid (3.4 mmol/L); lack of consciousness; dyspnea; severe abdominal distension. Indirect intra-abdominal pressure was 15 mmHg at this time. Arterial blood gas analysis showed metabolic acidosis and low levels of sodium and potassium. A blood routine test showed leukocytosis and mild anemia. Biochemistry showed a significant decrease in albumin. Conservative fluid resuscitation, ventilator support, and anti-infective therapy were used to maintain a stable internal environment. The General Pediatric Surgery Department was promptly consulted on the diagnosis and treatment of this child while we actively corrected his shock status as his abdominal distension was very severe.
Physical Examination
Poor response; rhonchi in both lungs when breathing; no evident rales; distended and soft abdomen; no palpable abnormal mass. Anal examination: smooth finger entry; a mass (about 6 * 5 cm in size, poorly circumscribed, poor mobility) was palpable in the sacrococcygeal area; no blood staining after finger stall withdrawal. Body temperature 38.2 °C, heart rate 200 beats/min, respiratory rate 50 breaths/min, blood pressure 55/30 mg.
Examination
Auxiliary examination: blood routine and biochemistry are shown in Table 1; tuberculosis antibody (-); fecal routine: occult blood (+), fecal culture (-); negative PPD test results (PPD is an intradermal test to diagnose tuberculobacter infection by injecting a small amount of tuberculin into the skin at the injection site.). Erect abdominal radiograph (Figure 1) indicated the intestine was pneumatic, dilated, rigid, and stiff, with a tendency to straighten, spaces between the intestine walls were thickened, and gas distribution was uneven. Intestinal obstruction was likely based on the erect abdominal radiograph. Abdominal B-mode ultrasound imaging: No evident causes of acute abdominal disease were detected, abundant gas accumulated in partial intestine, effusion was dilated in partial intestine, a hypoechoic area, about 5.1 * 4.9 * 4.8 cm in size was observed in the sacrococcygeal area, with a uniform echo, clear boundary, and regular shape. Color Doppler flow imaging (CDFI): punctate blood flow signal was observed, PSV: 25.6 cm/s, RI: 0.6. Whole blood was collected from the child before surgery. Serological tests revealed positive perinuclear antineutrophil antibody (P-ANCA) and elevated vasoactive intestinal peptide concentrations, both of which are markers that are currently highly distinctive in determining ulcerative colitis.
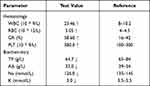 |
Table 1 Laboratory Investigations of the Patient |
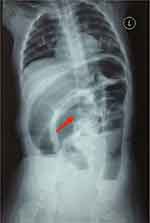 |
Figure 1 Erect abdominal radiograph reveals significant dilatation of the intestine, as the red arrow shows. |
Diagnosis and Differential Diagnosis
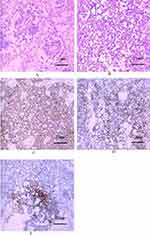
Prompt exploratory laparotomy and decompression were performed as the abdominal pressure of the child continued to rise to the ACS level (abdominal compartment syndrome). It was found that small intestinal blood circulation was good, and the entire colon was dilated with a diameter of more than 6.1 cm and there was no blood circulation disturbance. TM was determined to be likely based on the above data and the patient’s significant clinical manifestations of septic shock. Colonoscopy (Figure 2) revealed numerous purulent masses and ulcers on the surface of the colon. The pathology after colon biopsy (Figure 3) showed that the crypt structure had changed, and crypt abscess had formed. Mucosal and muscular layer eosinophils < 5 / HP, and ganglion cells can be seen in the muscular layer (Figure 4). After other diseases were ruled out and the clinical manifestations were combined with auxiliary examination results, the patient was diagnosed with UC.
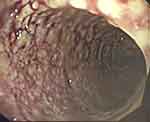 |
Figure 2 Endoscopic examination shows mucosal hyperemia, disappearance of vascular texture, and multiple ulcers. |
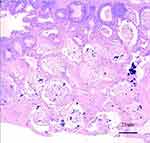 |
Figure 3 Colonic mucosal biopsy results show changes in the crypt structure and formation of crypt abscess. |
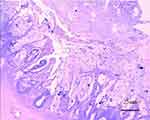 |
Figure 4 Colon pathology shows gangliocyte is normal. |
We conducted sacrococcygeal magnetic resonance imaging (MRI) (Figure 5) and surgically removed the sacrococcygeal mass, then sent it for pathological examination after surgery. The pathological change was diagnosed as yolk sac tumor based on imaging, pathomorphology, and immunohistochemical results (Figure 6).
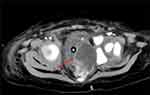 |
Figure 5 MRI shows a solid mass on the left side of the pelvis, as the red arrow shows. |
Treatment
The patient underwent an emergency exploratory laparotomy as his intra-abdominal pressure kept increasing after having been administered anti-shock medication, gastrointestinal decompression, an indwelling anal canal to lower intra-abdominal pressure, and other treatments. The entire colon was found to be highly dilated during the surgery. Appendectomy + colonic irrigation via appendiceal catheterization + intestinal exteriorization + colonic biopsy was performed after the colonoscopy showed ulcers and numerous purulent masses in the colon. Intestinal exteriorization + abdominal wall suspension was performed due to an unachievable primary abdominal closure caused by intra-abdominal hypertension. The child underwent enteroscopy + return of exteriorized intestine + terminal ileostomy when the abdominal distension was relieved on the 7th day after surgery. Colonoscopy + sacrococcygeal mass resection was performed after excluding surgical contraindications. Colonoscopy indicated that intestinal inflammation continued to exist without significant improvement. Postoperative pathology of the sacrococcygeal mass indicated yolk sac tumor. The child was given 6 courses of JEB chemotherapy (etoposide: 120 mg/m2, vgtt, d1-3; carboplatin: 600 mg/m2, vgtt, d2; bleomycin: 15 mg/m2, vgtt, d3). Enteroscopy was performed for the fourth time after these 6 courses and showed that intestinal inflammation disappeared completely. Stoma closure after terminal ileostomy was then performed. The child was treated for about 1 year, was hospitalized 9 times, underwent 4 surgeries, and received 6 courses of chemotherapy.
Treatment Results, Follow-Up, and Outcome of the Disease
Gastrointestinal symptoms of UC and TM in this patient completely disappeared after the sacrococcygeal YST resection, which was aided by postoperative chemotherapy. The child’s body grew and developed well during the 7-year follow-up with no recurrence of the tumor, and no recurrence of ulcerative colitis. The follow-up is still ongoing.
Discussion
Causes of Colitis in This Child
In the initial diagnosis and treatment, irritable bowel syndrome and allergic colitis were first excluded, Intraoperative colon biopsy excludes eosinophilic gastroenteritis and Hirschsprung disease. Infectious pathogens are not detected in the later diagnosis and treatment process, Infectious colitis was excluded (C. difficile, cytomegalovirus, Campylobacter jejuni, Yersinia, fungi, Epstein-Barr virus, schistosome, amoebae, human immunodeficiency virus, etc).4 Crohn’s disease and UC are frequently mixed up in diagnosis. Crohn’s disease lesions are mostly distributed in segments. The ulcers under endoscopes are mostly slit-like and longitudinal, with normal or cobblestone-like surrounding mucosa. Endoscopy did not reveal such findings. A biopsy of the patient’s intestinal mucosa revealed that the crypt structure had changed, and inflammation had infiltrated. This matched the distinctive histopathological characteristics of UC.5 Besides surgery, the main treatments for UC include nutritional therapy and drug therapy. However, as the gastrointestinal symptoms of the patient had improved and the patient was to undergo chemotherapy for the tumor after intestinal ileostomy, corresponding drug treatment for UC was not given at that time.
Diagnostic Points for Toxic Megacolon
Congenital megacolon was excluded after the first colon biopsy. Due to a lack of diagnostic criteria for TM diagnosis in children, the diagnostic criteria for TM in adults serves as the major reference:6 A. Having essential imaging evidence of colonic dilatation. B. Meeting at least three of the following items: a. body temperature > 38 °C; b. heart rate > 120 beats/min; c. neutrophil count > 10.5 * 109/L; d. anemia. C. Meeting any one of the following: a. dehydration; b. having a change in the level of consciousness; c. electrolyte imbalance; d. hypotension. D. Dilated colon diameter ≥ 56 mm. The child met all the conditions above. Benchimol concluded that a transverse colon diameter of 56 mm in imaging is the cut-off value for distinguishing the severity of inflammatory intestinal diseases, with a transverse colon diameter ≥ 56 mm indicating severe inflammatory intestinal diseases. He also concluded that fever, tachycardia, dehydration, and electrolyte disorders were more common in pediatric TM than in adults.7 It was also found that the lumen diameter of the transverse colon should be less than 40 mm in children under 11 years old.8 The child met the aforementioned criteria, hence, the diagnosis of TM is reasonable.
Therapeutic Experience in Children with Ulcerative Colitis Complicated by Toxic Megacolon
Cases of UC with TM are rarely reported in children. In current literature, children with UC complicated by TM are mostly treated with surgical intervention with drugs as adjuvant therapy.7 However, pediatric UC patients respond inadequately to hormone therapy and have a higher risk of steroid resistance than adults with paroxysmal UC (34% vs 29%).9–11 Furthermore, children have a much higher rate of colectomy within 10 years after UC occurrence than adults (30–40% vs 15–25%).10,12,13 According to studies, albumin levels are much lower in children who need colectomy,14 and albumin levels are significantly lower in these children at admission. This was consistent with other literature. From this, it can be inferred that the treatment of children with UC complicated by TM is highly challenging. After diagnosis, the patient did not undergo colectomy but recovered after resection of sacrococcygeal yolk sac tumor + adjuvant chemotherapy.
Monism of Paraneoplastic Syndrome to Explain the Case
UC is also known as chronic nonspecific ulcerative colitis. It is a chronic inflammatory disease of the rectum and colon whose etiology is not very clear, and it is a kind of intestinal immune inflammatory disease.15,16 Compared with adult patients, UC with childhood onset progresses more rapidly, has a wider range of lesions, and is at higher risk of serious complications and colectomy than in adults.17,18 The child has no genetic background, has an insidious onset, progresses rapidly, and UC symptoms resolve rapidly after resection of the sacrococcygeal tumor, we propose to explain this case with paraneoplastic syndrome. Instead of pathological changes caused by the tumor itself or metastases, paraneoplastic syndromes are pathological changes of single or multiple systems brought on by tumor products, abnormal immune responses, or other unknown causes. Some scholars proposed the concept of paraneoplastic syndrome as early as 1954. There have been more than 10,000 relevant reports around the world so far. Most of them are related to adults and involved multiple systems such as nerves, endocrine, skin, and other symptoms. Among them, there were also many cases of gastrointestinal symptoms induced by malignant tumors.19,20 Reports on children often focus on the neuroendocrine system, and no relevant reports of UC caused by paraneoplastic syndromes have been found. We propose that paraneoplastic syndromes can be used to explain the evolution and outcome of the disease. However, the cases collected and experience in diagnosis and treatment remain insufficient and can only be used as a reference when diagnosing and treating similar clinical cases.
Rare Complication of Extragonadal Yolk Cystoma
YST is a rare malignant germ cell tumor commonly found in the testes or ovaries, with a low extragonadal incidence of about 1%. YST occurring in extragonadal locations is more aggressive than intragonadal tumors and has a worse prognosis. Like other cancers, YST may be associated with PNS, involving the neurological, endocrine, and hematologic systems, with digestive symptoms as the less common. In our case, the YST location is located in the sacrococcygeal region, and inflammatory bowel disease is a typical presentation, which is rare. The clinical manifestations of different extragonadal YST vary. The MacDiarmid report describes a 54-year-old patient with extragonadal YST tumors who developed hypercalcaemia secondary to parathyroid hormone-related peptide production, disguised as metastases of renal cell carcinoma.21 In addition, Armstrong introduced a case of PNS caused by extragonadal nonseminoma germ cell tumor, the tumor location is located in the anterior mediastinum, the patient is mainly manifested by pericarditis and pulmonary artery stenosis circulatory symptoms, chemotherapy and surgery to remove the tumor, the patient has been performing well after surgery.22
In our case, the diagnosis of ulcerative colitis was clear, and after excluding other possible precipitating factors, the abnormal regulation of preoperative β-hCG concentration increased, and the symptoms completely disappeared after tumor resection, and there was no recurrence during follow-up. We speculate that tumor cells may release hormones or cytokines to promote the development of inflammation-related PNS. By introducing similar cases, it helps to fill the gaps in PNS in this area and provide more experience for dealing with similar cases in the future. The only downside is that we did not identify the mRNA of the relevant hormone in tumor tissue.
Conclusion
As paraneoplastic syndrome is an aberrant disease brought on by the onset and development of malignant tumors, treating primary tumors, mainly by surgical excision and chemoradiotherapy, can significantly alleviate the symptoms of paraneoplastic syndrome. Further immunotherapy after tumor resection also has a positive effect on relieving the symptoms. This case report uses the concept of paraneoplastic syndrome to explain the diagnosis and treatment process of the child, but there is still a theoretical lack of sufficient evidence to link UC and sacrococcygeal YST. It is the deficiency of this case report.
Sacrococcygeal YST with UC is a rare paraneoplastic syndrome with a hidden onset and complex clinical manifestations. It is often misdiagnosed, and the tumor is radiation-resistant. Surgical resection aided with chemotherapy is the primary treatment.23 More knowledge on the clinical symptoms of paraneoplastic syndrome can aid in the accurate diagnosis of this type of malignant tumor, thereby reducing treatment delays and improving the quality of life of patients.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Anhui Provincial Children’s Hospital, Children’s Hospital of Fudan University. A written informed consent was obtained from legal guardians of all participants.
Consent for Publication
Consent for publication was obtained from every individual (there parents) whose data are included in this manuscript.
Funding
Scientific research project of Anhui Provincial Health Commission (AHWJ2021a025).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ma GM, Chow JS, Taylor GA. Review of paraneoplastic syndromes in children. Pediatr Radiol. 2019;49(4):534–550. doi:10.1007/s00247-019-04371-y
2. Sweeney L, Howe T. Paraneoplastic syndromes. Medicine. 2016;44(1):69–72. doi:10.1016/j.mpmed.2015.10.006
3. Sharma R, Khera S, Sinha A, et al. Pure yolk sac tumor of sacrococcygeal region. Autops Case Rep. 2021;11:e2021287. doi:10.4322/acr.2021.287
4. Sanghavi SK, Rowe DT, Rinaldo CR. Cytomegalovirus, Varicella-Zoster virus, and Epstein-Barr virus. In: Clinical Virology Manual.
5. Levine A, Koletzko S, Turner D, et al. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795–806.
6. Jalan KN, Sircus W, Card WI, et al. An experience of ulcerative colitis. Gastroenterology. 1969;57:68–82. doi:10.1016/S0016-5085(19)33962-9
7. Benchimol EI, Turner D, Mann EH, et al. Toxic megacolon in children with inflammatory bowel disease: clinical and radiographic characteristics. Am J Gastroenterol. 2008;103:1524–1531. doi:10.1111/j.1572-0241.2008.01807.x
8. Turner D, Walsh CM, Benchimol EI, et al. Severe paediatric ulcerative colitis: incidence, outcomes and optimal timing for second-line therapy. Gut. 2008;57:331–338. doi:10.1136/gut.2007.136481
9. Turner D, Walsh CM, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5:103–110.
10. Gower-Rousseau C, Dauchet L, Vernier-Massouille G, et al. The natural history of pediatric ulcerative colitis: a population-based cohort study. Am J Gastroenterol. 2009;104:2080–2088. doi:10.1038/ajg.2009.177
11. Turner D, Griffiths AM. Acute severe ulcerative colitis in children: a systematic review. Inflamm Bowel Dis. 2011;17:440–449. doi:10.1002/ibd.21383
12. Van Limbergen J, Russell RK, Drummond HE, et al. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008;135:1114–1122. doi:10.1053/j.gastro.2008.06.081
13. Malaty HM, Mehta S, Abraham B, Garnett EA, Ferry GD. The natural course of inflammatory bowel disease-indeterminate from childhood to adulthood: within a 25 year period. Clin Exp Gastroenterol. 2013;115. doi:10.2147/CEG.S44700
14. Moore JC, Thompson K, LaFleur B, et al. Clinical variables as prognostic tools in pediatric-onset ulcerative colitis: a retrospective cohort study. Inflamm Bowel Dis. 2011;17:15–21. doi:10.1002/ibd.21393
15. Ajbar A, Cross E, Matoi S, et al. Diagnostic delay in pediatric inflammatory bowel disease: a systematic review. Dig Dis Sci. 2022;67(12):5444–5454. doi:10.1007/s10620-022-07452-5
16. Wlazło M, Meglicka M, Wiernicka A, et al. Dual biologic therapy in moderate to severe pediatric inflammatory bowel disease: a retrospective study. Children. 2022;10(1):11. doi:10.3390/children10010011
17. Lee KE, Faye AS, Vermeire S, et al. Perioperative management of ulcerative colitis: a systematic review. Dis Colon Rectum. 2022;65(S1):S5–S19. doi:10.1097/DCR.0000000000002588
18. Kori M, Zamir Y, Yermiyahu SO, et al. The association of inflammatory bowel disease with celiac disease and celiac autoimmunity in children and adults: a nationwide study from the epi-IIRN. J Crohns Colitis. 2022; 17(5):700.
19. Matsui H, Iwae S, Hirayama Y, et al. Bazex syndrome with hypoalbuminemia and severe ascites. Case Rep Oncol. 2016;9:405–408. doi:10.1159/000448168
20. Vassileva S, Drenovska K, Manuelyan K. Autoimmune blistering dermatoses as systemic diseases. Clin Dermatol. 2014;32:364–375. doi:10.1016/j.clindermatol.2013.11.003
21. MacDiarmid SA, Norman RW. Extragonadal nonseminomatous germ cell tumour with hypercalcemia, masquerading as renal cell carcinoma: a case report. Can J Surg. 1995;38(1):80–82.
22. Armstrong MA, Pollock GF. Pericarditis and pulmonary artery stenosis due to an extragonadal non-seminomatous germ cell tumor: case report and review of the literature. J Emerg Med. 2013;45(5):e157–e160. doi:10.1016/j.jemermed.2013.05.020
23. Esposito F, Di Serafino M, Oresta P. Atypical presentation of sacrococcygeal yolk sac tumor in infant: beware of the injuries of the gluteal region. J Ultrasound. 2014;19(3):227–229. doi:10.1007/s40477-014-0147-9
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.