Back to Journals » Journal of Inflammation Research » Volume 16
A Case of Postnatal Aortic Arch Atresia
Authors Yang ZX, Han Y, Zhang J, Yu YH, Zhu M
Received 18 July 2023
Accepted for publication 12 September 2023
Published 26 September 2023 Volume 2023:16 Pages 4265—4270
DOI https://doi.org/10.2147/JIR.S428231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Zi-Xin Yang,1 Ying Han,2 Jie Zhang,3 Yong-Hui Yu,4 Mei Zhu1
1Department of Ultrasound, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China; 2Department of Ultrasound, Zaozhuang Maternity and Child Health Care, Zaozhuang, Shandong, 277102, People’s Republic of China; 3Department of Cardiac Surgery, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China; 4Department of Neonatology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, 250021, People’s Republic of China
Correspondence: Mei Zhu, Department of Ultrasound, Shandong Provincial Hospital Affiliated to Shandong First Medical University, No. 324 Jingwu-Weiqi Road, Huaiyin District, Jinan, Shandong, 250021, People’s Republic of China, Tel +86 15653101616, Email [email protected]
Background: Aortic arch atresia is a rare congenital cardiac defect that may occur after birth. Pregnant women with gestational diabetes mellitus may increase the risk of aortic arch atresia in newborns after birth.
Case Description: A 16-day-old infant was referred to our hospital on the 15th postnatal day after an interrupted or atretic aortic arch was discovered. No obvious abnormality was detected in the infant during the prenatal ultrasound. Laboratory tests showed elevated inflammatory marker levels. Transthoracic echocardiography showed stenosis of the transverse arch of the aorta and a blind end at the distal end of the left subclavian artery. During surgery, it was found that the isthmus of the aorta was uninterrupted but completely occluded due to inflammation.
Conclusion: This case demonstrates that type A interrupted aortic arch and coarctation of the aorta can be acquired after birth, and if coarctation of the aorta is complicated by inflammation or if the pregnant women have gestational diabetes mellitus, it can result in aortic arch atresia as the patient’s condition worsens. It is advised to consider aortic arch atresia when imaging reveals type A interrupted aortic arch.
Keywords: aortic arch atresia, congenital heart disease, congenital obstruction and anomaly of the aortic arch, gestational diabetes mellitus
Background
Congenital obstruction and aortic arch anomalies are a group of anatomical malformations that includes coarctation of the aorta (CoA, localized partial occlusion caused by luminal shrinkage), interrupted aortic arch (IAA, lack of anatomical continuity in the aortic arch), and aortic arch atresia (AAA) that develops from severe luminal shrinkage.1 Although the aortic arch preserves its anatomical continuity, a specific segment of it is completely obstructed. Since AAA is extremely rare, it is seldom recorded in medical literature. AAA has typically been described as a solitary clinical case. Postnatal atresia and the formation of granulation tissue in local areas detected in tissue biopsy were not previously reported in other cases. In addition, all reported cases did not focus on the health status and perinatal condition of the pregnant women herself. This case report serves as a basis for further research into the development of postnatal AAA, the pathogenesis of AAA, the impact of maternal pathological state on fetal cardiovascular development, and the analysis of image-based diagnosis and differentiation of AAA.
Case Description
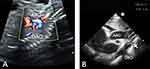
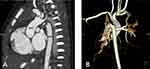
A 15-day-old infant with a purplish discoloration of the skin around the mouth was referred to our hospital for further clinical examination and surgical treatment. The female infant had poor response and oliguria. No evident abnormality was detected in the infant during prenatal ultrasonography (Figure 1A). The mother of the infant suffered from Hashimoto’s thyroiditis (HT) and gestational diabetes mellitus (GDM), although the father was healthy. Physical examination revealed that the infant had an even and regular heart rhythm, a slightly low timbre of heart sounds, and no heart murmur; snoring and a slightly mucus-filled whistling sound were audible on auscultation in both lungs; there was normal blood pressure in all four limbs and mild edema in both lower limbs. Laboratory tests showedelevated inflammatory marker levels, including peripheral white blood cells: 11.9×109/L, procalcitonin: 0.14 ng/mL, interleukin-6: 16.7 pg/mL, and hypersensitive C-reactive protein (hs-CRP) were within the normal range, all of which indicate late-onset inflammation in neonates. The infant had severe cardiomyocyte damage and heart failure. Transthoracic echocardiography findings (Figure 1B): the right ventricular wall was thickened and all segments of the left ventricle myocardium were mildly thickened; the transverse aortic arch had a 0.25 cm stenosis that extended from the opening of the left common carotid artery to the opening of the left subclavian artery, and the distal end of the transverse aortic arch was a blind end; the opening of the left subclavian artery was stenotic; the main pulmonary artery diameter was widened, with patent ductus arteriosus (PDA) and bi-directional shunt signals detected; the fossa ovalis in the interatrial septum had a defect, and a left-to-right shunt signal was detected; arteries within the chest cavity were thickened with abundant blood flow; and the spectral flow of the abdominal aorta was of the arterial type. Computed tomography angiography (CTA) examination of the great vessels of the heart (Figure 2A and B): the lumen of the aortic arch’s descending transition segment was obstructed, with an affected length of approximately 0.45 cm; bilateral thoracic arteries were tortuous and enlarged. The infant was diagnosed with type A of IAA (IAA-A), along with patent ductus arteriosus and stenosis at the opening of the left subclavian artery based on the results of multiple auxiliary examinations before surgery.
We performed total correction surgery of the IAA for the infant under general anesthesia and extracorporeal circulation. Surgical findings: the aortic isthmus remained continuous but was completely occluded, containing tissues resembling inflammation; the inner lining of the duct wall was thickened, and the texture was soft like cheese, with some parts showing worm-eaten-like changes; due to the invasion of proliferative tissue, the left subclavian artery’s opening was almost closed; the ascending aortic wall was thickened due to inflammatory response, while the descending aortic wall remained normal. During the surgery, we performed end-to-side vessel anastomosis to attach the ascending aorta curvature and the descending aorta curvature, excised the duct tissue, sutured the left subclavian artery, and preserved the patent foramen ovale. We took a sample of the aortic wall tissue for pathological examination, and the diagnosis based on postoperative pathological analysis is as follows (Figure 3): the arterial wall tissue had extensive mucoid degeneration and poor muscular structure; the pipe wall thickness varied, with some areas showing calcification and others showing the formation of granulation tissue. These conditions indicated congenital developmental defects with secondary degenerative changes. As shown by the transthoracic echocardiogram examination in postoperative follow-ups, the results of the surgery were good. The lumen of the transverse aortic arch and the descending part of the aortic arch had intact continuity and became wider than previously. The infant had an isthmus of the aortic arch with normal blood flow and no cardiac dysfunction.
 |
Figure 3 Arterial wall tissue had extensive mucoid degeneration with poor muscular structure and calcification and granulation tissue in some areas. |
Discussion
AAA is exceedingly rare compared to other malformation diseases of congenital aortic arch obstruction, and postnatal aortic atresia is even rarer.2 The patient in our study had no evident abnormality observed in the aortic arch in the prenatal stages but was diagnosed with AAA after birth. We analyzed the case from two angles: the embryonic development of the aortic arch and the etiology of aortic arch coarctation and atresia.
Multiple studies suggest that IAA-A is closely related to infant CoA (usually before the ductus arteriosus) or AAA in pathogenesis and is very different from type B of IAA (IAA-B) in development.3–5 It is generally believed that IAA-B results from early degeneration of the fourth aortic arch on the left. As IAA-A, CoA, and AAA occur at the distal end of the left subclavian artery, all of them tend to appear at a relatively late stage of development, ie, after the seventh intersegmental artery (LSA) has completed its proximal migration. Based on case review and the above theories, Van Mierop et al5 reported in their research that the IAA-A and CoA, and IAA-A and AAA pairs may be acquired prenatally but are secondary pathological changes at least in some patients. The left subclavian arteries in such patients have migrated to their normal positions, which clearly demonstrates that the isthmus of the aortic arch must have been normal at some point during development. Our case provides strong support for this inference.
The etiology of aortic coarctation or atresia is not clear. There are now two predominant theories regarding this. According to one theory, smooth muscle cells in the middle layer of the ductus arteriosus wall expand into the aortic wall during embryonic development. During the closure of the ductus arteriosus after birth, the contraction and fibrosis of smooth muscle lead to stenosis.6 Using histology for serial sectioning biopsy on 35 specimens, Ho et al7 confirmed that the aorta adjacent to the ductus arteriosus is completely surrounded by the ductus arteriosus tissue. However, this theory cannot explain the coexistence of CoA and PDA. According to an alternative theory, the inner diameter of the isthmus is smaller than other parts because the blood flow through it only accounts for 25% of the systemic circulation.8 If the blood flow through the isthmus of the aorta decreases, the isthmus will be underdeveloped, leading to coarctation of the aorta. Growing evidence shows there are defects in the ascending aortic vessel wall in neonatal patients with congenital CoA, including increased stiffness, increased collagen, decreased smooth muscle cells, and cystic medial necrosis (CMN) (disordered alignment of elastic fibers in the wall).9,10 Although the underlying mechanisms of the arterial wall abnormalities mentioned above are still unclear, they indicate that coarctation is not only a local problem of the aortic isthmus, and provides evidence for the hypothesis of aortic systemic vascular disease before coarctation. Therefore, we speculate that the infant in our study had an inconspicuous coarctation of the aorta in the uterus. Changes in blood flow in the uterus may lead to secondary changes in gene expression and regulation, resulting in dysfunction of endothelial cells and interstitial cells, which further causes endothelial cell damage and disordered alignment of elastic fibers. Local endarteritis caused by inflammatory mediators after birth aggravate the stenosis of the aortic arch and lead to AAA.
In previous case reports, patients who had been clearly diagnosed as CoA developed postnatal infection with obvious signs and symptoms and than underwent reimaging to reveal a vegetation in the coarctation site, leading to a diagnosis of CoA even aortic arch atresia combined with endarteritis.11,12 whereas in the present case, there was no obvious evidence of exogenous infection after birth, but inflammatory tissue formation at the site of atresia was still detected. In addition, all reported cases did not focus on the health status and perinatal conditions of the pregnant woman herself, who in this case had gestational diabetes mellitus and Hashimoto’s thyroiditis. Studies have shown that the fetoplacental vasculature from GDM manifests alterant maternal-fetal substance transport, and the fetus displays a pro-inflammatory state with an elevated level of pro-inflammatory cytokines in the circulation.13 In addition, cellular and molecular alterations associated with GDM can dysregulate adenosine kinase and make alterations in DNA methylation, leading to plausible fetal programming. If the expression of genes associated with preventing oxidative damage and markers of cardiovascular complications is altered, this may lead to the emergence of specific diseases in the offspring or cause an increased risk of cardiovascular disease.14 Unfortunately, there are no studies on the association of GDM-induced altered adenosine levels and DNA methylation with coarctation or even atresia of the fetal aortic arch after birth. Although Hashimoto’s thyroiditis is an autoimmune disease, it has a lesser impact on the development of the fetal cardiovascular system, especially in mid to late gestation. It is uncertain whether treatments targeting thyroid disease in pregnant women may improve adverse fetal outcomes. More research is needed to answer this question.
AAA and IAA-A are difficult to distinguish, and in most cases, it is impossible to judge them accurately. When the aortic arch is interrupted, its transverse part is completely missing, so there may be different distances between the ends of the patent part of the aortic arch. When the affected segment is atretic, there is no lumen patency, but it still exists as an anatomical entity with fibrous cords connecting it. The persistence of fibrous junctions limits the length of the gap between the atretic segments. According to a case reported by Stimato et al,15 as shown by gadolinium-enhanced magnetic resonance angiography, a patient with aortic atresia had diverticula on both sides of the interrupted zone. This indicates that there were fiber bundles in this area, which is consistent with the diagnostic criteria for AAA. The infant in our study had stenosis at the opening of left subclavian artery. Notably, left subclavian artery stenosis that starts from the starting point of the aorta was found in both patients with IAA-A and IAA-B,16 and is located adjacent to the starting point of the left subclavian artery in patients with IAA-A.17 Stenosis of the left subclavian artery is not a sign unique to coarctation or atresia of the aorta. It is a phenomenon that fits with the above-mentioned systemic arterial vascular disease hypothesis. As the clinical manifestations and hemodynamics of AAA and IAA-A are similar, and the presence or absence of fiber bundles between the two ends of the aorta is difficult to distinguish by CT (computed tomography) angiography and 3D (3-dimensional) reconstruction, it is necessary to consider AAA when the imageology indicates that the lesion is IAA-A. This can ensure more reasonable formulation of patient-specific treatment plans.
There are some differences in the choice of treatment options for aortic atresia and interrupted aortic arch because the lumen of aortic atresia is continuous. Traditionally, complete aortic atresia has been thought to require surgical repair; however, in patients found to be ineligible for surgery, cardiac catheterization and Simultaneous anterograde and retrograde aortogram can be performed, and carefully targeted perforation of the atresia site to create a new lumen using a CTO coronary wire, followed by balloon angioplasty and stenting, can be used to successfully alleviate the constriction, and there have been some successful cases.18–20 This non-surgical treatment has shown promising results at follow-up and may reduce the risk of postoperative infection and perioperative mortality in patients.19 Regardless of treatment modality, patients with AAA require long-term follow-up, blood pressure monitoring, and aortic arch and left ventricular outflow tract patency assessment on a regular basis. Intrauterine development is a stage where intergenerational transmission of pathological conditions and a process that can be effectively treated with interventions. Stricter prenatal management of pregnant women through weight control, dietary adjustments, glucose monitoring, and medications may reduce the risk of newborns being born with aortic coarctation or a sudden worsening of the coarctation or even atresia. Moreover, we should conduct more detailed prenatal screening for pregnant women with high or poorly controlled blood glucose.
Conclusion
The pathological process of AAA is progressive, and even manifests as atresia after birth. In the present case, there was no obvious evidence of exogenous infection after birth, but inflammatory tissue formation at the site of atresia was still detected. Combined with the fact that the pregnant woman has GDM, it suggests that maternal pathological state may increase the risk of newborns being born with aortic coarctation or a sudden worsening of the coarctation or even atresia. It is difficult to distinguish AAA from IAA-A during the diagnostic process; however, the length between the atretic segments and diverticula present at both ends can provide a basis for a differential diagnosis. Interventional treatment of aortic atresia is implementable and effective, and stricter prenatal management of pregnant women with GDM may be gained, but the single patient data in this case do not make it clear whether there is an association.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Shandong Provincial Hospital Affiliated to Shandong First Medical University (No.2017458). A written informed consent was obtained from legal guardians of participant.
Consent for Publication
Consent for publication was obtained from legal guardians of participant whose data are included in this manuscript.
Funding
Application of echocardiography in risk level assessment and management system of fetus-newborn congenital heart disease (No. 2018GSF118112).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hanneman K, Newman B, Chan F. Congenital variants and anomalies of the aortic arch. Radiographics. 2017;37(1):32–51. doi:10.1148/rg.2017160033
2. Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153(6):807–813. doi:10.1016/j.jpeds.2008.05.059
3. Kellenberger CJ. Aortic arch malformations. Pediatr Radiol. 2010;40(6):876–884. doi:10.1007/s00247-010-1607-9
4. Celoria GC, Patton RB. Congenital absence of the aortic arch. Am Heart J. 1959;58(3):407–413. doi:10.1016/0002-8703(59)90157-7
5. Van Mierop LH, Kutsche LM. Interruption of the aortic arch and coarctation of the aorta: pathogenetic relations. Am J Cardiol. 1984;54(7):829–834. doi:10.1016/S0002-9149(84)80215-5
6. Russell GA, Berry PJ, Watterson K, Dhasmana JP, Wisheart JD. Patterns of ductal tissue in coarctation of the aorta in the first three months of life. J Thorac Cardiovasc Surg. 1991;102(4):596–601. doi:10.1016/S0022-5223(20)31432-X
7. Ho SY, Anderson RH. Coarctation, tubular hypoplasia, and the ductus arteriosus. Histological study of 35 specimens. Br Heart J. 1979;41(3):268–274. doi:10.1136/hrt.41.3.268
8. Rudolph AM, Heymann MA, Spitznas U. Hemodynamic considerations in the development of narrowing of the aorta. Am J Cardiol. 1972;30(5):514–525. doi:10.1016/0002-9149(72)90042-2
9. Vogt M, Kühn A, Baumgartner D, et al. Impaired elastic properties of the ascending aorta in newborns before and early after successful coarctation repair: proof of a systemic vascular disease of the prestenotic arteries? Circulation. 2005;111(24):3269–3273. doi:10.1161/CIRCULATIONAHA.104.529792
10. Isner JM, Donaldson RF, Fulton D, Bhan I, Payne DD, Cleveland RJ. Cystic medial necrosis in coarctation of the aorta: a potential factor contributing to adverse consequences observed after percutaneous balloon angioplasty of coarctation sites. Circulation. 1987;75(4):689–695. doi:10.1161/01.CIR.75.4.689
11. Gnanam D, Bartelds B, van Leeuwen WJ, Frohn-Mulder IM, Koopman LP. A case report on endarteritis in a child with coarctation of aorta. Echocardiography. 2019;36(7):1427–1430. doi:10.1111/echo.14418
12. Jaleleddine Z, Sana C, Faker G, Adel K. Infective endarteritis and false mycotic aneurysm complicating aortic coarctation. Ann Pediatr Cardiol. 2012;5(2):197–199. doi:10.4103/0974-2069.99627
13. Sáez T, de Vos P, Kuipers J, Sobrevia L, Faas MM. Fetoplacental endothelial exosomes modulate high d-glucose-induced endothelial dysfunction. Placenta. 2018;66:26–35. doi:10.1016/j.placenta.2018.04.010
14. Silva L, Plösch T, Toledo F, Faas MM, Sobrevia L. Adenosine kinase and cardiovascular fetal programming in gestational diabetes mellitus. Biochim Biophys Acta Mol Basis Dis. 2020;1866(2):165397. doi:10.1016/j.bbadis.2019.01.023
15. Nigro Stimato V, Didier D, Beghetti M, Tissot C. Atresia of the aortic arch in 4-year-old child: a clinical case study. Front Pediatr. 2015;3:19. doi:10.3389/fped.2015.00019
16. Dische MR, Tsai M, Baltaxe HA. Solitary interruption of the arch of the aorta. Clinicopathologic review of eight cases. Am J Cardiol. 1975;35(2):271–277. doi:10.1016/0002-9149(75)90012-0
17. Zetterqvist P. Atypical coarctation of the aorta with bilateral vertebral-subclavian pathway. A report of the first known case of preligamental aortic arch interruption and its surgical correction. Scand J Thorac Cardiovasc Surg. 1967;1(1):68–75. doi:10.3109/14017436709131845
18. Momenah TS, Khan MA, Qureshi S, Hijazi ZM. Acquired aortic atresia: catheter therapy using covered stents. Catheter Cardiovasc Interv. 2015;86(6):1063–1067. doi:10.1002/ccd.26008
19. Chessa M, Favoccia C, Jha NK, et al. Long-term follow-up after recanalisation of aortic arch atresia. EuroIntervention. 2021;16(15):e1274–e1280. doi:10.4244/EIJ-D-18-00857
20. Musso TM, Slack MC, Nowlen TT. Balloon angioplasty with stenting to correct a functionally interrupted aorta: a case report with three-year follow-up. Catheter Cardiovasc Interv. 2008;72(1):87–92. doi:10.1002/ccd.21523
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.