Back to Journals » OncoTargets and Therapy » Volume 15
7β-22 Dihydroxyhopane, Isolated from the Sub-Antarctic Lichen, Inhibits the Viability and Stemness in Glioma Stem Like Cells
Authors Kim HJ, Suh SS, Park J, Shin MJ, Koo MH , Lee SJ, Jeon YJ, Lee S, Youn UJ , Kim SH
Received 1 June 2022
Accepted for publication 28 October 2022
Published 15 November 2022 Volume 2022:15 Pages 1375—1383
DOI https://doi.org/10.2147/OTT.S371042
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Hyun-Jin Kim,1,* Sung-Suk Suh,2,* Jeongwon Park,3 Min-Ji Shin,2 Man Hyung Koo,4 Suk Jun Lee,5 Young-Jun Jeon,6 Seongsoo Lee,3 Ui-Joung Youn,7 Sung-Hak Kim1
1Department of Animal Science, Chonnam National University, Gwangju, 61186, Korea; 2Department of Biosciences, Mokpo National University, Muan, 58554, Korea; 3Gwangju Center, Korea Basic Science Institute (KBSI), Gwangju, 61186, Korea; 4Research Unit of Cryogenic Novel Material, Korea Polar Research Institute, Incheon, 21990, Korea; 5Department of Biomedical Laboratory Science, College of Health & Medical Sciences, Cheongju University, Chungbuk, 28503, Korea; 6Department of Integrative Biotechnology, Sungkyunkwan University, Suwon, 16419, Korea; 7Division of Life Sciences, Korea Polar Research Institute (KOPRI), Incheon, 21990, Korea
*These authors contributed equally to this work
Correspondence: Ui-Joung Youn, Division of Life Sciences, Korea Polar Research Institute (KOPRI), Incheon, 21990, Korea, Tel +82 32 760 5562, Email [email protected] Sung-Hak Kim, Department of Animal Science, Chonnam National University, Gwangju, 61186, Korea, Tel +82 62 530 2115, Email [email protected]
Background: Glioma stem cells (GSCs) have been reported to contribute to tumor initiation and relapse, therapy resistance, and intra-tumoral heterogeneity of glioblastoma multiforme. Therefore, inhibiting GSCs presents a critical therapeutic tactic to suppress the aggressiveness of tumors.
Methods: In this study, we examined the effects of 7β-22 dihydroxyhopane (AP 18), isolated from the sub-Antarctic lichen, Pseudocyphellaria freycinetii. The cytotoxic effect of AP 18 and its effects on cell proliferation were assessed by alamarBlue assay and 5-ethynyl-2′-deoxyuridine (EdU) assay. Real-time confluence analysis was performed with a Celloger automatic live cell imaging system. Western Blotting and 3-D optical diffraction tomography (ODT) imaging were performed to determine whether apoptosis was triggered by AP 18. A Limiting dilution assay and qRT-PCR were performed to investigate the impact of AP 18 on GSC stemness.
Results: AP 18 significantly reduced GSCs viability and proliferation, inducing programmed cell death identified by Annexin V/PI staining and had effects on morphologic features determined by 3-D ODT. Interestingly, treatment with AP 18 suppressed stemness features.
Conclusion: Taken together, our results suggest that AP 18 might be a potential therapeutic agent to target GSCs.
Keywords: Glioblastoma, glioma, Pseudocyphellaria freycinetii, hopane triterpenoid
Introduction
Glioblastoma multiforme (GBM) is the most aggressive type of primary brain tumor in adults.1 Despite the conventional treatments for GBM (eg, surgery, chemotherapy, and radiotherapy) cancer patients have a median overall survival of about 1 year.1–3 GBM has histological features including high invasiveness in surrounding normal brain areas, angiogenesis, and necrosis.1 It has been reported that it retains a specific cell population known as glioma stem cells (GSCs), which are involved in tumor initiation, resistance to disease treatments, and invasion, as well as the ability to induce intra-tumoral heterogeneity.4,5 Therefore, inhibiting GSCs is considered a major therapeutic strategy for GBM.
Recently, natural plant extracts were reported to be a potential source of cancer treatment agents on various cancers such as liver, brain, colon, and stomach cancer due to low toxicities to non-cancer cells.6–13 Existing chemotherapy attacks cancer cells, but side effects are more prevalent.
Pseudocyphellaria (Lobariaceae) is widely distributed in the southern temperate regions, and contains about 170 species.14 In previous studies, secondary metabolites such as zeorin, tenuiorin, constictic acid, cryptostictic acid, norstictic acid, hyposalazinic acid, stictic acid, and leucotylin have been isolated from the genus Pseudocyphellaria, which was reported to have anti-parasitic, anti-fungal, and anti-cancer activities.15–21 In addition, there was a chemical study on the lichen, P. freycinetii, found in the sub-Antarctic region.21
As part of the effort to discover naturally occurring anti-cancer agents from the Antarctic and sub-Antarctic lichens, acetone extracts of P. freycinetii had an inhibitory effect on cell cytotoxicity and the self-renewal ability of GSCs.
This preliminary result encouraged us to study P. freycinetii further, resulting in the isolation of 7β-22 dihydroxyhopane (AP 18), which was reported for the first time in this species. However, no studies have evaluated the anti-cancer effects of AP 18 in brain cancer. Here, we examined whether AP 18 isolated from P. freycinetii exerted anti-cancer effects on brain cancer cells. We report that AP 18 induced apoptotic cell death in GSCs.
Materials and Methods
Lichen Material
P. freycinetii was collected in January 2017 from San Juan, Chile, and identified by Dr. Ji Hee Kim through the analysis on morphological characteristics using the microscope (Zeiss Boom Stand Stemi 2000 Stereo Microscope). A voucher specimen was deposited at the Natural Product Chemistry Laboratory of the Korea Polar Research Institute.
Extraction and Isolation
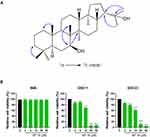
The air dried and powered lichen, P. freycinetii (130.2 g) was extracted with methanol (MeOH, 3×1 L) at room temperature. The solvent was concentrated in vacuo to yield 15.2 g of crude extract, which was then suspended in distilled water (1.0 L) and extracted successively with n-hexane (1.0 L), ethyl acetate (EtOAc) (1.0 L), and n-butanol (1.0 L) to yield soluble layers of hexane (1.5 g), EtOAc (2.9 g), BuOH (3.2 g), respectively. The n-hexane extracts (1.5 g) were subjected to silica gel column chromatography (CC; 230–400 mesh), using a gradient solvent system, n-hexane and EtOAc [9:1 (2L), 8:2 (2L), and 7:3 (1L), respectively], to yield 12 subfractions (HS. 1 – HS. 12). Subfraction HS. 6 containing a major spot in the TLC analysis (n-hexane:EtOAc=8:2) was separated by Sephadex-LH-20 gel column, using an isocratic solvent mixture, MeOH:H2O [70:30 (2L)] to yield 5 subfractions (HS. 6.1 – HS. 6.5). AP 18 (43.0 mg) was obtained by recrystallization using a solvent mixture, n-hexane:EtOAc [50:50 (10 mL)] from subfractions HS. 6.2 and HS. 6.3, which was identified as 7β-22 dihydroxyhopane by means of 1H and 13C NMR and 1H-13C HMBC NMR analyses, as well as the comparison of the spectroscopic and physical data with reported values (Figure 1A).
Cell Culture and Reagents
Normal human astrocytes (NHA) were purchased from ScienceCell Research Laboratories (USA) and cultured in Astrocyte Medium (ScienCell Research Laboratories, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific, USA) and 1% penicillin/streptomycin (Welgene, Korea). GBM patient-derived GSC11 and GSC23 were obtained from the University of Texas MD Anderson Cancer Center.22 GSCs were maintained using neurobasal medium (NBE, serum-free Neurobasal media supplemented with basic FGF and EGF). NBE conditions were as follows: Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Welgene) was supplemented with 2% B27 (50×) (Gibco), 1% penicillin/streptomycin (Welgene), epidermal growth factor (EGF; 20 ng/mL; R&D Systems, USA), and basic fibroblast growth factor (bFGF; 20 ng/mL; R&D Systems). Serum-free conditions were as follows: Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Welgene) was supplemented with 5% UltraGROTM–Advanced Cell Culture Supplement (AventaCell) and 1% penicillin/streptomycin (Welgene).
Cell Viability
Cell viability was conducted with an alamarBlue® solution (Invitrogen, USA). NHA and GSCs were seeded at a density of 3000 cells per well in 96-multiwell plates (n = 6). Then, the cells were treated with or without AP 18 at different concentrations. After incubation for 48 hrs, alamarBlue solution was added to each well and then cells were incubated for 6 hrs. The solution fluorescence was measured at wavelengths of 570 and 600 nm in a Synergy HTX Multi-Mode Reader (BioTek Instruments Inc., USA).
Real-Time Cell Confluence Analysis
Real-time cell confluence analysis was conducted with a Celloger Mini automatic live cell imaging system (Curiosis Inc., South Korea). GSC11 and GSC23 were seeded at a density of 300,000 cells per well in 6-multiwell plates. Then, GSCs were treated with AP 18 (14 μM) or DMSO (mock). Cell confluence was measured and quantified every two hours after the drug was added.
5-Ethynyl-2′-Deoxyuridine (EdU) Staining
GSCs and NHA were treated with AP 18 (14 μM, 5 hrs) or DMSO (mock). After treatment, the medium was replaced with a new medium. After incubated with EdU for 45 min, the cells were washed with in PBS and fixed in 4% paraformaldehyde (PFA) for 15 min. After washing with 1% BSA in PBS for three times, 1% BSA in PBS containing 0.5% Triton X-100 was used for permeabilization at room temperature (RT) for 15 min. The cells were incubated with Click-It® Plus (Thermo Fisher Scientific, USA) reaction mixture for 30 min, protected from light. The nuclei were stained with DAPI (1:1000). The percentage of EdU staining was calculated from at least four microscopic visual fields.
Annexin-V and Propidium Iodide Staining
NHA, GSC11, and GSC23 were treated with DMSO or AP 18 (10 μM) for 24 hrs. The cells were then harvested and washed with cold PBS. The cells were incubated with Annexin-V-FITC and propidium iodide (Invitrogen) at room temperature for 15 min and then analyzed by flow cytometry (Beckman Coulter, USA).
In vitro Limiting Dilution Assay
For limiting dilution assays, cells that decreased twice per well (50, 25, 12, 6, 3, and 1) were seeded on a 96-multi well plate, and the tumorsphere formation of each well was observed. The calculation of stem cell frequency was conducted using software on a website available at http://bioinf.wehi.edu.au/software/elda.23
Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNA was extracted using RiboEx reagent (GeneAll, Korea) and purified using a Hybrid-R kit (GeneAll), according to the manufacturer’s instructions. Five hundred nanograms of mRNA were used to synthesize cDNA with a Revert Aid First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, USA). The qRT-PCR reaction was conducted on a Qtower3 real-time PCR thermal cycler (Analytik Jena, Germany) using SYBR Premix Ex Taq ITM (Takara Bio, Japan). The results of qRT-PCR were evaluated as the Ct values, and, in turn, were quantified using the 2−ΔΔCt method. Primer sequences used for RT-qPCR amplification were as follows (5’ to 3’): human 18S rRNA forward: 5’-CAGCCACCCGAGATTGAGCA-3’, reverse:5’-TAGTAGCGACGGGCGGTGTG-3’; human CD133 primer forward: 5’-CAGGTAAGAACCCGGATCAA-3’, reverse: 5’-TCAGATCTGTGAACGCCTTG-3’.
Neuro-Sphere Assay
GSCs treated with AP 18 (14 μM, 24 hrs) were seeded onto 24-well plates at a density of 1×104 cells/well and incubated at 5% CO2 and 37°C for 7 d without disturbing the plates and without replenishing the medium. At the end of 7 days incubation, images of neuro-spheres were taken to measure their size using a digital microscope (Logos Biosystems, Korea).
Apoptosis Imaging by 3-D ODT (Optical Diffraction Tomography)
According to the method presented by Sangwoo Park et al, apoptotic morphology was monitored in GSCs by 3-D ODT (Tomocube, Korea).24 To observe live cells with ODT, GSCs (1 × 103 cells) were seeded onto a central glass-bottom Tomodish. For apoptosis imaging, the GSCs were treated with AP 18 (20 μM) for 24 hrs for apoptosis imaging. Then, GSC11 and GSC23 were washed out with phosphate-buffered saline (PBS) (pH 7.4) and imaged by 3-D ODT.
Western Blot Analysis
GSCs treated with AP 18 were lysed in RIPA buffer and phosphatase inhibitor cocktail 2 (ApexBio, USA). The protein contents were determined by a BCA Protein Assay Kit (Thermo Scientific). The proteins were separated by 10% and 15% SDS–polyacrylamide gel and transferred to a PVDF membrane. The membranes were blocked with 5% skim milk in PBST for 1 hr at room temperature and then incubated with the indicated primary antibodies overnight at 4°C with gentle shaking. After washing with PBST, the membrane was developed with a secondary antibody for 1 hr at room temperature. After washing with PBST, membranes were visualized by chemiluminescence (Invitrogen) according to the manufacturer’s protocol.
Statistical Analysis
For statistical analyses, Microsoft Excel and GraphPad Prism Ver.9.0 were used. Comparisons between two groups were verified by the Student’s t-test in GraphPad Prism. Statistical significance among groups was determined by a one-way ANOVA, followed by Tukey’s multiple comparison test. P-values less than 0.05 were considered significant.
Results
AP 18 Inhibits the Viability of GSCs
GSC11, GSC23, and NHA were treated with different concentrations of AP 18 (0–50 μM) for 48 hrs. The cytotoxicity of AP 18 was detected by alamarBlue cell viability analysis. As shown in Figure 1B, AP 18 had acute toxic properties to GSCs in a dose-dependent manner in vitro but had no toxic effect on NHA cells. At concentrations of 25 and 50 μM, the viability of GSC11 and GSC23 was below 20%, whereas NHA cells survived at these concentrations. We checked again at serum-free medium to identify if this difference in effect was caused by blood protein such as serum. Similar to that, only GSC shows a decrease in viability in the serum-free media (Supplementary Figure 1). These results suggested that AP 18 had a selective anti-cancer effect on GSCs compared with NHA.
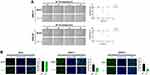
AP 18 Suppresses the Proliferation of GSCs
To investigate whether AP 18 suppresses GSCs proliferation, real-time cell confluence analysis was conducted after AP 18 treatment. As a result of the experiment, cell confluence was decreased within three hours of exposure to AP 18 (14 μM) (Figure 2A). After treating AP 18 in NHA cells, it did not affect cell confluence even after 72 hours (Supplementary Figure 2). Moreover, EdU staining was conducted to determine the inhibitory effect of AP 18 on cell growth. Compared with the MOCK group, EdU-positive cells were decreased in response to AP 18 in GSCs (Figure 2B). Taken together, AP 18 inhibited the proliferation of GSCs.
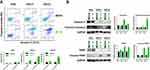
AP 18 Induces GSCs Apoptosis-Like Features
To determine whether AP 18 induces programmed cell death, we performed Annexin V-FITC/PI staining using flow cytometry (facs) after treatment with AP 18. After exposure to AP 18 (14 μM, 24 hours), the proportions of early and late apoptotic GSC11 and GSC23 cells were increased (Figure 3A). Immunoblot analysis showed that AP 18 increased cleaved Caspase 3 and cleaved PARP, implying that AP 18 induced apoptosis in GSCs, not NHA (Figures 3B).
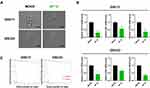
AP 18 Induces Apoptotic-Like Cell Morphology in GSC
To examine the morphological features of GSC and NHA after AP 18 treatment, we used 3-D ODT at an individual cell level. Figure 4A shows 3-D images of mock GSCs with elongated morphologic features in laminin-coated dishes. Conversely, the AP 18 treated GSCs exhibited compressed intracellular components, membrane bleb formations, and cell shrinkage, which are well-known programmed cell death phenotypes. Quantitative measurements in Figure 4B indicated that AP 18 reduced the cell volume, surface area, projected area, and dry mass suggesting that AP 18 induces apoptotic features in GSCs.
AP 18 Suppresses GSC Self-Renewal Ability and Stemness Markers in GSCs
To examine whether AP 18 changed GSC stemness, we examined their self-renewal ability and mRNA levels of the stemness marker, CD133, NES, and OLIG2. The neuro-sphere size was significantly reduced in AP 18 treated GSCs compared with the mock group (Figure 5A). Real-time PCR revealed that AP 18 significantly reduced the mRNA levels of the stemness marker CD133, NES, and OLIG2 (Figure 5B). In Figure 5C, using limiting dilution analysis, we demonstrated that AP 18 impaired GSCs self-renewal ability, as measured by neuro-sphere formation. To determine whether AP 18 targets GSC specifically, compound was also treated on the glioma cell line. As a result, there was little or no effect of AP 18 on the glioma cell line (Supplementary Figure 3). These results indicate that AP 18 suppresses the stemness feature of GSCs.
Discussion
Glioblastoma, one of the most aggressive tumors, is resistant to conventional radio- and chemotherapies.1,2 It has been reported that tumor relapse is caused by the presence of GSCs, a small proportion of cells with stemness features.4,5,25 Therefore, research on drugs inhibiting GSCs is essential for new cure strategies against malignant brain tumors.
Lichens are fungi and photosynthetic algae that have a symbiotic relationship.20 Lichens are found in a wide range of environments, from desert climates to polar tundra, and there are over 25,000 species worldwide.26 Lichens can synthesize a wide range of secondary metabolites and are home to over 600 distinct compounds found nowhere else in the living world due to their special physiology and environments.27 Lichens have been used in traditional cultures for a variety of purposes, including the preparation of pharmaceuticals, nutraceuticals, spices, dyes, and perfumes, due to the existence of secondary metabolites.28 Several researchers have investigated the antiviral, antibacterial, antioxidant, anti-inflammatory, and anti-cancer properties of lichen extracts and metabolites, which offer tremendous potential for biopharmaceutical applications and the development of novel medicines. Considering the availability of lichens, we extracted 7β-22 dihydroxyhopane (AP 18) isolated from the lichen, Pseudocyphellaria freycinetii.
The first limitation of this study was the in vitro nature of the experiments. For the drug to treat a brain tumor, the drug must reach the brain. Therefore, further research using an orthotropic xenograft mouse model is needed to determine whether AP 18 reaches tumors through the blood-brain barrier.
The second limitation is that we did not investigate the mechanisms associated with the suppression of glioma stem cell proliferation and stemness by AP 18. To address these limitations, transcriptomic analysis might determine which transcripts change after AP 18 treatment.
Conclusion
In this study, AP 18 reduced the viability of GSCs but not NHA. Furthermore, AP 18 triggered programmed cell death (apoptosis) with changes in cell morphology measured by 3-D ODT. Moreover, this extract inhibited the tumor cell sphere-forming ability of GSCs and reduced stemness in GSCs. Given the findings, AP 18 may contain cytotoxic molecules to GSCs when combined with extract alone or standard therapy.
Acknowledgments
This research was supported by a grant (no. 2019R1I1A3A01059211, and NRF-2021R1F1A1055541) from the National Research Foundation (NRF) funded by the Ministry of Science and ICT (MSIT), Republic of Korea. Also, this work was part of a project titled “Development of potential antibiotic compounds using polar organism resources (15250103, KOPRI Grant PM22030)”, funded by the Ministry of Oceans and Fisheries, Korea. Finally, this study was supported in part by a grant from the Korea Basic Science Institute (C280200).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lapointe S, Perry A, Butowski NA. Primary brain tumours in adults. Lancet. 2018;392(10145):432–446. doi:10.1016/S0140-6736(18)30990-5
2. Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy–Temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi:10.1056/NEJMoa1308345
3. Mohtashami E, Shafaei-Bajestani N, Mollazadeh H, et al. The current state of potential therapeutic modalities for glioblastoma multiforme: a clinical review. Curr Drug Metab. 2020;21(8):564–578. doi:10.2174/1389200221666200714101038
4. Bradshaw A, Wickremesekera A, Brasch HD, et al. Cancer stem cells in glioblastoma multiforme. Front Surg. 2016;3:1203–1217.
5. Prager BC, Bhargava S, Mahadev V, Hubert CG, Rich JN. Glioblastoma stem cells: driving resilience through chaos. Trends Cancer. 2020;6(3):223–235. doi:10.1016/j.trecan.2020.01.009
6. Afshari AR, Mollazadeh H, Mohtashami E, et al. Protective role of natural products in glioblastoma multiforme: a focus on nitric oxide pathway. Curr Med Chem. 2020;28(2):377–400.
7. Afshari AR, Jalili-Nik M, Abbasinezhad-moud F, et al. Anti-tumor effects of curcuminoids in glioblastoma multiforme: an updated literature review. Curr Med Chem. 2020;28(39):8116–8138.
8. Memari F, Mirzavi F, Jalili-Nik M, Afshari AR, Ghorbani A, Soukhtanloo M. Tumor-inhibitory effects of zerumbone against HT-29 human colorectal cancer cells. Int J Toxicol. 2022;41(5):402–411.
9. Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci. 2011;16(3):980–996.
10. Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55(1):32–45. doi:10.1002/mnfr.201000412
11. Woźniak Ł, Skąpska S, Marszałek K. Ursolic acid - a pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules. 2015;20(11):20614–20641. doi:10.3390/molecules201119721
12. Patwardhan B, Vaidya ADB, Chorghade M. Current science association ayurveda and natural products drug discovery. Curr Sci. 2004;86:789–799.
13. Lahlou M. Screening of natural products for drug discovery. Expert Opin Drug Discov. 2007;2(5):697–705. doi:10.1517/17460441.2.5.697
14. Kirk P, Cannon P, Minter DJS, editors. Dictionary of the Fungi.
15. Piovano M, Chamy MC, Garbarino JA. Studies on Chilean lichens xxxi: additions to the chemistry of pseudocyphellaria. Bol Soc Chil Quím. 2001;46(1):23–27.
16. Maass WSG. Lichen Substances VIII. Phenolic constituents of pseudocyphellaria quercifolia. Bryologist. 1975;78(2):183. doi:10.2307/3242049
17. Elix JA, Wilkins AL, Wardlaw JH. Five new fully substituted depsides from the lichen Pseudocyphellaria pickeringii. Aust J Chem. 1987;40(12):2023–2029. doi:10.1071/CH9872023
18. Fritis MC, Lagos CR, Sobarzo NQ, et al. Depsides and triterpenes in Pseudocyphellaria coriifolia (lichens) and biological activity against Trypanosoma cruzi. Nat Prod Res. 2013;27(17):1607–1610. doi:10.1080/14786419.2012.740033
19. BT Kannangara, RSCG Rajapaksha, Paranagama PA. Nature and bioactivities of endolichenic fungi in Pseudocyphellaria sp., Parmotrema sp. and Usnea sp. at Hakgala montane forest in Sri Lanka. Lett Appl Microbiol. 2009;48(2):203–209. doi:10.1111/j.1472-765X.2008.02512.x
20. Yang Y, Park SY, Nguyen TT, et al. Lichen secondary metabolite, physciosporin, inhibits lung cancer cell motility. PLoS One. 2015;10(9):1–16.
21. Howarth OW, Rickard TMA, Sainsbury M. The Antarctic lichens: 1—the stereochemistry of 7β-acetoxy-22-hydroxyhopane from Pseudocyphellaria freycinettii, indigenous to S. Georgia J Magn Reson. 1983;21(1):56–59. doi:10.1002/omr.1270210115
22. Bhat KPL, Balasubramaniyan V, Vaillant B, et al. Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. doi:10.1016/j.ccr.2013.08.001
23. Hu Y, Smyth GK. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J Immunol Methods. 2009;347(1–2):70–78. doi:10.1016/j.jim.2009.06.008
24. Park S, Ahn JW, Jo Y, et al. Label-free tomographic imaging of lipid droplets in foam cells for machine-learning-assisted therapeutic evaluation of targeted nanodrugs. ACS Nano. 2020;14(2):1856–1865. doi:10.1021/acsnano.9b07993
25. Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi:10.1056/NEJMra061808
26. Thiyagarajan T. Lichens: a myriad hue of Bioresources with medicinal properties. Int J Life Sci. 2017;5(3):387–393.
27. Molnár K, Farkas E. Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C J Biosci. 2010;65(3–4):157–173. doi:10.1515/znc-2010-3-401
28. Upreti DK, Divakar PK, Nayaka S. Commercial and ethnic use of lichens in India. Econ Bot. 2017;59(3):269–273. doi:10.1663/0013-0001(2005)059[0269:CAEUOL]2.0.CO;2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.