Back to Journals » Infection and Drug Resistance » Volume 16
A Case of Kaposi’s Sarcoma Associated with Disseminated AIDS: The Management Challenges
Authors Shi J , Ying G, Zhang Z
Received 28 July 2023
Accepted for publication 18 September 2023
Published 27 September 2023 Volume 2023:16 Pages 6367—6374
DOI https://doi.org/10.2147/IDR.S428945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Jinchuan Shi, Gaoxiang Ying, Zhongdong Zhang
The Second Infectious Disease Department, Xixi Hospital of Hangzhou, Hangzhou, 310023, People’s Republic of China
Correspondence: Zhongdong Zhang, The Second Infectious Disease Department, Xixi Hospital of Hangzhou, No. 2 Hengbu Street, Liuxia Town, Xihu District, Hangzhou, 310023, People’s Republic of China, Email [email protected]
Background: As a malignant tumor derived from vascular endothelial cells, Kaposi’s sarcoma (KS) is quite common in AIDS patients. Nonspecific clinical symptoms often lead to timely diagnosis or wrong treatment, leading to recurrent disease and poor prognosis. Anti-retroviral therapy (ART) could significantly reduce its morbidity and aggressiveness. As one of the ARTs, liposome anthracyclines are the preferred chemotherapy regimen for disseminated KS with multiple organs or tissue invasion. The curative effect is highly related to the degree of immunosuppression. This is the first case of AIDS with Kaposi’s sarcoma, who was cured after ART and two consecutive chemotherapy with doxorubicin liposome without recurrence. This case may provide new ideas and methods for the clinical management of AIDS with Kaposi’s sarcoma.
Case Description: The patient, a male aged 60 years, was hospitalized on 21/11/2018 following having a cough, expectoration, and difficulty breathing. He was infected with HIV eight years ago and presented symptoms of blood-stained sputum. The patient complained that he had not received ART before. After admission, he was diagnosed as KS with disseminated AIDS after multiple biopsies and histopathological examinations. The patient was treated with ten months of ART (lamivudine+tenofovir+dolutegravir) and 14 times of chemotherapy with doxorubicin liposome (20 mg/m2, three times per week, seven times per course of treatment). The patient’s disease was finally alleviated, and there was no recurrence during the follow-up.
Conclusion: The reconstitution of immune function and consecutive chemotherapy with doxorubicin liposome play a vital role in treating KS. In addition, for the early general symptoms of AIDS patients, such as thrombocytopenia and hemorrhagic purple papules, it is necessary to increase vigilance and obtain the results of histopathological verification as soon as possible to diagnose KS patients at an earlier stage and realize clinical intervention in time.
Keywords: AIDS, Kaposi’s sarcoma, human herpesvirus 8, doxorubicin liposome
Introduction
According to the incidence of cancer patients with AIDS, Kaposi’s sarcoma, non-Hodgkin’s lymphoma, and invasive cervical cancer were classified as AIDS-related tumors by the CDC in the US in 1993.1 KS is the most common malignant tumor related to AIDS, which is vascular endothelial cells characterized by red or purple-brown maculopapular lesions. KS can occur alone or involve the oral gastrointestinal tract and respiratory systems. Human herpesvirus 8 (HHV-8) was closely related to the occurrence of KS.2
The standard therapy method for late-stage KS is still anti-retroviral therapy (ART) combined with systemic chemotherapy.3 A case of disseminated AIDS-KS was diagnosed and treated in the Second Department of Infection in Xixi Hospital, Hangzhou. The lesions of this case involve the skin and mucosa, respiratory system, and bone marrow hematopoietic function. The early clinical manifestations include hemorrhagic purpura and thrombocytopenia. Before diagnosis, the patients have been treated with idiopathic thrombocytopenic purpura and pulmonary infection. After hospitalization, the patient was significantly relieved by ART and 14 times of chemotherapy of doxorubicin liposome. This is the first case of AIDS with Kaposi’s sarcoma, who was cured after ART and two consecutive chemotherapy with doxorubicin liposome without recurrence. Hence, the reconstitution of immune function and consecutive chemotherapy with doxorubicin liposome play a vital role in treating KS.
Case Presentation
The patient, a male aged 60 years, was hospitalized on 21/11/2018 following having a cough and expectoration for more than one month and having difficulty breathing for more than four days. He was infected with HIV eight years ago and presented symptoms of cough and sputum one month ago and blood-stained sputum two weeks ago. The patient complained that he had not received ART before.
The patient was first hospitalized at a community hospital and Zhejiang Provincial People’s Hospital with a cough and expectoration. His pulmonary CT showed interstitial changes in both lungs. The laboratory examination showed a platelet count of 10×109/L, diagnosed as pneumonia and thrombocytopenia. One week after treatment, the platelet count was 174×109/L. Then, the patient presented difficulty breathing four days ago and was admitted to our hospital.
The physical, laboratory, imageological, and histopathological examinations were performed after admission. The physical examination parameters are as follows: temperature of 36.9°C; pulse of 127 times/min; respiration of 20/min; blood pressure of 118/76 mmHg. The acute sickly look and congestion of the throat were visible. The large purple-red macules could be observed on the oral mucosa (Figure 1A). The dark brown and dark red scattered petechiae can be seen in the neck, chest, abdomen, and lower limbs (Figure 1B). Breath sound was slightly rough, with no lung dry, moist rales. Heart rate was consistent, with no murmur. The abdomen was soft without tenderness, the liver, spleen, and ribs were unaffected, and there was no prominent edema in both lower limbs.
 |
Figure 1 The observation and physical examination of disseminated AIDS-KS. (A) Purple-red macula on the oral mucosa. (B) Dark red maculopapular on chest and abdomen skin. |
The laboratory examination results were as follows: WBC of 11.11×109/L, RBC of 4.22×1012/L, Hb of 135 g/L, PLT of 9×109/L, CRP of 25 mg/L, PCT of 0.2 ng/mL. The occult blood test (stool) was positive (+). The antiplatelet antibody was negative (-). Lymphocyte subset test results showed a total lymphocyte count of 550/μL, CD4+/CD8+ of 0.08, CD4+T of 34/μL, and HIV-RNA of 1.12×105 IU/mL. TB infects T-cells was 2.4 pg/mL. EB-DNA with whole blood was 2.28×104 cp/mL.
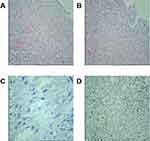
The histopathological examination (4/12/2018) was as follows: (skin biopsy) Ill-defined nodular areas were found in the dermis. Novel and immature vessels can be seen in the nodes; hyperplasia for the mesenchymal spindle cells; Angiomatous changes can be observed in the focal area, which follows the character of KS; Immunohistochemical indices: D34(+); Fli-1(+); HHV8(-); Ki-67(30%+); D2-40(+) (Figure 2). Histopathology examination percutaneous pulmonary biopsy (18/12/2018) showed that neovascularization with varying-sized red blood cells could be seen in the alveolar stroma, spindle cell hyperplasia with mild stroma atypia. Immunohistochemical examination/specific stain of pathology of CD34(+), D2-40(+), Fli-1(+) HHV8(+), Ki-67(20%+), PAS(-), acid-fast staining (-), and Silver hexamine staining (-) (Figure 3).
During this period, the patient was treated as follows: moxifloxacin injection was given for 0.4 g qd intravenous drip for anti-bacterial treatment for seven days. SMZ-TMP was given orally for anti-PCP and anti-fungal at a dose of 1.44 g tid and then changed to 0.96 g qd after 21 days of treatment until CD4+T lymphocytes increased to 200/μL and continued for six months. Fosinate sodium injection was given for 3.0 g intravenous qd for antiviral therapy. Voriconazole tablet was given for 0.2 g oral bid treatment for 15 days. Immunoglobulin 20 g intravenous drip qd treatment for five days. Methylprednisolone treatment was given for anti-inflammatory for 40 mg iv qd, tapering after five days. Recombinant thrombopoietin was given to promote platelets. 15000U was given for subcutaneous injection once daily for nine days.
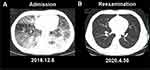
Chest CT (8/12/2018) showed that multiple lesions were observed in both lungs, with a small amount of exudation and a small amount of fluid in both pleural cavities (Figure 4A). Finally, he was diagnosed with AIDS-KS, lung infection, and thrombocytopenia.
According to the guideline of Clinical Pathways for AIDS and related diseases (second edition), ART started on 6/12/2018, and chemotherapy on 12/12/2018. ART was given for lamivudine 0.3g qd, tenofovir 0.3g qd and dolutegravir 0.05g qd. To reduce the potential adverse reactions of the drug, it was adjusted to lamivudine 0.3g qd and dolutegravir 0.05g qd on 11/9/2020. The first chemotherapy was administered with one liposomal doxorubicin treatment (20 mg/m2, three times per week, seven times per course of treatment). New lesions on the right lower extremity after seven times of treatment, and pathologically diagnosed as KS. And pathological biopsy showed tumor recurrence. In general, if the effect of chemotherapy is not ideal, it is generally recommended to adjust the treatment plan according to the relevant literature, such as changing to paclitaxel liposome therapy. However, considering the drug was unavailable in our hospital, the second course of treatment of the original scheme (liposomal doxorubicin) was given after communicating with the patient. Surprisingly, the patient’s condition was completely relieved after the second chemotherapy, and there has been no recurrence. During this period, no severe opportunistic infection occurred, the curative effect was significant, skin lesions gradually vanished, and lung lesions were absorbed.
A reexamination of chest CT (30/4/2020) showed a marked improvement compared with the last examination. The ground-glass attenuation was absorbed for both lungs (Figure 4B). No recurrence was observed after two years of follow-up. Reexamination results showed CD4+T 282/μL and HIV-1 RNA was not detected. Follow-up is still underway. The timeline of patient diagnosis and treatment is shown in Figure 5.
 |
Figure 5 The timeline shows the entire diagnosis and treatment process of this case. |
Discussion
HHV-8 could infect B cells latently and invade vascular endothelial cells when the immune system is compromised.4–6 HHV-8 was first identified from the case of KS in 1994and then detected in all KS subtypes, thus referred to as Kaposi’s sarcoma herpesvirus.7 The genome of HHV-8 encodes many genes, which include Bcl-2, Cyclin D1, interleukin-6 (IL-6), etc. It is worth noting that IL-6 enhances cell proliferation and angiogenesis of KS and causes joint pain and thrombocytopenia by mediating related systemic inflammatory syndrome.8–10 This case of disseminated AIDS-KS is characterized by thrombocytopenia and hemorrhagic purpura. The cause of thrombocytopenia is speculated to be related to the systemic inflammatory response and tumor invasion of bone marrow. Although this patient presented a systemic purple-brown maculopapular rash early on, the clinical diagnosis was easily missed when the characteristic lesions overlapped with hemorrhagic purple maculopapular caused by thrombocytopenia. AIDS with KS-related lung CT presents the bronchial enlargement of the blood vessel bundle, nodules distributed around the bronchovascular bundle, the edges of the nodules are marked by irregular flares, and ground glass image appears if KS invades the pulmonary alveolus. It is difficult to distinguish with interstitial pneumonia due to opportunistic infection when etiological evidence is absent.11,12 In this case, pneumonia-like exudation was the main sign for pulmonary imaging in the early course of the disease, and the typical flame sign was absent.
ART can inhibit the replication of HIV effectively and promote the body’s immune reconstruction, thus is the most effective way to control AIDS.13 However, KS still occurs in persons living with HIV (PLHIV) who have received ART.14 It is speculated that it could be related to the failure of antiviral therapy or persistently low CD4+T cell count in the first year after ART initiation.15,16 Some studies have also indicated that the lesions of KS can remain stationary for a long time or even fade spontaneously, so it is believed that the skin lesions of KS do not necessarily need treatment.17,18 However, for disseminated KS with multiple organ tissue invasion, chemotherapy with anthracycline or paclitaxel is still the first-line treatment for these patients, although it has some inherent drug toxicity.19,20 The effect of the immunomodulatory ability of HHV-8 may suggest the potential role of immunotherapy and immunomodulatory drugs.21–24 However, the efficacy of this new treatment needs to be determined by large-scale evidence-based medical evidence.
In our case, the patient was treated with ART for ten months and finally achieved remission after 14 rounds of chemotherapy with adriamycin liposome and no recurrence was observed after follow-up for more than two years. The patient is still being followed up.
KS is mainly characterized by HHV-8 infection, spindle cell proliferation, and abnormal neovascularization.25 Typical skin lesions are commonly found in the nasal tip, oral mucosa, trunk, limbs, and other areas.26 The skin lesions start with a pink macula, grow dark, form mauve or brown macula or plaques, and finally become hemorrhage skin lesions and nodules.26 ART not only rebuilds immunity and improves immune activation but also significantly reduces the incidence and invasiveness of related tumors, such as KS.27 For disseminated KS with multiple organ tissue invasion, chemotherapy with liposome anthracyclines has significant efficacy and high safety.28 In addition, the degree of immunosuppression is highly correlated to prognosis.29 KS patients with poor immune reconfiguration are more challenging to treat for ART, the course of treatment is longer, and it is more prone to relapse. Thus, the reconstruction of immune function is significant for the treatment and prognosis of AIDS-KS.
Conclusion
This is the first case of AIDS with Kaposi’s sarcoma, who was cured after ART and two consecutive chemotherapy with doxorubicin liposome without recurrence. This further validates the importance of reconstitution of immune function in treating KS. Last but not least, it is necessary to increase vigilance for the symptoms of thrombocytopenia and hemorrhagic purple papulesand obtain the results of histopathological verification as soon as possible to timely diagnose and treat KS patients. This case may provide new ideas and methods for the clinical management of AIDS with Kaposi’s sarcoma.
Ethics Approval and Informed Consent
The authors certify that the patient consent form has been obtained. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. All procedures performed in the study involving human participants were in accordance with the ethical standards of the Ethics Committee of the Hangzhou Xixi Hospital. The ethics committee approved the waiver in this case report, based on the ethical standards to publish the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; have drafted, revised, or critically reviewed the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Key projects of Hangzhou health science and technology plan (ZD20200005).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Barros JP, de Paula T, Mediano MFF, et al. The effects of acute aerobic exercise on blood pressure, arterial function, and heart rate variability in men living with HIV. Front Physiol. 2021;12:685306. doi:10.3389/fphys.2021.685306
2. Hengge UR, Ruzicka T, Tyring SK, et al. Update on Kaposi’s sarcoma and other HHV8 associated diseases. Part 1: epidemiology, environmental predispositions, clinical manifestations, and therapy. Lancet Infect Dis. 2002;2:281–292. doi:10.1016/s1473-3099(02)00263-3
3. Bower M, Dalla Pria A, Coyle C, et al. Prospective stage-stratified approach to AIDS-related Kaposi’s sarcoma. J Clin Oncol. 2014;32:409–414. doi:10.1200/JCO.2013.51.6757
4. Akula SM, Pramod NP, Wang F-Z, Chandran B. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi’s sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi:10.1016/s0092-8674(02)00628-1
5. Hahn AS, Kaufmann JK, Wies E, et al. The ephrin receptor tyrosine kinase A2 is a cellular receptor for Kaposi’s sarcoma–associated herpesvirus. Nat Med. 2012;18:961–966. doi:10.1128/mBio.02892-18
6. Sakurada S, Katano H, Sata T, Ohkuni H, Watanabe T, Mori S. Effective human herpesvirus 8 infection of human umbilical vein endothelial cells by cell-mediated transmission. J Virol. 2001;75:7717–7722. doi:10.1128/JVI.75.16.7717-7722.2001
7. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated kaposi’s sarcoma. Science. 1994;266:1865–1869. doi:10.1126/science.7997879
8. Aoki Y, Jaffe ES, Chang Y, et al. Increased expression of vascular endothelial growth factor (VEGF) in Castleman’s disease: proposed pathomechanism of vascular proliferation in the affected lymph node. Blood. 1999;93:4034–4043. doi:10.3109/10428190009087030
9. Uldrick TS, Wang V, O’Mahony D, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infec Dis. 2010;51:350–358. doi:10.1086/654798
10. Polizzotto MN, Uldrick TS, Hu D, Yarchoan R. Clinical manifestations of Kaposi sarcoma herpesvirus lytic activation: multicentric Castleman disease (KSHV–MCD) and the KSHV inflammatory cytokine syndrome. Front Microbiol. 2012;3:73. doi:10.3389/fmicb.2012.00073
11. Sivit CJ, Schwartz AM, Rockoff S. Kaposi’s sarcoma of the lung in AIDS: radiologic-pathologic analysis. Am J Roentgenol. 1987;148:25–28. doi:10.2214/ajr.148.1.25
12. Naidich DP, Tarras M, Garay SM, Birnbaum B, Rybak BJ, Schinella R. Kaposi’s sarcoma: CT-radiographic correlation. Chest. 1989;96:723–728. doi:10.1378/chest.96.4.723
13. Rojas Rojas T, Poizot-Martin I, Rey D, et al. Incidence of cervical, breast and colorectal cancers between 2010 and 2015 in people living with HIV in France. PLoS One. 2022;17(3):e0261069. doi:10.1371/journal.pone.0261069
14. Maurer T, Ponte M, Leslie K. HIV-associated Kaposi’s sarcoma with a high CD4 count and a low viral load. N Engl J Med. 2007;357:1352–1353. doi:10.1056/NEJMc070508
15. Papalini C, Brescini L, Curci L, et al. Kaposi Sarcoma in People Living with HIV: is it Water under the Bridge? Mediterr J Hematol Infect Dis. 2023;15(1):e2023027. doi:10.4084/MJHID.2023.027
16. Guihot A, Dupin N, Marcelin A-G, et al. Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194:1078–1088. doi:10.1086/507648
17. Zhu X, Guo Y, Yao S, et al. Synergy between Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein in promotion of angiogenesis and oncogenesis: role of the AKT signaling pathway. Oncogene. 2014;33:1986–1996. doi:10.1038/onc.2013.136
18. Vincenzi B, D’Onofrio L, Frezza AM, et al. Classic Kaposi Sarcoma: to treat or not to treat? BMC Res Notes. 2015;8:1–4. doi:10.1002/(sici)1097-0215(19990118)80:2<178::aid-ijc3>3.0.co;2-l
19. Kondo Y, Izumi T, Yanagawa T, Kanda H, Katano H, Sata T. Spontaneously regressed Kaposi’s sarcoma and human herpesvirus 8 infection in a human immunodeficiency virus‐negative patient. Pathol Int. 2000;50:340–346. doi:10.1046/j.1440-1827.2000.01043.x
20. Windebank AJ, Grisold W. Chemotherapy‐induced neuropathy. J Peripher Nerv Syst. 2008;13:27–46. doi:10.1002/ana.24951
21. Yarchoan R, Uldrick TS. HIV-associated cancers and related diseases. N Engl J Med. 2018;378:1029–1041. doi:10.1056/NEJMc1804812
22. Polizzotto MN, Uldrick TS, Wyvill KM, et al. Pomalidomide for symptomatic Kaposi’s sarcoma in people with and without HIV infection: a Phase I/II study. J Clin Oncol. 2016;34:4125. doi:10.1200/JCO.2016.69.3812
23. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. 2019;5:1–21. doi:10.5858/arpa.2012-0101-RS
24. Fromentin R, DaFonseca S, Costiniuk CT, et al. PD-1 blockade potentiates HIV latency reversal ex vivo in CD4+ T cells from ART-suppressed individuals. Nat Commun. 2019;10:1–7. doi:10.1038/s41467-019-08798-7
25. Galanina N, Goodman AM, Cohen PR, Frampton GM, Kurzrock R. Successful treatment of HIV-associated Kaposi sarcoma with immune checkpoint blockade. Cancer Immunol Res. 2018;6:1129–1135. doi:10.1158/2326-6066.CIR-18-0121
26. Lodi S, Guiguet M, Costagliola D, et al. Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst. 2010;102:784–792. doi:10.1093/jnci/djq134
27. Morris SR, Chen B, Mudd JC, et al. Inflammescent CX3CR1+CD57+CD8+ T cells are generated and expanded by IL-15. JCI Insight. 2020;5(11):e132963. doi:10.1172/jci.insight.132963
28. Cao W, Mehraj V, Kaufmann DE, Li T, Routy JP. Elevation and persistence of CD8 T‐cells in HIV infection: the Achilles heel in the ART era. J Int AIDS Soc. 2016;19:20697. doi:10.7448/IAS.19.1.20697
29. Lambert M, Gannagé M, Karras A, et al. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108:3871–3880. doi:10.1182/blood-2006-03-014225
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.