Back to Journals » OncoTargets and Therapy » Volume 13
5-HT7 Receptor Contributes to Proliferation, Migration and Invasion in NSCLC Cells
Authors Du X, Wang T, Wang Z, Wu X, Gu Y, Huang Q, Wang J, Xie J
Received 31 December 2019
Accepted for publication 25 February 2020
Published 9 March 2020 Volume 2020:13 Pages 2139—2151
DOI https://doi.org/10.2147/OTT.S244339
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Xiaohui Du, Ting Wang, Zhihua Wang, Xiaomei Wu, Yiya Gu, Qian Huang, Jianmiao Wang, Jungang Xie
Department of Respiratory and Critical Care Medicine, National Clinical Research Center of Respiratory Disease, Key Laboratory of Pulmonary Diseases of Health Ministry, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430030, People’s Republic of China
Correspondence: Jungang Xie
Department of Respiratory and Critical Care Medicine, National Clinical Research Center of Respiratory Disease, Key Laboratory of Pulmonary Diseases of Health Ministry, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, 1095 Jiefang Avenue, Wuhan, Hubei, People’s Republic of China
Email [email protected]
Introduction: Because only a small portion of NSCLC (non-small-cell lung cancer) patients benefit from molecular targeted therapy or immunotherapy and do not develop therapeutic resistance, continued research on new targets is warranted. Serotonin has recently emerged as a growth factor for tumor cells, and its receptors may be potential therapeutic targets. The mechanism related to the behavior of the 5-HT7 receptor in NSCLC remains unknown.
Methods: Both gene expression analysis and immunohistochemical analysis were conducted to evaluate 5-HT7 receptor expression in NSCLC tissues. The correlation between 5-HT7 receptor expression and clinicopathological features was also examined. Cell proliferation was measured using a CCK8 (Cell Counting Kit-8) assay and colony formation, migration and invasion were evaluated by the Transwell assay. siRNA transfection and stimulation with the selective agonist LP211 were used to identify the involvement of molecules in proliferation, migration and invasion. Quantitative real-time chain reaction (qRT-PCR) and Western blotting were used to quantifiy mRNA and protein levels, respectively. Pathway inhibitors facilitated the exploration of possible signaling pathways regulated by the 5-HT7 receptor in migration and invasion.
Results: The 5-HT7 receptor was overexpressed in NSCLC tumor tissues compared with adjacent normal lung tissues. High 5-HT7 receptor expression levels were correlated with lymph node metastasis (P=0.007) and advanced TNM stage (P=0.000) in NSCLC patients. The 5-HT7 receptor positively regulated cell proliferation, migration and invasion in NSCLC cells. The stimulatory effect of the 5-HT7 receptor on A549 cell migration and invasion may occur through the P38 pathway. In H1299 cells, the 5-HT7 receptor might positively regulate Src to promote cell migration and invasion.
Conclusion: Our findings suggest that the 5-HT7 receptor, which mediates NSCLC progression, may be a potential therapeutic target.
Keywords: non-small cell lung cancer, progression, 5-HT7 receptor, LP211
Introduction
The GLOBOCAN (Global Cancer Observatory http://gco.iarc.fr/) 2018 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer across 20 world regions showed that lung cancer was the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer-related death (18.4% of the total cancer-related deaths) in both sexes combined.1
A large proportion of lung cancer patients have a group of histological subtypes collectively known as NSCLC (non-small-cell lung cancer),2 of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma (LUSC) are the most common subtypes.3 Along with the development of immune-checkpoint inhibitors (ICIs) and targeted therapy, NSCLC treatment prospects have notably progressed.3–6 However, only a small portion of patients benefit from molecular targeted therapy or immunotherapy and do not develop therapeutic resistance.7,8 Therefore, to extend the clinical benefit to more patients, continued research on new targets or novel combination therapies is warranted.
The 5-HT7 receptor (HTR7), one of the most recently identified serotonin receptors, belongs to a family of G-protein coupled receptors9. Since it was discovered in 1993, there has been extensive research into its role in the central nervous system.10 In addition to its well-established role in cognition,11 circadian rhythms12 and depression,13 its involvement in various cancers has also been reported.14–18 Although the 5-HT7 receptor has been implicated in many lung-associated pathologic processes in rats19,20 and guinea-pigs,21,22 research on the 5-HT7 receptor in human lungs is limited.23
In our study, an exploration of mRNA expression using bioinformatics analysis and the results of immunohistochemistry analysis both showed higher 5-HT7 receptor expression levels in the tumor tissues of NSCLC than in adjacent normal tissues, which indicates that the 5-HT7 receptor may play a role in the progression of NSCLC.
Materials and Methods
NSCLC Tissue Specimens
Formalin-fixed, paraffin-embedded lung tissue sections (tumor with or without paired adjacent normal tissue) were collected from NSCLC (mainly LUSC and LUAD) patients who underwent thoracic surgery at Tongji Hospital between January 2016 and June 2019. No patients had a history of pulmonary fibrosis, chronic obstructive pulmonary disease, or any other severe pulmonary disease, and all enrolled patients provided written informed consent. Approvals were obtained from the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Cell Culture
The NSCLC cell line A549 was obtained from Genechem (Shanghai, China), and H1299 cells were obtained from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). These cell lines were cultured in Roswell Park Memorial Institute-1640 medium supplemented with 10% fetal bovine serum (FBS) in a humidified atmosphere with 5% CO2 at 37°C.
Reagents
Antibodies against the 5-HT7 receptor (5-hydroxytryptamine receptor 7), MMP9 (matrix metallopeptidase 9), PCNA (proliferating cell nuclear antigen), and survivin (baculoviral IAP repeat-containing protein 5) were purchased from Proteintech Group, Inc. (Wuhan, Hubei, China), while phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204), p44/42 MAPK (Erk1/2), phospho-SAPK/JNK (Thr183/Tyr185), SAPK/JNK, phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, phospho-Akt (Ser473), Akt (pan), phospho-Src (Ser17), and Src antibodies were purchased from Cell Signaling Technology Inc. (Beverly, MA, USA), and the β-actin antibody was obtained from Sungene Biotech Co, Ltd (Tianjin, China). LP211 was purchased from MedChemExpress LLC (Monmouth Junction, NJ, USA). BMS582949, MK2206, SP600125 and AZD0530 were obtained from Selleck Chemicals LLC (Houston, TX, USA).
UCSC Xena
Gene profiles (gene expression RNAseq Illumina HiSeq, log2 (x+1)-transformed RSEM-normalized data) of the 5-HT7 receptor in tumors and correlating adjacent normal lung tissues from male smokers in the LUSC (TCGA lung squamous cell carcinoma, n=28) and LUAD (TCGA lung adenocarcinoma, n=11) datasets were retrieved from the UCSC Xena browser (https://genome-cancer.ucsc.edu/). Additionally, we obtained 5-HT7 receptor expression data for tumors in male smokers with NSCLC of different pathological stages.
TCGA
The mRNA expression (raw read counts), clinical, meta- and manifest data of the aforementioned paired tissues were downloaded from The Cancer Genome Atlas (TCGA http://cancergenome.nih.gov/) using the Genomic Data Commons Data Portal (GDC Data Portal https://portal.gdc.cancer.gov/). Then, mRNA expression data were converted using the R language (http://www.r-project.org/) and Perl software (http://www.cpan.org). Replicate within-array probes were replaced with the average, genes with very low counts were filtered out, and differentially expressed genes were identified by the R package “edgeR” in Bioconductor (http://www.bioconductor.org/). A false discovery rate (FDR) of 0.05 and a log-fold change of 2 were considered as cutoffs for a significant difference. Volcano plots and heat maps were generated by using the “gplot” package and the “TBtools” software. GO (Gene Ontology) enrichment and KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway analyses were performed with the packages “clusterProfiler”, “enrichplot” and “org.Hs.eg.db”.
Immunohistochemistry (IHC)
Formalin-fixed, paraffin-embedded lung tissue sections (4 μm thick) were deparaffinized and then rehydrated. Antigen retrieval was performed at 100°C using citrate buffer (pH 6.0). Then sections were incubated in 3% H2O2 for 20 min to inactivate endogenous peroxidase. The sections were then blocked in 10% bovine serum albumin (BSA) for 20 min, and then incubated with primary antibody overnight at 4°C. After washing, the samples were incubated with HRP-conjugated secondary antibody. Then, subsequent detection step was conducted using 3,3ʹ-diaminobenzidine (DAB) substrate. Finally, the sections were stained with hematoxylin, dehydrated and placed with coverslips.
Transfection
Small interfering RNA (siRNA) targeting the 5-HT7 receptor and nontargeting negative control siRNA (siNC) were synthesized by RiboBio (Guangzhou, China). Lipofectamine 3000 Reagent (Invitrogen, USA) was used to enhance cellular uptake. The media was changed to remove the Lipofectamine 3000 after 6 h. Then, the cells were harvested for RNA isolation or protein extraction at an appropriate time after transfection. After 48 h, the transfected cells were collected for further experiments. The siRNA sequence was as follows: CTCTACCGCAGTGGCATTT.
Real-Time PCR
Total cellular RNA was isolated using the RNAiso Plus Kit (Takara, Dalian, China) according to the manufacturer’s instructions. Then, cDNA was synthesized using PrimeScript™ RT Master Mix (Takara, Dalian, China) and was amplified by TB Green® Premix Ex Taq™ (Takara, Dalian, China) in an ABI PRISM Fast 7500 sequence detection system (Applied Biosystems, Foster, CA). The level of gene expression was determined by the ΔΔCt method. The primers used were as follows:
β-Actin: F-5ʹ-AGAAAATCTGGCACCACACCT-3ʹ, R-5ʹ-GATAGCACAGCCTGGATAGCA-3ʹ;
5-HT7 receptor: F-5ʹ-ACTCTACCGCAGTGGCATTT-3ʹ, R-5ʹ-TGTGTTTGGCAGCACTCTTC-3ʹ.
Western Blotting
Cellular protein was extracted using phenylmethylsulfonyl assay (RIPA) lysis buffer with phenylmethylsulfonyl fluoride, cocktail, and phosphorylation protease inhibitor and centrifuged at 4°C to extract the supernatant. The protein concentration was determined by BCA assay. Before loading for SDS-polyacrylamide gel electrophoresis (SDS-PAGE), the protein homogenates were added with loading buffer and boiled for 10 min. Then, proteins were transferred to microporous polyvinylidene difluoride (PVDF) membranes (Roche Diagnostics, Mannheim, Germany) using a Bio-Rad blotting system. After blocking with 5% skim milk in Tris-buffered saline-Tween 20 (TBST) for 1 h at room temperature, the membranes were incubated with the appropriate dilution of primary antibodies overnight, followed by incubation with secondary antibodies for 1 h. The western ECL substrate (Bio-Rad, California, USA) and the ChemiDocTM-XRS+imaging system (Bio-Rad, California, USA) were used to visualize the bands.
Cell Proliferation Assay
The role of the 5-HT7 receptor in NSCLC cell proliferation was demonstrated by CCK-8 assay. After transfection with siRNA, A549 or H1299 cells were routinely trypsinized, and the cell count was determined. A549 cells were seeded in 96-well plates with an average of 3500 cells per well, and H1299 cells were plated at a density of 3000 cells per well. Each experimental group had six replicate wells. Then, 10 μL CCK8 (Cell Counting Kit-8, Dojindo, Japan) reagent was added into the wells and incubated for 30 min at the indicated times. The absorbance at 450 nm was determined by an ELx800 Universal Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT).
Colony Formation Assay
Transfected cells were seeded into 6-well plates at an average of 400 cells per well for A549 cells and 300 cells per well for H1299 cells. The same density of cells was plated in 6-well plates incubated with or without the indicated concentration of LP211. Visible cell clusters took at least 8 days to form. Following fixation with 4% paraformaldehyde for 20 min, the cells were stained with 0.1% crystal violet for 30 min, and then the colonies were photographed and counted.
Migration and Invasion Assay
The migration and invasion capacities of A549 and H1299 cells were assayed using 24-well plates. In the migration assay, 200 μL serum-free cell suspension was added into the upper chamber, and 600 μL medium containing 10% FBS was placed into the lower chamber. In the invasion assay, the upper chambers were first coated with Matrigel (BD Bioscience, MA, USA) at a dilution of 1/8 in serum-free medium for at least 1 h in a 37°C incubator, and the cell suspension volume in the upper chamber was 100 μL. Once the cells migrated into the lower chamber, the migrated or invaded cells on the lower side of the insert membrane were fixed with 4% paraformaldehyde and stained with crystal violet, while cells on the upper layer of the chamber were removed with cotton swabs.
Statistical Analysis
All data are presented as the means ± standard deviation. The significance of differences between two groups was assessed by Student’s t-test or the χ2 test. The significance of differences between multiple groups was assessed by one-way analysis of variance. All statistical analyses were performed using R3.5.1 (http://www.r-project.org/) and SPSS 21.0 software (Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software Inc, San Diego, CA, USA). P<0.05 was considered statistically significant.
Results
5-HT7 Receptor Is Overexpressed in NSCLC Tumor Tissues Compared with Matched Adjacent Normal Lung Tissues
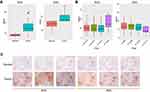
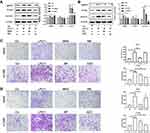
Demographic data and gene profiles (gene expression RNAseq Illumina HiSeq, log2 (x+1)-transformed RSEM-normalized data) of NSCLC lung tissues from TCGA were downloaded from the UCSC Xena browser. Data from male smokers were explored. The mRNA expression of the 5-HT7 receptor in 28 paired tumor and adjacent normal lung tissues from the LUSC (TCGA lung squamous cell carcinoma, SCC) datasets, 11 paired tumor and correlating normal lung tissues from LUAD(TCGA lung adenocarcinoma, ADC) datasets, samples from 309 LUSC patients with different pathological stages, and samples from 166 LUAD patients with different pathological stages were included in the analysis. We found that tumor tissues expressed 5-HT7 receptor mRNA at a higher level than normal tissues (Figure 1A). In addition the mRNA expression of the 5-HT7 receptor in SCC patients tended to be increased in stage IV, while the data of ADC patients did not show the same trend (Figure 1B).
To verify the higher expression of 5-HT7 receptors in NSCLC tumors in male smokers, we stained 6 paired lung tissues for the 5-HT7 receptor using immunohistochemistry (Figure 1C). The expression of 5-HT7 receptor in tumor tissues was higher than that in the paired normal lung tissues. To avoid bias in smoking history and sex, we also stained paired lung tissues of male nonsmokers and female nonsmokers (Figure S1). The same results were obtained.
Further evaluation of the correlations between 5-HT7 receptor expression and clinicopathological features in patients with NSCLC was also conducted using immunohistochemistry, and the expression levels of 5-HT7 receptor in tumor tissues were presented as average optical density (AOD) values. In total, 51 patients were divided into low and high expression groups according to the median expression value. As shown in Table 1, higher 5-HT7 receptor expression levels were correlated with lymph node metastasis (P=0.007) and advanced TNM stage (P=0.000) in NSCLC patients. However, 5-HT7 receptor expression levels were not associated with age, sex, smoking status or tumor size. Taken together, our data suggest that the 5-HT7 receptor may be involved in the progression of NSCLC.
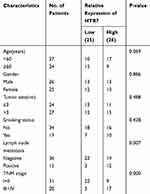 |
Table 1 Association of 5-HT7 Receptor (HTR7) Expression with Clinicopathologic Characteristics of NSCLC |
Exploring Gene Expression Profiles of the Aforementioned Paired Samples Using the TCGA Database
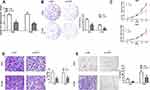
The raw read count data of the aforementioned paired tissues from The Cancer Genome Atlas (TCGA) were explored. Genes that met the criteria of an FDR<0.05 and a log-fold change of greater than or equal to 2 were considered to be significantly differentially expressed genes (DEGs). After the management of replicate probes and specifically low expression level probes, we obtained 3540 differentially expressed genes from the SCC data, among which 1681 genes were upregulated and 1859 genes were downregulated in tumors. The 5-HT7 receptor gene was one of the significantly elevated genes. Regarding the ADC data, we obtained 1642 DEGs, which contained 747 genes with elevated expression and 895 genes with reduced expression in tumors. Although 5-HT7 receptor expression was not markedly increased in ADC tumor samples, we found a log-fold change of 0.7345 when we reviewed the primary data, which means 5-HT7 receptor expression still showed an increasing trend. The above results were visualized using volcano plots (Figure 2A). Both the top 50 elevated DEGs and top 50 downregulated DEGs were represented via heatmaps (Figure 2B).
To further understand the role of the 5-HT7 receptor gene in these DEGs, we investigated the GO enrichment in three categories, biological process (BP), cellular component (CC), and molecular function (MF), based on the DEGs. A substantial proportion of SCC DEGs were enriched in the “biological process” term “G-protein-coupled peptide receptor” (Figure 2C), while a significant number of ADC DEGs were enriched in the “cellular component” term “G-protein-coupled peptide receptor” (Figure 2C). Obviously, the 5-HT7 receptor belongs to the GPCR (G-protein-coupled peptide receptor) super family9. In addition, the KEGG pathway database was employed to detect biological pathway enrichment. The majority of the DEGs were enriched in the “neuroactive ligand-receptor interaction” pathway. (Figure 2D) The 5-HT7 receptor can serve as a neurotransmitter receptor,24 which has been reported in many studies.
Overall, we found a functional significance of the 5-HT7 receptor gene in the differences between NSCLC from normal samples, and it is worth exploring the effect of the 5-HT7 receptor gene in NSCLC progression.
5-HT7 Receptor Downregulation Can Suppress NSCLC Cell Proliferation, Decrease Colony Formation and Inhibit Cell Migration and Invasion
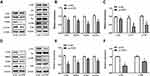
To identify the role of the 5-HT7 receptor in NSCLC progression, we used small interfering RNA to knockdown the gene expression of 5-HT7 receptor in two NSCLC cell lines: A549 and H1299. The knockdown efficiency was shown via determination of mRNA levels (Figure 3A) and protein levels (Figure 4A and D).
We conducted CCK-8 and colony formation assays to assess the effect of 5-HT7 receptor depletion on cell proliferation. As shown, 5-HT7 receptor knockdown notably reduced the colony formation of the two NSCLC cell lines (Figure 3B) and decreased the rate of NSCLC cell proliferation (Figure 3C). We detected the effects of 5-HT7 receptor knockdown on migration and invasion by the Transwell assay. We observed a decreased number of cells on the lower side of the upper chamber in transfected A549 and H1299 cells (Figure 3D and E).
Changes Downstream After 5-HT7 Receptor Knockdown in NSCLC Cells
To confirm the effect of 5-HT7 receptor depletion on cell proliferation, we chose to examine PCNA and survivin, which are oncogenes associated with proliferation. Knockdown of the 5-HT7 receptor caused decreased PCNA and survivin expression compared with that seen in NSCLC cells treated with the nontargeting negative control (Figure 4A, B, D, E). There was a significant downregulation of the expression of MMP9 (matrix metalloproteinase-9), a positive regulator of cancer cell metastasis, in 5-HT7 receptor knockdown A549 and H1299 cells (Figure 4A, B, D, E).
We next investigated the signaling molecules downstream of the 5-HT7 receptor involved in the aforementioned phenotypes. We examined the mTOR, Akt, Src and MAPK family signaling pathways and discovered that the phosphorylation of Akt, Src and P38 was notably inhibited in A549 cells (Figure 4A and C) after transfection, but the phosphorylation of mTOR, JNK, and ERK was not inhibited. In H1299 cells, we detected that 5-HT7 receptor deficiency markedly suppressed the phosphorylation of JNK and Src (Figure 4D and F), but it did not suppress the phosphorylation of mTOR, Akt, ERK and P38.
LP211, an Agonist of the 5-HT7 Receptor, Stimulates NSCLC Cell Colony Formation, Migration and Invasion
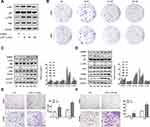
LP211, a selective agonist of the 5-HT7 receptor,25–28 was employed to activate 5-HT7 receptor downstream signaling in NSCLC cells. After a 2 h incubation with LP211, at concentrations that varied from 10 nM to 100 nM, A549 cells were harvested to detect the phosphorylation of Akt, Src and P38 signaling pathway proteins. We observed activation of only the Akt and P38 signaling pathways, and the activation was not evident when the concentration of LP211 reached 100 nM (Figure 5A). Further validation was displayed using colony formation assays. Two NSCLC cell lines were cultured with different concentrations of LP211 until visible cell clusters formed (8 days). The 10 nM concentration of LP211 enhanced in the formation of colonies compared with the control in A549 and H1299 cells (Figure 5B). We used 10 nM LP211 for subsequent investigation.
After treatment with or without 10 nM LP211, NSCLC cells were harvested at various times. LP211 stimulation induced upregulation of MMP9, PCNA and survivin expression in both NSCLC cell lines, and the highest levels of MMP9 and survivin were observed at 12 h in A549 cells (Figure 5C), while the highest expression of MMP9, PCNA and survivin was observed at 24 h in H1299 cells (Figure 5D). We also evaluated the phosphorylation state of the signaling pathways described above. The highest phosphorylation of P38 was obtained at 2 h, and the highest p-Akt level was observed at 6 h in A549 cells (Figure 5C). As with H1299 cells, p-JNK and p-Src both reached their maximum levels at 2 h (Figure 5D).
Given these findings, A549 cells were incubated with 10 nM LP211 for 12 h before testing for migration and invasion, while H1299 cells were treated for 24 h. As expected, LP211 notably enhanced the migration and invasion capacities of the two NSCLC cell lines (Figure 5E and F).
The 5-HT7 Receptor Regulates Cell Migration and Invasion Through P38 or Src Signaling
Based on the previous results, NSCLC cells were pretreated with various inhibitors and then stimulated with 10 nM LP211, and the cell proteins were extracted. To explore the pathways involved in MMP9, PCNA and survivin expression, A549 cells and H1299 cells were incubated with 10 nM LP211 for 12 h and 24 h, respectively, following pretreatment with the corresponding inhibitors. We found that the P38 inhibitor BMS582949 (5 μM, 2 h) significantly reversed the effect of LP211 on MMP9 expression, and the Akt inhibitor MK2206 (5 μM, 1 h) suppressed the expression of survivin, in A549 cells (Figure 6A). We also validated that the Src inhibitor AZD0530 (8 μM, 3 h) could attenuate the effcts of LP211 on MMP9, PCNA and survivin in H1299 cells, and the JNK inhibitor SP600125 (40 μM, 1 h) partly reversed the effect of LP211 on survivin expression. (Figure 6B).
Then we conducted Transwell assays and obtained the same results. LP211-induced migration (Figure 6C) and invasion (Figure 6D) were significantly suppressed by BMS582949 in A549 cells. In H1299 cells, the enhanced migration (Figure 6C) and invasion (Figure 6D) were reversed by AZD0530.
Discussion
Although serotonin receptors and serotonin synthesis pathways are considered potential chemotherapeutic targets for the treatment of several cancers,29 studies investigating the 5-HT7 receptor in cancer are scarce. Human glioblastoma cell lines express functional 5-HT7 receptors,30 and treatment with a 5-HT7 receptor agonist increased resistance to apoptosis and mitosis via the ERK1/2 signaling pathway in glioblastomas.15 When small intestinal (SI) neuroendocrine neoplasms (NENs) metastasize to the liver, hepatocytes respond to elevated 5-HT levels produced by specific SI NENs with increased secretion of IGF-1 via the 5-HT7 receptor/Akt pathway, and the paracrine production of IGF-1, in turn, supports tumor cell proliferation.16 In triple-negative breast cancer (TNBC), the autocrine effects of 5-HT on MDA-MB-231 cell proliferation and invasion were mediated through the 5-HT7 receptor.17,18
Considering the markedly high expression of 5-HT7 receptors in the lung tissues of NSCLC patients, the 5-HT7 receptor functional significance in GO term enrichment and KEGG pathway enrichment analyses of the DEGs, and the lack of investigation of 5-HT7 receptors in NSCLC, we carried out this study.
The present study showed that proliferation, migration and invasion were inhibited in both A549 cells and H1299 cells when the 5-HT7 receptor expression was downregulated. This was accompanied by a significant decrease in the expression of PCNA, survivin and MMP9 in two NSCLC cell lines. To further confirm this phenomenon, we identified that the highly selective agonist LP211 promoted proliferation, migration and invasion in both A549 cells and H1299 cells and also induced upregulation of the aforementioned proteins. The results also illustrated that the 5-HT7 receptor might be an upstream regulator of P38 and Akt and mediates proliferation or metastasis in A549 cells by activating these pathways. In H1299 cells, the pathways that might be regulated by the 5-HT7 receptor to influence proliferation and metastasis were the JNK and Src signaling pathways.
The loss of growth regulation in cancer is characterized by proliferation and subsequent invasion. These two phenotypes are mutually incompatible and can be achieved when subclones of phenotypes coexist in one tumor.31–33 This view indicates that the downstream signaling pathways of these disparate phenotypes may be quite different and appear to act independently. This likely accounts for the results that the 5-HT7 receptor might regulate the P38 pathway to influence A549 cell migration and invasion, but might be through the Akt pathway to impact the expression of survivin to mediate cell proliferation. In addition, in H1299 cells, the Src signaling pathway might be involved in the 5-HT7 receptor-mediated regulation of proliferation, migration and invasion, while the JNK pathway might also contribute to the modulation of survivin expression.
We also observed the different downstream targets in A549 and H1299 cells. Similar discrepancies in cell lines have been reported in several previous studies. There might exist one target signaling pathway involved in both NSCLC cell lines that was not discussed in our study,34 which would need future investigation. The different levels of endogenous activation of specific signaling pathways35 or the innate difference between cell lines35,36 might also account for the discrepancy, and further exploration is needed.
For most GPCRs, acute exposure to high agonist concentrations may contribute to immediate desensitization via phosphorylation.37,38 Furthermore, prolonged stimulation with GPCR agonists may produce suppressive effects.39,40 After a 2 h incubation with LP211, changes in signaling activation appeared when the concentration of LP211 reached 100 nM in A549 cells. In the results of the colony formation assays, we demonstrated that the effects of LP211 were not concentration-dependent. These findings may be explained by the previously described unique characteristics of GPCRs. Therefore, we chose 10 nM LP211 for further investigations.
Conclusion
In the current study, for the first time, we investigated the effects of overexpression of the 5-HT7 receptor in NSCLC lung tissues and explored the role of the 5-HT7 receptor in NSCLC progression. Additionally, we demonstrated that the 5-HT7 receptor mediated cell proliferation, migration and invasion in NSCLC cells. We also conducted a preliminary investigation of pathways that might be involved in 5-HT7 receptor-mediated regulation of different phenotypes in both A549 cells and H1299 cells. This work provides insights into the involvement of the 5-HT7 receptor in NSCLC progression and suggests a target for intervention in NSCLC metastasis. However, whether a selective antagonist for the 5-HT7 receptor can be used for NSCLC therapy will require future studies.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81973986, 81570033, 81370145), the National Key Basic Research and Development Program (973 Program, No. 2015CB553403), and the National Key R&D Program of China (2016YFC1304500, 2018YFC1311900).
Author Contributions
X.J.G and D.X.H. conceived and designed research; D.X.H. and W.T. performed experiments; W.Z.H. and W.X.M. collected and summarized data, and interpreted the results; G.Y.Y. and H.Q. collected the lung tissue samples and modified the paper; D.X.H. and W.J.M. did the bioinformatics analysis; D.X.H. drafted the manuscript; X.J.G revised the manuscript and contributed to figure and table design and format. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi:10.1016/S0025-6196(11)60735-0
3. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553(7689):446–454. doi:10.1038/nature25183
4. Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi:10.1016/S0140-6736(16)30958-8
5. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol. 2018;15(11):694–708.
6. Doroshow DB, Herbst RS. Treatment of advanced non-small cell lung cancer in 2018. JAMA Oncol. 2018;4(4):569–570. doi:10.1001/jamaoncol.2017.5190
7. Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer. 2017;17(11):637–658. doi:10.1038/nrc.2017.84
8. Heigener DF, Reck M. Lung cancer in 2017: giant steps and stumbling blocks. Nat Rev Clin Oncol. 2018;15(2):71–72. doi:10.1038/nrclinonc.2017.178
9. Leopoldo M, Lacivita E, Berardi F, Perrone R, Hedlund PB. Serotonin 5-HT7 receptor agents: structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol Ther. 2011;129(2):120–148. doi:10.1016/j.pharmthera.2010.08.013
10. Rague A, Tidgewell K. Pharmacophore comparison and development of recently discovered long chain arylpiperazine and sulfonamide based 5-HT7 ligands. Mini Rev Med Chem. 2018;18(7):552–560. doi:10.2174/1389557517666170913111533
11. Santello M, Bisco A, Nevian NE, Lacivita E, Leopoldo M, Nevian T. The brain-penetrant 5-HT7 receptor agonist LP-211 reduces the sensory and affective components of neuropathic pain. Neurobiol Dis. 2017;106:214–221. doi:10.1016/j.nbd.2017.07.005
12. Hedlund PB, Huitron-Resendiz S, Henriksen SJ, Sutcliffe JG. 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol Psychiatry. 2005;58(10):831–837. doi:10.1016/j.biopsych.2005.05.012
13. Mullins UL. Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology. 1999;21(3):352–367. doi:10.1016/S0893-133X(99)00041-X
14. Louiset E, Isvi K, Gasc JM, et al. Ectopic expression of serotonin7 receptors in an adrenocortical carcinoma co-secreting renin and cortisol. Endocr Relat Cancer. 2008;15(4):1025–1034. doi:10.1677/ERC-08-0085
15. Kast RE. Glioblastoma chemotherapy adjunct via potent serotonin receptor-7 inhibition using currently marketed high-affinity antipsychotic medicines. Br J Pharmacol. 2010;161(3):481–487. doi:10.1111/j.1476-5381.2010.00923.x
16. Svejda B, Kidd M, Timberlake A, et al. Serotonin and the 5-HT7 receptor: the link between hepatocytes, IGF-1 and small intestinal neuroendocrine tumors. Cancer Sci. 2013;104(7):844–855. doi:10.1111/cas.12174
17. Gautam J, Banskota S, Regmi SC, et al. Tryptophan hydroxylase 1 and 5-HT7 receptor preferentially expressed in triple-negative breast cancer promote cancer progression through autocrine serotonin signaling. Mol Cancer. 2016;15(1):75. doi:10.1186/s12943-016-0559-6
18. Gautam J, Bae YK, Kim JA. Up-regulation of cathepsin S expression by HSP90 and 5-HT7 receptor-dependent serotonin signaling correlates with triple negativity of human breast cancer. Breast Cancer Res Treat. 2017;161(1):29–40. doi:10.1007/s10549-016-4027-1
19. Cadirci E, Halici Z, Bayir Y, et al. Peripheral 5-HT7 receptors as a new target for prevention of lung injury and mortality in septic rats. Immunobiology. 2013;218(10):1271–1283. doi:10.1016/j.imbio.2013.04.012
20. Tawfik MK, Makary S. 5-HT7 receptor antagonism (SB-269970) attenuates bleomycin-induced pulmonary fibrosis in rats via downregulating oxidative burden and inflammatory cascades and ameliorating collagen deposition: comparison to terguride. Eur J Pharmacol. 2017;814:114–123. doi:10.1016/j.ejphar.2017.08.014
21. Segura P, Vargas MH, Cordoba-Rodriguez G, et al. Role of 5-HT2A, 5-HT4 and 5-HT7 receptors in the antigen-induced airway hyperresponsiveness in guinea-pigs. Clin Exp Allergy. 2010;40(2):327–338. doi:10.1111/cea.2010.40.issue-2
22. Cordoba-Rodriguez G, Vargas MH, Ruiz V, et al. Allergic sensitization modifies the pulmonary expression of 5-hydroxytryptamine receptors in guinea pigs. Respir Physiol Neurobiol. 2016;223:9–15. doi:10.1016/j.resp.2015.11.018
23. Ayaz G, Halici Z, Albayrak A, Karakus E, Cadirci E. Evaluation of 5-HT7 receptor trafficking on in vivo and in vitro model of Lipopolysaccharide (LPS)-induced inflammatory cell injury in rats and LPS-treated A549 cells. Biochem Genet. 2017;55(1):34–47. doi:10.1007/s10528-016-9769-2
24. Adriani W, Leo D, Greco D, et al. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31(9):1946–1956. doi:10.1038/sj.npp.1300962
25. Costa L, Spatuzza M, D’Antoni S, et al. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol Psychiatry. 2012;72(11):924–933. doi:10.1016/j.biopsych.2012.06.008
26. Di Pilato P, Niso M, Adriani W, et al. Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: an overview. Rev Neurosci. 2014;25(3):401–415. doi:10.1515/revneuro-2014-0009
27. Speranza L, Giuliano T, Volpicelli F, et al. Activation of 5-HT7 receptor stimulates neurite elongation through mTOR, Cdc42 and actin filaments dynamics. Front Behav Neurosci. 2015;9:62. doi:10.3389/fnbeh.2015.00062
28. Speranza L, Labus J, Volpicelli F, et al. Serotonin 5-HT7 receptor increases the density of dendritic spines and facilitates synaptogenesis in forebrain neurons. J Neurochem. 2017;141(5):647–661. doi:10.1111/jnc.2017.141.issue-5
29. Sarrouilhe D, Mesnil M. Serotonin and human cancer: a critical view. Biochimie. 2019;161:46–50. doi:10.1016/j.biochi.2018.06.016
30. Mahe C, Bernhard M, Bobirnac I, et al. Functional expression of the serotonin 5-HT7 receptor in human glioblastoma cell lines. Br J Pharmacol. 2004;143(3):404–410. doi:10.1038/sj.bjp.0705936
31. Gao CF, Xie Q, Su YL, et al. Proliferation and invasion: plasticity in tumor cells. Proc Natl Acad Sci U S A. 2005;102(30):10528–10533. doi:10.1073/pnas.0504367102
32. Hatzikirou H, Basanta D, Simon M, Schaller K, Deutsch A. ‘Go or grow’: the key to the emergence of invasion in tumour progression? Math Med Biol. 2012;29(1):49–65. doi:10.1093/imammb/dqq011
33. Hecht I, Natan S, Zaritsky A, Levine H, Tsarfaty I, Ben-Jacob E. The motility-proliferation-metabolism interplay during metastatic invasion. Sci Rep. 2015;5:13538. doi:10.1038/srep13538
34. Rao PS, Satelli A, Moridani M, Jenkins M, Rao US. Luteolin induces apoptosis in multidrug resistant cancer cells without affecting the drug transporter function: involvement of cell line-specific apoptotic mechanisms. Int J Cancer. 2012;130(11):2703–2714. doi:10.1002/ijc.v130.11
35. Li C, Ahlborn TE, Kraemer FB, Liu J. Oncostatin M-induced growth inhibition and morphological changes of MDA-MB231 breast cancer cells are abolished by blocking the MEK/ERK signaling pathway. Breast Cancer Res Treat. 2001;66(2):111–121. doi:10.1023/A:1010614724664
36. Lin SQ, Jia FJ, Zhang CY, et al. Actinomycin V suppresses human non-small-cell lung carcinoma A549 cells by inducing G2/M phase arrest and apoptosis via the p53-dependent pathway. Mar Drugs. 2019;17:10. doi:10.3390/md17100572
37. Thomas DR, Gittins SA, Collin LL, et al. Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br J Pharmacol. 1998;124(6):1300–1306. doi:10.1038/sj.bjp.0701946
38. Srinivas BN, Subhash MN, Vinod KY. Cortical 5-HT(1A) receptor downregulation by antidepressants in rat brain. Neurochem Int. 2001;38(7):573–579. doi:10.1016/S0197-0186(00)00123-6
39. El Mansari M, Lecours M, Blier P. Effects of acute and sustained administration of vortioxetine on the serotonin system in the hippocampus: electrophysiological studies in the rat brain. Psychopharmacology (Berl). 2015;232(13):2343–2352. doi:10.1007/s00213-015-3870-9
40. Carton L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280–3297. doi:10.2174/1381612821666150619092903
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.