Back to Journals » Open Access Journal of Contraception » Volume 14
A Brief History and Advancement of Contraceptive Multipurpose Prevention Technology (cMPT) Products
Authors Dohadwala S, Politch JA, Barmine JH, Anderson DJ
Received 15 March 2023
Accepted for publication 27 May 2023
Published 13 June 2023 Volume 2023:14 Pages 83—94
DOI https://doi.org/10.2147/OAJC.S375634
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Igal Wolman
Sarah Dohadwala,1 Joseph A Politch,2 Jessica H Barmine,2 Deborah J Anderson1– 3
1Department of Virology, Immunology and Microbiology, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, USA; 2Department of Medicine, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, USA; 3Department of Obstetrics and Gynecology, Boston University Chobanian and Avedisian School of Medicine, Boston, MA, USA
Correspondence: Deborah J Anderson, Professor of Medicine, Obstetrics and Gynecology, and Microbiology, Boston University Chobanian and Avedisian School of Medicine, 670 Albany St Suite 516, Boston, MA, 02118, USA, Tel +617-358-2480, Email [email protected]
Abstract: The high incidence of HIV and other sexually transmitted infections (STIs), and an unmet need for modern contraception resulting in a high unintended pregnancy rate, are major problems in reproductive health. The concept of multipurpose prevention technology (MPT) was introduced following the failure of several leading microbicide candidates to prevent human immunodeficiency virus type 1 (HIV-1) transmission in large clinical trials in the early 2000s. MPTs are defined as products designed to simultaneously prevent at least two of the following conditions: unintended pregnancy, HIV-1, or other major STIs. The goal of contraceptive MPT products (cMPTs) is to provide contraception and protection against one or more major STI pathogen (eg, HIV-1, herpes simplex virus (HSV) type 2, Neisseria gonorrhoeae (gonorrhea), Treponema pallidum (syphilis), Trichomonas vaginalis, Chlamydia trachomatis (Chlamydia). This new field has great potential and will benefit from lessons learned from the early microbicide trials. The cMPT field includes candidates representing various categories with different mechanisms of action including pH modifiers, polyions, microbicidal peptides, monoclonal antibodies, and other peptides that target specific reproductive and infectious processes. More preclinical research is being conducted to ensure minimal side effects and maximum efficacy in vivo. Effective proven and novel candidates are being combined to maximize efficacy, minimize side effects, and avoid drug resistance. More attention is being paid to acceptability and new delivery systems. cMPTs have a very promising future if adequate resources can be mobilized to sustain the effort from preclinical research to clinical trials to bring effective, acceptable, and affordable products to market.
Keywords: contraceptive, microbicide, multipurpose prevention technology, sexually transmitted infection
Introduction
The Multipurpose Prevention Technology (MPT) Initiative formally emerged from the microbicide field in 2009 to provide women and men protection against multiple current reproductive health risks, including unintended pregnancies, HIV-1, and/or other major sexually transmitted infections (STIs).1 In this review, we delineate contraceptive MPTs (cMPT), products that specifically provide contraception, and protection against at least one pathogenic STI. Approximately half of all pregnancies worldwide are unintended due to lack of use or incorrect use of effective modern contraceptives, with an estimated gap of 257 million women who would benefit from additional contraceptive options.2 In addition, over 350 million bacterial and parasitic pathogens (eg, N. gonorrhoeae, T. pallidum, T. vaginalis, C. trachomatis) and millions of incurable pathogenic viruses (eg, HIV-1, HSV-2) are sexually transmitted each year globally.3 The emergence of antibiotic resistant strains of various STI pathogens highlights the need for investment in prevention strategies in addition to new treatments.3 For these reasons, many sexually active women and men indicate that they would welcome a product that is not only contraceptive but also protective against STIs.4 Many factors, including efficacy, formulation, timing of use, side effects, messiness, and dose volume, can affect user acceptance of an MPT product, and survey data suggest that geographic and cultural factors play a role in user preferences, highlighting the need to bring diverse products to market.4 This current review of the cMPT field presents candidate products representing various categories with different mechanisms of action including pH modifiers, polyions, microbicidal peptides, monoclonal antibodies, and other peptides that target specific reproductive and infectious processes.
History of MPTs
3000 BCE-1990
Human sexually transmitted infections (STIs) have been described since antiquity. The genetic record shows that HSV-2 evolved into its present form and infected humans over 1.5 million years ago.5 Symptoms of STIs, such as genital ulcers, inflammation, and discharge, were described by ancient Egyptians, Greeks, and Romans.5 In the early 15th century, these conditions were given the name “venereal disease” after Venus, the goddess of love, but it was not until the mid-19th century, following the development of powerful compound microscopes, that individual bacterial STI organisms would be identified and characterized.6 Sexually transmitted viruses would not be differentiated until the invention of the electron microscope in the mid-20th century.6
Male barrier methods (penile sheaths) were used to protect against STIs in ancient Egypt and Rome, and a female condom was described in Greek literature from 3000 BCE.5 Early condoms were made from animal bladders and intestines or from chemically-treated linen.7 Their use dramatically increased in the 16th century when a severe syphilis epidemic devastated Europe. An Italian anatomist, Gabriele Falloppio, conducted one of the first recorded clinical trials to test the efficacy of chemical-soaked linen male condoms to prevent syphilis; of the 1100 participants he studied, reportedly none became infected.8 Condoms became more accessible and popular in the 1920s following the invention of latex and were widely used by American troops in World War II.7 The AIDS epidemic, starting in the 1980s, further boosted the use of condoms for STI prevention (Figure 1).7
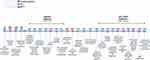 |
Figure 1 History of MPTs. Created with BioRender.com. |
The history of contraception also has roots in antiquity. Male penile barriers have been primarily used for STI prevention throughout history, but their contraceptive properties have also been recognized. The modern latex condom was introduced in the 1920s.7 Women have used vaginal poultices for contraception since at least 2000 BC, employing soft materials such as cotton or wool soaked in various mixtures of oils, fruit juice, herbs, and other substances to form a tampon-like plug.10 The solid material would have provided a partial barrier, and some of the ingredients (eg, the acidic nature of lemon juice) could have impaired sperm function and inhibited STIs.30 The modern female condom which, like the male condom, effectively provides both contraception and protection against STIs, was introduced in 1993.30 While progress has been made in the fields of contraception and STI treatment and prevention, barrier methods remain the only widely available approved cMPT product to date. Today, many couples use a combination of condoms and other contraception methods for added protection.
Microbicide Research: 1998-2010
The Alliance for Microbicide Development, founded in 1998, brought together researchers, developers, and stakeholders globally to fast-track microbicide development.25 Over the next 10 years, six microbicide candidates were evaluated in large clinical trials: two surfactant compounds (nonoxynol-9 and C31G) that disrupt cell membranes, three polyanion compounds (PRO2000, cellulose sulfate, and Carraguard) that block viral binding, and one pH modifier (BufferGel). All of these microbicides also had contraceptive activity. The trials involving these microbicides are summarized in Table 1, Supplementary Tables 1 and 2. None of the candidates protected against HIV transmission in clinical trials, and the field was severely reprimanded for poor coordination among interested parties and the choice of non-validated scientific targets.31 Subsequently, the Alliance for Microbicide Development was disbanded, and funding for microbicide research was drastically reduced. The term “multipurpose prevention technology” (MPT) was introduced in 2009 to redefine and reinvigorate the field. MPTs are defined as methods that provide protection against at least two of the following: unintended pregnancy, HIV-1, or other STIs. cMPTs provide contraception and protection against at least one prominent STI.
 |
Table 1 Summary of Efficacy of Leading Contraceptive MPTsa |
Current Status of MPTs and Prospects
Broadly Acting MPTs
Barrier Methods
Condoms, which are currently the only widely available MPT product, are relatively inexpensive, widely available directly to the end-user, provide effective contraception, and protect against most sexually transmitted infections (STIs).3 However, the time needed to apply a condom can interrupt intimacy, and many users report decreased sexual sensation. Under perfect use, male condoms are 98% effective in preventing pregnancy, but in actual use, they are only 87% effective.32 They also provide >90% protection against STI pathogens that are transmitted through the exchange of sexual secretions (HIV-1, Hepatitis-B, N. gonorrhoeae, T. vaginalis) but are less effective against those primarily transmitted by skin contact (HPV, HSV-2, T. pallidum).33 The male condom requires compliance from male partners, reducing women’s agency in protecting themselves from unintended outcomes. The female or internal condom, while having the same protective characteristics as the male condom and offering the distinct advantage of being controlled by the female partner, has not gained widespread popularity primarily because it is less accessible, more costly, indiscrete, and difficult to use. Female condoms in the United States are currently made from nitrile, a stronger, though less pliable rubber than natural latex, and are held in place by two large rings which must be inserted manually.23 In India, female condoms are made from natural rubber latex and use a sponge to secure them in place.34 The female condom was introduced in over 90 countries, but only accounts for 1.6% of global condom distribution due to low user acceptability and high cost.35 Ideal barrier methods should be easier to use, more discrete and intimate, and coitally independent to overcome the current shortcomings.
Chemical barrier products prevent sperm and pathogens from passing through the mucus layer that covers the vaginal or rectal epithelium. One example is the “invisible condom”, a poloxamer gel that coats the entire vaginal epithelium using a novel applicator and impedes the passage of pathogens and sperm.36 The gel is combined with sodium lauryl sulfate (SLS), a surfactant spermicide/microbicide which immobilizes sperm and kills pathogens in vitro.37 In a clinical trial to assess acceptance and safety, the invisible condom was found to be imperceptible and to adhere to the mucosa up to 4–8 hours after coitus. The product did not irritate the vagina or disrupt the vaginal pH.37 In a pre-phase III efficacy trial, the invisible condom immobilized 99% of sperm in a post-coital test; four out of 30 women became pregnant after 3 months of use, but this was attributed to user failure.38 This unique and promising product prevented STI transmission in animal models, but the invisible condom needs more extensive testing for contraceptive efficacy and STI prevention in clinical trials before coming to market.
Topical chitosan is another potential barrier product. In a sheep model, it crosslinked cervical mucus to prevent the progression of sperm and did not irritate the vaginal tissue on initial safety tests.39 Interestingly, its mechanism of action was purely reinforcing the mucus barrier, with no additional spermicidal activity.39 Further developments in gel and cream formulations that alter the rheological characteristics of cervicovaginal mucus provide a promising novel approach to increase the suite of cMPT barrier products.
Surfactant MPTs
Surfactants reduce surface tension, making hydrophilic-hydrophobic interfaces unstable. As contraceptives, they disrupt the lipid bilayer of sperm cells, killing sperm. As microbicides, surfactants disrupt the lipid envelope of various pathogens, including bacteria and viruses that are enveloped in a lipid bilayer. Surfactants are nonspecific and do not promote viral or bacterial resistance; however, this mechanism can damage host epithelia, leading to lesions that may increase STI risk and be uncomfortable for users. Some examples of surfactant products are nonoxynol 9 (N-9), Savvy (C31G), sodium lauryl sulfate (SLS), and Consap (saponin-based).
N-9 has been available as a nonprescription topical contraceptive since the 1960s. In addition to killing sperm, N-9 was a leading early MPT candidate because it was shown to kill HIV-1 in vitro and block the vaginal transmission of simian immunodeficiency virus (SIV) in a nonhuman primate (NHP) model.40 However, a series of clinical trials revealed that N-9 did not decrease the transmission of HIV or other STIs, and disrupted the vaginal epithelium. In one study of sex workers that used N-9 more than 3.5 times a day, severe vaginal lesions and significantly increased HIV transmission were recorded.41 N-9 remains on the market as a contraceptive for populations with low HIV risk; the product insert states that N-9 should not be used as a microbicide to prevent HIV transmission.
Savvy is a mixture of two surfactants (known in chemical repositories as Glyminox) with a similar profile to N-9 but is designed to be less irritating to the vaginal mucosa.42 Its efficacy as a microbicide was assessed in a phase III trial, but it did not reduce HIV acquisition.43 SLS is another surfactant, commonly used in several cosmetic products. It was tested in a preliminary efficacy trial as part of the invisible condom (described above). SLS demonstrated contraceptive efficacy but has not been tested for protection against HIV or other STIs in clinical trials. Consap (saponin-based) is a contraceptive cream derived from the Sapindus plant. It was tested in a phase IIIa trial in India to assess safety and contraceptive efficacy. Initial results have given a Pearl index of 2.3 (the number of pregnancies per 100 women over a year).44,45
While surfactants may be promising in their broad abilities, there remains a need to balance their microbicidal and spermicidal effects with their potential to damage the vaginal and penile epithelia, and to enhance their in vivo efficacy. The future of surfactant MPTs depends on the selection of less inflammatory products and improvement of delivery methods to enhance their in vivo efficacy.
Polyanion MPTs
Polyanions, or polymers with a negative charge, are viral entry inhibitors that disrupt the interaction between host entry receptors and positively charged surface proteins on viral membranes, such as between host CXCR4 and HIV gp120.46 In addition, polyanions are potential contraceptive MPT candidates because they interfere with the sperm enzymes hyaluronidase and acrosin and impede sperm passage through mucus.45 Polyanionic polymers that have been investigated as MPTs include polyphenylene carboxymethylene (PPCM), naphthalene sulfonate (PRO2000), Carrageenan (Carraguard/PC-515), cellulose sulfate (Ushercell), cellulose acetate phthalate (CAP), and SPL7013 Dendrimer (Vivagel). Three of these candidates, PRO2000, Carraguard, and cellulose sulfate progressed to phase III efficacy trials, but none reduced HIV infection. It was later shown that seminal plasma dramatically decreases the antiviral activity of many polyanionic MPTs, which may explain their reduced in vivo efficacy.47 Furthermore, a phase III trial with Carraguard measured gel use with an applicator dye test and estimated adherence to the regimen to be 50% lower than the self-reported values. Low adherence may also explain the failure of these candidates in clinical trials.48 Cellulose sulfate was another candidate which demonstrated microbicidal activity against N. gonorrhoeae, C trachomatis, HPV, Gardnerella vaginalis, and HIV in vitro. However, the phase III trial was halted as a precaution due to a higher observed incidence of HIV in women using cellulose sulfate gel compared to the placebo group.49
Other anionic cMPT candidates include PPCM, CAP, and Vivagel. PPCM is an anionic condensation polymer that is chemically distinct from prior candidates due to its lack of sulfonation which has many positive downstream effects including retaining functionality in the presence of seminal plasma.50 In preclinical models, PPCMs demonstrated microbicidal activity against HIV-1, HSV-1 and 2, HPV and N. gonorrhoeae. Its contraceptive activity is not spermicidal but it disrupts the acrosomal reaction.50 Currently, PPCM is being developed by Yaso Therapeutics. CAP blocks HIV gp120 binding sites, and demonstrates activity against HSV. It was tested for safety as both a film and a micronized gel, but the phase I trial was discontinued because participants experienced heavy vaginal discharge.
Dendrimers are highly branched, typically spherical polymers composed of a core and charged surface moieties that also target viral entry. Because dendrimers have multiple charged sites, they can bind to multiple locations on cells or viruses. Vivagel, a dendrimer with high anionic surface charge, was assessed for safety and efficacy in a phase I trial. For determining efficacy, ex vivo cervicovaginal fluid (CVF) was tested for anti-HSV and HIV activity.51 Strong antimicrobial activity was observed in CVF immediately after delivery of the product and 3 hours post-delivery in the presence of seminal plasma.51 However, elevated levels of proinflammatory cytokines were also observed, and when the MTN-004 clinical trial revealed a significant increase in grade 1/2 genital adverse events (notably vulvovaginal burning) and a shift in vaginal microflora, the product was withdrawn from further development as a microbicide.52,53 Vivagel was also tested in phase III trials as a treatment for bacterial vaginosis (BV) and was efficacious. As a result, Vivagel is available as a treatment for BV in the EU, US, and Australia. However, the manufacturer of Vivagel, Starpharma, has not pursued further trials. Other research groups are pursuing combination dendrimers as MPTs in preclinical investigations.54,55 Second generation polyanions such as dendrimers remain potential MPT candidates, particularly as combination therapies in concert with other molecules.
pH Modifiers
One mechanism of innate immune defense in the female reproductive tract (FRT) is low vaginal pH (3.5–4.5) maintained by lactic acid produced by endogenous lactobacilli. Acidic pH can kill sperm and many STI pathogens.56 Furthermore, low pH is deleterious to N. gonorrhoeae, T. pallidum, H. ducreyi, and BV-associated bacteria.57 After intercourse, vaginal pH increases due to alkaline semen (pH 7.2–7.8) to create a permissive environment for conception.58 Several MPT products aimed at maintaining low vaginal pH after ejaculation have been developed. BufferGel is an aqueous gel product containing Carbopol 974P, a cross-linked acrylic acid polymer with a pH of 3.9 that buffers the neutralizing effect of seminal plasma after intercourse.54,59 In preclinical trials, BufferGel was found to be spermicidal and microbicidal against HIV, HSV-2, HPV, and C. trachomatis.59 In clinical trials, BufferGel was safe and had significant contraceptive activity when used with a diaphragm-like device; however, it did not protect women against HIV infection.57 Acidform (Phexxi) is a vaginal gel composed of lactic acid, citric acid, and potassium bitartrate.60 Clinical trials demonstrated contraceptive efficacy and protection against chlamydia and gonorrhea, although a second phase IIb/III trial to assess its microbicidal activity against C. trachomatis and N. gonorrhoeae did not indicate efficacy.61 Future directions of pH modulation in cMPT development include combining the effects of pH modulation with the stronger profiles of other contraceptives and microbicides to achieve synergistic protection from infection.
Metallic MPTs
Metals such as copper and zinc are toxic to sperm and cause mild inflammation in the FRT, making them potential contraceptives. They also can disrupt multiple viral components, including proteases, internal proteins such as p24, and nucleic acids.62 These nonspecific mechanisms of pathogen disruption do not promote drug resistance, but can induce unanticipated side effects in vivo such as toxicity/inflammation. The copper intrauterine device (IUD), which has been used for contraception for over 40 years, has not been shown to affect HIV acquisition rates in clinical trials, most likely as it is placed in the uterus and has little effect in the lower FRT where first contact with STI pathogens occurs.63 A copper intravaginal ring product is being developed to deliver copper to the lower FRT for microbicidal and contraceptive effects.64 Another metal-based product in development as a cMPT is zinc acetate. This compound has both contraceptive and microbicidal activities in vitro. Zinc ions disrupt HIV RNA transcription.65 Vaginal treatment of macaques with zinc acetate protected against simian-human immunodeficiency virus (SHIV) and HSV-2 challenge.66 Zinc acetate is spermicidal, likely due to the acetate moiety reducing oxygen availability in the FRT.62 Zinc has also been pursued as a candidate for reducing dysmenorrhea in women, as it interacts with prostaglandins which are responsible for pain and inflammation during menses.63 Zinc acetate has been tested in a few clinical trials, but mostly as in combination with antiretroviral drugs or formulated in a carrageenan gel.66,67 Future directions for metal MPTs could involve their delivery in mucoadhesive gels or nanoparticles to provide longer term protection.
Peptide MPTs
Antimicrobial peptides (AMPs) are naturally secreted in the FRT to provide innate immune defense and other functions.68 Levels of these peptides vary throughout the menstrual cycle, and their role in STI defense has been studied in clinical cohorts such as the HIV-exposed seronegative groups (HESN) that are resistant to HIV acquisition.69
Use of antimicrobial peptides as MPTs remains an attractive proposition due to their broad-spectrum activities against pathogenic viruses and bacteria. To date, these peptides do not appear to cause irritation of the vaginal epithelium or disrupt the endogenous lactobacilli microbiome.70 Furthermore, twelve antimicrobial peptides have been found to be spermicidal, including LL37, Nisin A, and Magainin 2.71 The best characterized AMP MPT is LL37, which has both spermicidal and broad microbicidal activity. In addition to its endogenous expression in the FRT, LL37 is produced in the vagina 2–6 hours after intercourse from a precursor peptide in seminal plasma, hCAP-18. It exerts contraceptive and microbicidal activity by disrupting membranes through interaction with anionic surface lipids abundant on microbes and sperm. LL37 did not irritate the vaginal epithelium in a mouse model.72 Furthermore, sperm treated with LL37 underwent a premature acrosome reaction and became immotile.72 The peptide also has broad antimicrobial activity, with demonstrated in vitro activity against HIV, N. gonorrhoeae and Candida albicans. To enable its topical use, the peptide has been engineered to be protease resistant, with truncated variants such as 17BHEP aiming to have longer residence times in the FRT.73 The next step will be to test this cMPT candidate for safety in phase I clinical trials, and contraceptive and microbicidal efficacy in stage II and III clinical trials. Characterizing other less studied AMP MPT candidates and comparing their properties to LL37 could enable rational design of such peptides, as it has for other peptide classes.74,75
Targeted MPT Agents
Small Molecule Natural Products
Another area of interest is biologically active natural products, a classic place to search for small molecules that may have specific contraceptive or antimicrobial activity.76 There are many bioactive small molecules of contraceptive interest, including curcumin, which is derived from turmeric. Curcumin has been widely investigated for its immunosuppressive properties in wound healing, among other applications.72 Naz et al investigated its use as a potential MPT in vitro, finding that a >250 μM dose had contraceptive activity and microbicidal activity against yeast and bacteria.77 Natural products have many strengths, such as a high acceptance in communities that may be resistant to synthetic medications, biocompatibility, and a history of use outside of Western medicine. However, natural products have the potential for non-specific activity, unknown mechanisms of action, or expensive purification procedures limiting their use as medication.
Monoclonal Antibodies
Immunocontraception is the concept of using the immune response for contraception. One avenue for reversible immunocontraception is topical delivery of anti-sperm monoclonal antibodies (mAbs). Isojima et al isolated a potent IgM antisperm antibody from an infertile woman; this mAb has been engineered as an IgG1 antibody in Nicotiana benthamiana (tobacco plants) for use as a contraceptive.78,79 The antibody targets a male reproductive tract-specific glycan on CD52 (CD52g) and potently causes sperm agglutination and trapping in cervical mucus. This antibody, named the Human Contraception Antibody (HCA), was incorporated into a film (ZB-06), and was found to be safe and efficacious in a phase I trial using a postcoital test.80 Furthermore, HCA may have additional functions that can target pathogens. The GPI-anchored glycopeptide, CD52, inserts into the plasma membrane of sperm as part of their maturation in the epididymis, and may incorporate into the lipid bilayer of pathogens and infected cells present in the male reproductive tract (MRT). We speculate that this may play a role in clearing HIV-1 infected leukocytes from mucosa before they can infect the vaginal epithelium and enable Fc mediated functions such as complement-mediated lysis of MRT-associated pathogens with CD52g on their surface. Further development of monoclonal antibody-based cMPTs include generating more effective antibody variants, pursuing alternative delivery mechanisms and use of antibody combinations that have synergizing effects.81
Combination MPT Products
Many first generation MPTs were single agents aimed to simultaneously prevent infection and unintended pregnancy through nonspecific mechanisms such as membrane dissolution or inhibition of binding to receptors. So far, these single MPT compounds have failed in clinical trials due to nonspecific damage to the vaginal epithelium and inactivity in the vaginal environment. One way to potentially overcome these shortcomings is to use combinations of synergistic MPT agents.
A new class of cMPTs combine potent antiretroviral drugs (ARVs) with previously approved contraceptive hormones. Daily hormonal birth control pills and daily antiretroviral therapy (ART) are pre-existing medications that, if combined, would reduce patient burden in maintaining therapy. A combination product, a dual prevention pill, containing emtricitabine and tenofovir alafenamide for ART and ethinyl estradiol and levonorgestrel for contraception, is currently slated for acceptability studies. Levonorgestrel was added to a monthly Dapivirine intravaginal ring developed to prevent HIV sexual transmission, and this contraceptive ARV ring demonstrated safety and effective drug release profiles in phase I clinical trials. However, the dapivirine ring failed to gain Food and Drug Administration (FDA) approval for HIV prevention due to poor efficacy (29%) in phase III clinical trials, and the dapivirine contraceptive MPT ring has for now been withdrawn from further development.82 However, this may be revisited as subsequent data analysis from the clinical trials indicates anti-HIV efficacy exceeding 50% among women who consistently used the Dapivirine ring; the World Health Organization (WHO) has approved this product for HIV prevention.83 Three other potent ARVs, tenofovir alafenamide, islatravir, and dolutegravir, have been formulated into long-acting subdermal implants that release effective drug concentrations for 6 months to 1 year. Plans are underway to add a contraceptive hormone (eg, levonorgestrel) to these implants to develop long-acting contraceptive MPTs.84,85 Oral and injectable anti-HIV/contraception combinations are also being explored. Long-acting cabotegravir/rilpivirine injections were recently approved for HIV prevention and could be combined with effective injectable contraceptives such as Depo-Provera (medroxyprogesterone acetate) or NET-EN (norethisterone enanthate).
Two combination cMPT ring products are also under development: a 90-day silk fibroid ring containing etonogestrel/ethinyl estradiol and QGriffithsin (being developed by the Population Council) and a specially designed ring containing pods that release an engineered high-valency antisperm antibody and HIV-specific antibodies (being developed by Mucommune).
There are a few gel and film products in preclinical development, including an islatravir/progestin intravaginal film, an organic acids/QGriffithsin fast dissolving film, and a Carrageenan/Griffithsin (CG/GR) gel. The CG/GR gel was tested preclinically in macaques and found to be protective against HSV-2 and HPV pseudoviruses.86 Long-term alternative delivery methods in preclinical development that combine ART and hormonal contraception include a long-term injectable product, an injectable in-situ forming implant, and an MPT microarray patch similar to a bandage.1
Design Considerations for Future cMPTs
Several lessons have been learned from the history of microbicide development. First, it is essential to avoid damaging the vaginal mucosa and/or microbiome, as this could cause discomfort to the user and may promote the transmission of HIV-1 and other STIs. Several of the surfactant and polyanionic products used in early microbicide clinical trials induced vaginal inflammation and lesions to varying degrees, leading to their discontinuation after preliminary clinical trials. Second, it is essential to confirm product activity in the genital environment. Several of the polyanion candidates apparently failed in clinical trials because they were neutralized in the presence of semen. This effect should have been detected in preclinical studies before the products were advanced into clinical trials at considerable expense and risk to human subjects. Polyanionic microbicides were also later found to be less effective against HIV strains that use the CCR5 receptor for entry which accounts for a majority of heterosexually transmitted strains.87 It is essential to perform extensive preclinical research before advancing cMPT candidates into clinical trials. A third design consideration is combining products with known in vitro efficacy to synergize effects at lower drug doses, minimize side effects, and prevent the development of drug resistance by involving multiple protective mechanisms. Furthermore, investing in delivery modalities already widely used, such as injectables, films, rings, and oral medications, enables new drugs to fit into existing contraceptive user paradigms. The contraception field has learned that long-acting reversible contraception (LARC) methods such as implants and intrauterine devices that release drugs over an extended period and bypass the need for daily or monthly patient adherence are highly effective and such approaches should be explored for cMPTs. These new modalities should also be extensively tested for acceptability to confirm their potential demand in target populations. Lastly, phase III efficacy trials for MPT products are very difficult and costly. Many promising cMPT products are stymied at the preclinical development stage because of the large investment required to move them forward into advanced clinical trials. Developing strong preclinical markers for contraceptive and microbicidal activity will allow for efficient and objective prioritization of candidates to move into clinical trials.
Conclusion
The future of cMPTs holds considerable promise. The landscape for MPTs is constantly evolving, with many different academic and commercial entities at various stages of product development and clinical testing, reflected in the iMPT database of products in the pipeline.1 The potential of new drug products and delivery modalities to reduce both unintended pregnancy and sexually transmitted infections can have a huge impact on the livelihood and agency of women and men around the globe. Lessons from historical developments in the MPT field, such as the failure of nonoxynol 9, and the lack of cross-institution prioritization of clinical candidates for large, expensive clinical trials, can inform future efforts. In particular, the increasing importance of acceptability studies and surveys to create products that people actually want to use will prioritize delivery modalities with favorable end user characteristics for investment and development. Furthermore, successful development of cMPT products specifically addressing contraception and STI prevention could enable the use of similar technologies to enhance reproductive health more broadly, such as reducing bacterial vaginosis or vaginal inflammation in response to infections or other factors, preventing miscarriage and premature birth, preventing cancer (eg, targeting HPV and related neoplastic mechanisms), easing menstrual cramps and dysmenorrhea, or enhancing wound healing after trauma or childbirth. We hope that future characterization and efficacy testing of potential cMPT products at the preclinical and clinical stages will lead to new investment in this therapeutic area that can bring products from the clinical pipeline into the market to reduce the global burden of STI transmission and unintended pregnancy. The massive investment in vaccines and testing due to the COVID pandemic highlights the potential of similar financial and organizational investments in prevention of unintended pregnancy and STIs, particularly as these therapeutic indications are difficult to test in clinical trials and require extensive cross-institutional coordination.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by P50HD096957 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) branch of the National Institutes of Health (NIH). This manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors report no conflicts of interest in this work.
References
1. The Initiative for Multipurpose Prevention Technologies. IMPT for reproductive health. Available from: https://theimpt.org/.
2. United Nations. “Staggering number” of unintended pregnancies reveals failure to uphold women’s rights. UN News; 2022. Available from: https://news.un.org/en/story/2022/03/1115062.
3. World Health Organization. Sexually transmitted infections (STIs). Available from: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis).
4. Morrow KM, Fava JL, Rosen RK, et al. Willingness to use microbicides is affected by the importance of product characteristics, use parameters, and protective properties. J Acquir Immune Defic Syndr. 2007;45(1):93–101. doi:10.1097/QAI.0b013e3180415ded
5. Gruber F, Lipozenčić J, Kehler T. History of venereal diseases from antiquity to the renaissance. Acta Dermatovenerol Croat ADC. 2015;23(1):1–11.
6. Burg G. History of sexually transmitted infections (STI). G Ital Dermatol E Venereol Organo Uff Soc Ital Dermatol E Sifilogr. 2012;147(4):329–340.
7. Khan F, Mukhtar S, Dickinson IK, Sriprasad S. The story of the condom. Indian J Urol IJU J Urol Soc India. 2013;29(1):12–15. doi:10.4103/0970-1591.109976
8. Fallopio G. De Morbo Gallico Liber (The French Disease) Describing the Earliest Documented Use of Condoms; 1564. Available from: https://books.google.com/books?id=lQk8AAAAcAAJ&printsec=frontcover&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false.
9. Wertheim JO, Smith MD, Smith DM, Scheffler K, Kosakovsky Pond SL. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol Biol Evol. 2014;31(9):2356–2364. doi:10.1093/molbev/msu185
10. Briggs P, Kovacs G, Guillebaud J. Contraception: A Casebook from Menarche to Menopause. Cambridge University Press; 2013.
11. Conrad LI, Neve M, Nutton V, Porter R, Wear A. The Western Medical Tradition: 800 BC to AD 1800. Cambridge University Press; 1995.
12. Rothschild BM. History of syphilis. Clin Infect Dis. 2005;40(10):1454–1463. doi:10.1086/429626
13. Heckel NJ. A Study of the Pathologic Alterations in the Female Bladder and Urethra Resulting from Infection with Trichomonas Vaginalis1. J Urol. 1936;35(5):520–523. doi:10.1016/S0022-5347(17)72217-3
14. Tjia KF, van Putten JPM, Pels E, Zanen HC. The interaction between Neisseria gonorrhoeae and the human cornea in organ culture. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):341–345. doi:10.1007/BF02172964
15. Beem MO, Saxon EM. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with chlamydia trachomatis. N Engl J Med. 1977;296(6):306–310. doi:10.1056/NEJM197702102960604
16. Alharbi SA, Wainwright M, Alahmadi TA, Salleeh HB, Faden AA, Chinnathambi A. What if Fleming had not discovered penicillin? Saudi J Biol Sci. 2014;21(4):289–293. doi:10.1016/j.sjbs.2013.12.007
17. Korber B, Muldoon M, Theiler J, et al. Timing the Ancestor of the HIV-1 Pandemic Strains. Science. 2000;288(5472):1789–1796. doi:10.1126/science.288.5472.1789
18. Watkins ES. How the Pill Became a Lifestyle Drug: the Pharmaceutical Industry and Birth Control in the United States Since 1960. Am J Public Health. 2012;102(8):1462–1472. doi:10.2105/AJPH.2012.300706
19. Roepke CL, Schaff EA. Long tail strings: impact of the dalkon shield 40 years later. Open J Obstet Gynecol. 2014;04(16):996. doi:10.4236/ojog.2014.416140
20. Nakashima AK, Fleming PL. HIV/AIDS Surveillance in the United States, 1981-2001. J Acquir Immune Defic Syndr. 2003;32:S68.
21. Gissmann L, Wolnik L, Ikenberg H, Koldovsky U, Schnürch HG, Zur Hausen H. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci. 1983;80(2):560–563. doi:10.1073/pnas.80.2.560
22. Kolata G. FDA Approves AZT. Science. 1987;235(4796):1570.
23. Peters A, Jansen W, van Driel F. The female condom: the international denial of a strong potential. Reprod Health Matters. 2010;18(35):119–128. doi:10.1016/S0968-8080(10)35499-1
24. Ray M, Logan R, Sterne JA; HIV-Causal Collaboration. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS Lond Engl. 2010;24(1):123–137. doi:10.1097/QAD.0b013e3283324283
25. Harrison PF. A new model for collaboration: the alliance for microbicide development. Int J Gynaecol Obstet. 1999;67(Suppl 2):S39–S53. doi:10.1016/s0020-7292(99)00145-9
26. Food and Drug Administration. Approval Package for Mirena (levonorgestrel-releasing intrauterine system). FDA; 2015. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/021225orig1s031.pdf.
27. McLemore MR. Gardasil: introducing the new human papillomavirus vaccine. Clin J Oncol Nurs. 2006;10(5):559–560. doi:10.1188/06.CJON.559-560
28. Bowers R. FDA issues warning for nonoxynol-9 products. AIDS Alert. 2008;23(4):44–45.
29. McKenna M The last drug that can fight gonorrhea is starting to falter. Wired. Available from: https://www.wired.com/story/the-last-drug-that-can-fight-gonorrhea-is-starting-to-falter/.
30. Suthutvoravut S, Kamyarat O. Spermicidal effects of lemon juice and juices from other natural products. Agric Nat Resour. 2016;50(2):133–138. doi:10.1016/j.anres.2015.09.004
31. Grant M, Hamer D, Hope T, et al. Whither or Wither Microbicides? Science. 2008;321(5888):532–534. doi:10.1126/science.1160355
32. Planned Parenthood. What Is the Effectiveness of Condoms? Available from: https://www.plannedparenthood.org/learn/birth-control/condom/how-effective-are-condoms.
33. Marfatia YS, Pandya I, Mehta K. Condoms: past, present, and future. Indian J Sex Transm Dis AIDS. 2015;36(2):133–139. doi:10.4103/0253-7184.167135
34. Smita J, Neelam J, Rochelle DY, et al. Comparative acceptability study of the Reality® female condom and the version 4 of modified Reddy female condom in India. Contraception. 2005;72(5):366–371. doi:10.1016/j.contraception.2005.05.014
35. Fasehun LK, Lewinger S, Fasehun O, Brooks M. Barriers and facilitators to acceptability of the female condom in low- and middle-income countries: a systematic review. Ann Glob Health. 2016;88(1):20. doi:10.5334/aogh.3612
36. Omar RF, Trottier S, Brousseau G, Lamarre A, Gagnon Null A, Bergeron MG. Distribution of a vaginal gel (Invisible Condom) before, during and after simulated sexual intercourse and its persistence when delivered by two different vaginal applicators: a magnetic resonance imaging study. Contraception. 2008;77(6):447–455. doi:10.1016/j.contraception.2008.01.015
37. Mbopi-Keou FX, Trottier S, Omar RF, et al. A randomized, double-blind, placebo-controlled Phase II extended safety study of two Invisible Condom formulations in Cameroonian women. Contraception. 2010;81(1):79–85. doi:10.1016/j.contraception.2009.07.002
38. Omar RF, Leboeuf M, Lemyre M, et al. A pre-phase iii efficacy trial of the spermicide/contraceptive effect of the invisible condom, a non-hormonal vaginal gel, in women from Canada. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC. 2022;44(2):175–181. doi:10.1016/j.jogc.2021.09.018
39. Schimpf U, Caldas-Silveira E, Katchan L, et al. Topical reinforcement of the cervical mucus barrier to sperm. Sci Transl Med. 2022;14(673):eabm2417. doi:10.1126/scitranslmed.abm2417
40. Miller CJ, Alexander NJ, Sutjipto S, et al. Effect of virus dose and nonoxynol-9 on the genital transmission of siv in rhesus macaques. J Med Primatol. 1990;19(3–4):401–409. doi:10.1111/j.1600-0684.1990.tb00445.x
41. Stephenson J. Widely used spermicide may increase, not decrease, risk of HIV transmission. JAMA. 2000;284(8):949. doi:10.1001/jama.284.8.949
42. Burke AE, Barnhart K, Jensen JT, et al. Contraceptive Efficacy, Acceptability, and Safety of C31G and Nonoxynol-9 Spermicidal Gels: a Randomized Controlled Trial. Obstet Gynecol. 2010;116(6):1265. doi:10.1097/AOG.0b013e3181fc3b1a
43. Feldblum PJ, Adeiga A, Bakare R, et al. SAVVY vaginal gel (C31G) for prevention of HIV infection: a randomized controlled trial in Nigeria. PLoS One. 2008;3(1):e1474. doi:10.1371/journal.pone.0001474
44. Bhosale VV, Asthana OP, Gaur SPS. Efficacy and safety of herbal spermicidal contraceptive, Consap. Curr Sci. 2013;104(12):1701–1703.
45. Rajasekaran M, Nair AGR, Hellstrom WJG, Sikka SC. Spermicidal activity of an antifungal saponin obtained from the tropical herb Mollugo pentaphylla. Contraception. 1993;47(4):401–412. doi:10.1016/0010-7824(93)90037-8
46. Pirrone V, Wigdahl B, Krebs FC. The rise and fall of polyanionic inhibitors of the human immunodeficiency virus type 1. Antiviral Res. 2011;90(3):168–182. doi:10.1016/j.antiviral.2011.03.176
47. Patel S, Hazrati E, Cheshenko N, et al. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196(9):1394–1402. doi:10.1086/522606
48. Skoler-Karpoff S, Ramjee G, Ahmed K, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet Lond Engl. 2008;372(9654):1977–1987. doi:10.1016/S0140-6736(08)61842-5
49. Tan S, Lu L, Li L, et al. Polyanionic candidate microbicides accelerate the formation of semen-derived amyloid fibrils to enhance HIV-1 infection. PLoS One. 2013;8(3):e59777. doi:10.1371/journal.pone.0059777
50. Weitzel M, North BB, Waller D. Development of multipurpose technologies products for pregnancy and STI prevention: update on polyphenylene carboxymethylene MPT gel development†. Biol Reprod. 2020;103(2):299–309. doi:10.1093/biolre/ioaa087
51. Price CF, Tyssen D, Sonza S, et al. SPL7013 Gel (VivaGel®) retains potent HIV-1 and HSV-2 inhibitory activity following vaginal administration in humans. PLoS One. 2011;6(9):e24095. doi:10.1371/journal.pone.0024095
52. Moscicki AB, Kaul R, Ma Y, et al. Measurement of mucosal biomarkers in a Phase 1 trial of intravaginal 3% StarPharma LTD 7013 gel (VivaGel) to assess expanded safety. J Acquir Immune Defic Syndr. 2012;59(2):134–140. doi:10.1097/QAI.0b013e31823f2aeb
53. McGowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004). AIDS Lond Engl. 2011;25(8):1057–1064. doi:10.1097/QAD.0b013e328346bd3e
54. Fernandes T, Baxi K, Sawarkar S, Sarmento B, Das Neves J. Vaginal multipurpose prevention technologies: promising approaches for enhancing women’s sexual and reproductive health. Expert Opin Drug Deliv. 2020;17(3):379–393. doi:10.1080/17425247.2020.1728251
55. Caminade AM. Dendrimers, an emerging opportunity in personalized medicine? J Pers Med. 2022;12(8):1334. doi:10.3390/jpm12081334
56. Mirmonsef P, Gilbert D, Zariffard MR, et al. The effects of commensal bacteria on innate immune responses in the female genital tract. Am J Reprod Immunol. 2011;65(3):190–195. doi:10.1111/j.1600-0897.2010.00943.x
57. Mayer KH, Peipert J, Fleming T, et al. Safety and tolerability of BufferGel, a novel vaginal microbicide, in women in the United States. Clin Infect Dis off Publ Infect Dis Soc Am. 2001;32(3):476–482. doi:10.1086/318496
58. Bouvet JP, Grésenguet G, Bélec L. Vaginal pH neutralization by semen as a cofactor of HIV transmission. Clin Microbiol Infect. 1997;3(1):19–23. doi:10.1111/j.1469-0691.1997.tb00246.x
59. Zeitlin L, Hoen TE, Achilles SL, et al. Tests of buffergel for contraception and prevention of sexually transmitted diseases in animal models. Sex Transm Dis. 2001;28(7):417.
60. Su S, Vincent KL. Lactic acid, citric acid, and potassium bitartrate non-hormonal prescription vaginal pH modulator (VPM) gel for the prevention of pregnancy. Expert Rev Clin Pharmacol. 2022;15(6):659–670. doi:10.1080/17512433.2022.2100347
61. Seeking Alpha. Evofem’s Phexxi fails in Phase 3 trial to prevent chlamydia, gonorrhea in women; 2022. Available from: https://archive.vn/M2K3r.
62. Styczynski AR, Anwar KN, Sultana H, et al. In vitro antiretroviral activity and in vivo toxicity of the potential topical microbicide copper phthalocyanine sulfate. Virol J. 2015;12:132. doi:10.1186/s12985-015-0358-5
63. Hannaford PC, Ti A, Chipato T, Curtis KM. Copper intrauterine device use and HIV acquisition in women: a systematic review. BMJ Sex Reprod Health. 2020;46(1):17–25. doi:10.1136/bmjsrh-2019-200512
64. National Institutes of Health. Copper Intravaginal Contraception. RePORTER. Available from: https://reporter.nih.gov/project-details/10018526.
65. Haraguchi Y, Sakurai H, Hussain S, Anner BM, Hoshino H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43(2):123–133. doi:10.1016/s0166-3542(99)00040-6
66. Kenney J, Rodríguez A, Kizima L, et al. A modified zinc acetate gel, a potential nonantiretroviral microbicide, is safe and effective against simian-human immunodeficiency virus and herpes simplex virus 2 infection in vivo. Antimicrob Agents Chemother. 2013;57(8):4001–4009. doi:10.1128/AAC.00796-13
67. Friedland BA, Hoesley CJ, Plagianos M, et al. First-in-human trial of miv-150 and zinc acetate coformulated in a carrageenan gel: safety, pharmacokinetics, acceptability, adherence, and pharmacodynamics. J Acquir Immune Defic Syndr. 2016;73(5):489–496. doi:10.1097/QAI.0000000000001136
68. Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353–377. doi:10.1093/humupd/dmu065
69. Levinson P, Choi RY, Cole AL, et al. HIV-Neutralizing Activity of Cationic Polypeptides in Cervicovaginal Secretions of Women in HIV-Serodiscordant Relationships. PLoS One. 2012;7(2):e31996. doi:10.1371/journal.pone.0031996
70. Tanphaichitr N, Srakaew N, Alonzi R, et al. Potential Use of Antimicrobial Peptides as Vaginal Spermicides/Microbicides. Pharm Basel Switz. 2016;9(1):13. doi:10.3390/ph9010013
71. Broliden K. Innate molecular and anatomic mucosal barriers against HIV infection in the genital tract of HIV‐exposed seronegative individuals. J Infect Dis. 2010;202(Suppl 3):S351–355. doi:10.1086/655964
72. Srakaew N, Young CD, Sae-wu A, et al. Antimicrobial host defence peptide, LL-37, as a potential vaginal contraceptive. Hum Reprod. 2014;29(4):683–696. doi:10.1093/humrep/deu018
73. Lee SG, Kiattiburut W, Khongkha T, et al. 17BIPHE2, an engineered cathelicidin antimicrobial peptide with low susceptibility to proteases, is an effective spermicide and microbicide against Neisseria gonorrhoeae. Hum Reprod. 2022;37(11):2503–2517. doi:10.1093/humrep/deac188
74. Perez-Caballero D, Zang T, Ebrahimi A, et al. Tetherin inhibits HIV-1 release by directly tethering virions to cells. Cell. 2009;139(3):499–511. doi:10.1016/j.cell.2009.08.039
75. Bhardwaj G, Mulligan VK, Bahl CD, et al. Accurate de novo design of hyperstable constrained peptides. Nature. 2016;538(7625):329–335. doi:10.1038/nature19791
76. Hifnawy MS, Aboseada MA, Hassan HM, Tohamy AF, El Naggar EMB, Abdelmohsen UR. Nature-inspired male contraceptive and spermicidal products. Phytochem Rev. 2021;20(4):797–843. doi:10.1007/s11101-020-09721-5
77. Naz RK, Lough ML. Curcumin as a potential non-steroidal contraceptive with spermicidal and microbicidal properties. Eur J Obstet Gynecol Reprod Biol. 2014;176:142–148. doi:10.1016/j.ejogrb.2014.01.024
78. Baldeon-Vaca G, Marathe JG, Politch JA, et al. Production and characterization of a human antisperm monoclonal antibody against CD52g for topical contraception in women. EBioMedicine. 2021;69:103478. doi:10.1016/j.ebiom.2021.103478
79. Kyurkchiev SD, Shigeta M, Koyama K, Isojima S. A human-mouse hybridoma producing monoclonal antibody against human sperm coating antigen. Immunology. 1986;57(3):489–492.
80. ZabBio Inc. An Exploratory Phase 1 Mechanism-of-Action Study of ZB-06, a Vaginal Film Containing HC4-N, an Anti-Sperm Monoclonal Antibody. clinicaltrials.gov; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04731818.
81. Anderson DJ, Politch JA, Cone RA, et al. Engineering monoclonal antibody-based contraception and multipurpose prevention technologies†. Biol Reprod. 2020;103(2):275–285. doi:10.1093/biolre/ioaa096
82. Riddler SA, Balkus JE, Parikh UM, et al. Clinical and Virologic Outcomes Following Initiation of Antiretroviral Therapy Among Seroconverters in the Microbicide Trials Network-020 Phase III Trial of the Dapivirine Vaginal Ring. Clin Infect Dis off Publ Infect Dis Soc Am. 2019;69(3):523–529. doi:10.1093/cid/ciy909
83. Obiero J, Mwethera PG, Hussey GD, Wiysonge CS. Vaginal microbicides for reducing the risk of sexual acquisition of HIV infection in women: systematic review and meta-analysis. BMC Infect Dis. 2012;12(1):289. doi:10.1186/1471-2334-12-289
84. Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA. Long-acting antiretrovirals: a new era for the management and prevention of HIV infection. J Antimicrob Chemother. 2022;77(2):290–302. doi:10.1093/jac/dkab324
85. Brown ER, Hendrix CW, van der Straten A, et al. Greater dapivirine release from the dapivirine vaginal ring is correlated with lower risk of HIV-1 acquisition: a secondary analysis from a randomized, placebo-controlled trial. J Int AIDS Soc. 2020;23(11):e25634. doi:10.1002/jia2.25634
86. Derby N, Lal M, Aravantinou M, et al. Griffithsin carrageenan fast dissolving inserts prevent SHIV HSV-2 and HPV infections in vivo. Nat Commun. 2018;9(1):3881. doi:10.1038/s41467-018-06349-0
87. Le AQ, Taylor J, Dong W, et al. Differential evolution of a CXCR4-using HIV-1 strain in CCR5wt/wt and CCR5∆32/∆32 hosts revealed by longitudinal deep sequencing and phylogenetic reconstruction. Sci Rep. 2015;5(1):17607. doi:10.1038/srep17607
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
