Back to Journals » International Journal of Nanomedicine » Volume 18
3D Bioprinting: An Important Tool for Tumor Microenvironment Research
Authors Li Y, Liu J, Xu S, Wang J
Received 17 August 2023
Accepted for publication 16 December 2023
Published 28 December 2023 Volume 2023:18 Pages 8039—8057
DOI https://doi.org/10.2147/IJN.S435845
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Jie Huang
Yilin Li,1,* Jiaxing Liu,2,* Shun Xu,1 Jiajun Wang1
1Department of Thoracic Surgery, The First Hospital of China Medical University, Shenyang, People’s Republic of China; 2Department of General Surgery, The Fourth Hospital of China Medical University, Shenyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shun Xu; Jiajun Wang, Email [email protected]; [email protected]
Abstract: The tumor microenvironment plays a crucial role in cancer development and treatment. Traditional 2D cell cultures fail to fully replicate the complete tumor microenvironment, while mouse tumor models suffer from time-consuming procedures and complex operations. However, in recent years, 3D bioprinting technology has emerged as a vital tool in studying the tumor microenvironment. 3D bioprinting is a revolutionary biomanufacturing technique that involves layer-by-layer stacking of biological materials, such as cells and biomaterial scaffolds, to create highly precise 3D biostructures. This technology enables the construction of intricate tissue and organ models in the laboratory, which are utilized for biomedical research, drug development, and personalized medicine. The application of 3D bioprinting has brought unprecedented opportunities to fields such as cancer research, tissue engineering, and organ transplantation. It has opened new possibilities for addressing real-world biological challenges and improving medical treatment outcomes. This review summarizes the applications of 3D bioprinting technology in the context of the tumor microenvironment, aiming to explore its potential impact on cancer research and treatment. The use of this cutting-edge technology promises significant advancements in understanding cancer biology and enhancing medical interventions.
Keywords: 3D bioprinting, tumor microenvironment research, tumor microenvironment
Introduction
In the late 1980s, the emergence and advancement of tissue engineering opened up possibilities for repairing damaged human tissues and organs.1 In a groundbreaking achievement in 1988, Klebe demonstrated the first successful bioprinting experiment by employing a standard inkjet printer to precisely deposit cells using a cell patterning technique.2 By accurately assembling biological materials, seed cells, and growth factors, this technology enables the precise fabrication of skin, blood vessels, internal organs, bone tissues, and other biologically active tissues and organs. The distribution of cells and biological materials can be tailored as needed, allowing the creation of intricate microstructures.3,4 Referred to as “additive manufacturing technology”, 3D bioprinting involves computer-aided design modeling and layer-by-layer printing of products in STL file format under computer control.5 This novel approach surpasses the limitations of traditional methods, enabling precise control of stent porosity and pore size. It also allows customization of stents with adjustable mechanical properties, and the process is simple and replicable, making it widely utilized in stent manufacturing. In contrast to the conventional method of cell inoculation after scaffold preparation, biological 3D printing ensures even distribution of cells within the scaffold, thanks to the precise distribution of cells and biomaterials. This advancement facilitates the successful construction of human organs.6,7
In the field of medicine, 3D bioprinting technology has demonstrated high value in regenerative medicine, such as in the regeneration and repair of bones and cartilage, as well as the regeneration and repair of skin and vascular networks. It aids in the functional restoration of these non-regenerative human tissues or organs.7–12 On the other hand, with the increasing incidence of tumors, many researchers are attempting to leverage 3D bioprinting technology to provide research strategies for the mechanisms of tumor occurrence and development, as well as personalized treatment plans. In current research on tumor mechanisms and treatments, there has been significant attention on the tumor microenvironment (TME) in recent years. Moreover, recreating the components of TME and their interplay in vitro can provide more robust evidence for targeted therapy against the tumor microenvironment, subsequently offering prospects for the clinical translation of tumor treatment drugs. Given that tumor tissues possess their unique complex structures and the three-dimensional organization and distribution of cells and extracellular matrix (ECM), accurately summarizing the complexity of tumor tissue from a structural and functional perspective remains a formidable challenge. Due to the progress in rapid prototyping and biomanufacturing technologies, researchers are now delving into diverse 3D bioprinting techniques and materials to fabricate intricate three-dimensional cancer models.13 Unlike conventional modeling methods, 3D bioprinting provides a significant advantage in developing in vitro tumor models, as it enables precise definition of perfusable networks and the positioning of various cell types in a high-throughput manner. Notably, in simulating the TME, 3D bioprinting excels at generating high-resolution microstructures that faithfully replicate the complexity of TME. Extensive research is focused on exploring 3D drug-tumor interactions and constructing preclinical models for tumor biology and pharmaceutical applications. These models are instrumental in advancing personalized medicine, offering a promising approach for diverse research applications within the fields of oncology and pharmaceuticals.14–16
This review aims to focus on the simulation of TME models using 3D bioprinting technology. It summarizes the research progress in the application of 3D bioprinting to simulate TME in various cancers, encompassing the technology itself, the simulated components of the models, and their interactions. Furthermore, it examines the existing issues and challenges in this field and provides prospects for future research directions.
3D Bioprinting Technology
3D Bioprinting Method
Based on the printing principle, biological 3D printing can be categorized into three types: extrusion-based bioprinting, droplet-based bioprinting, and photocuring-based bioprinting (Figure 1).
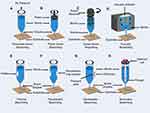 |
Figure 1 Pattern diagrams of different types of 3D bioprinting. Notes: This figure is drawn by Figdraw. |
Extrusion-Based Bioprinting
Extrusion-based bioprinting is a versatile method widely used for printing a diverse array of biomaterials. Extrusion-based 3D bio-printing uses computer-controlled fluid distribution and automated robotic systems to accurately deposit materials based on 3D computer-aided design (CAD) files. Typically, a temperature-controlled material reservoir extrudes bioprinting ink through a fine nozzle by applying various forms of extrusion forces, such as pressure-time or displacement-time. The printhead then moves consistently in the x, y, and z directions, allowing for continuous deposition of filaments onto the build platform.17 This approach enables the printing of bioinks with high cell density, ensuring cost-effectiveness, and facilitating the fabrication of intricate 3D structures consisting of different cell types and materials.18
There are two main types of force-driven extrusion mechanisms in bio-printing: pneumatic (pressure-driven) and mechanical (displacement-driven) systems. In pneumatic systems, compressed air is used to generate force and push the bioprinting ink out of the syringe. The ink used in this process typically has a rheological range (30 to over 6×107 mPa s) and is extruded through the pneumatic bio-printing nozzle. On the other hand, mechanical systems utilize pistons or screw mechanisms to directly distribute the bioprinting ink from the material reservoir. The ink used in this method usually has a higher viscosity (>6×107 mPa s).19 Commonly used biomaterials include alginate, gelatin, poly(ε-caprolactone) (PCL), fibroin, and gelatin at different concentrations.20 In the subsequent discussion, we will further summarize the important role of bioprinting ink in 3D bio-printing and its selection in different bio-printing strategies. Extrusion-based bio-printing techniques can be improved by combining bio-printing processes such as modifying needle diameter, pressure and printing speed, using supports, and employing coaxial nozzles to achieve higher quality bio-printed structures.21 This also provides a broad prospect for 3D bio-printing to simulate the TME.
Droplet-Based Bioprinting
In the 3D bioprinting of simulated tissues with different cell types, the resulting tissues should ideally achieve a similar level of layered cellular patterning to capture the complexity and functionality of real tissues. This goal requires high-precision bioprinting capabilities, ideally achieving a single-cell level of accuracy.22 Droplet-based bioprinting can control the deposition of bioinks in volume, allowing for the spatial construction of heterogeneous cell populations and precise cellular positioning at predefined locations.23 Droplet-based bioprinting constructs structures by layering droplets, and it further subdivides into inkjet, acoustic-droplet-ejection and micro-valve bioprinting methods.24–26
Inkjet-based bioprinting, similar to traditional 2D printers, a printhead is utilized to eject bioink layer by layer, progressively building tissues or organs. Although it may not achieve the physiological cell density in bioprinting and has limitations in bioink selection, the incorporation of multiple printheads allows for rapid simultaneous printing of various cells, biomaterials, or growth factors.27 It can also be divided into continuous-inkjet bioprinting, drop-on-demand inkjet bioprinting and electrohydrodynamic jet bioprinting.28 In continuous-inkjet bioprinting, the liquid jet of the bioink solution is influenced by several factors under pressure, leading to the breakup of the stream into droplets for bioprinting. Drop-on-demand inkjet bioprinting demonstrates greater efficiency than the former method, as it generates droplets on demand, making it more cost-effective, easier to control, and more suitable for shaping bio-products. Drop-on-demand bioprinters consist of single or multiple print heads, with each print head comprising a fluid chamber and one or multiple nozzles. The bioink is stored in the fluid chamber and held in place by surface tension at the nozzle opening. As pressure pulses are introduced into the fluid chamber through a thermal actuator, piezoelectric actuator, or electrostatic actuator, the bioink overcomes surface tension and is ejected as droplets.28
Acoustic-droplet-ejection printers consist of single or an array of 2D microfluidic channels, where the bioink is maintained in the proper position due to surface tension at the exit of the small channels. This method utilizes gentle acoustic forces to eject droplets from an open liquid reservoir, eliminating the need for nozzle-based droplet ejection. This also ensures that the bioink and the composed living cells are not exposed to potentially harmful stressors such as heat, high pressure, high voltage, and shear stresses during the droplet ejection process. Although there is limited research on bioprinting based on this method, some studies have shown the ability to print cell clusters within droplets at the millimeter scale, as well as single-cell-sized droplets. This multiscale acoustic bioprinting technology holds promise for preserving cell viability and creating complex and personalized 3D hydrogel structures loaded with cells.29 Micro-valve bioprinting typically involves using an electromagnet coil and a plunger to block the nozzle opening, thereby generating droplets. When the bioink in the fluid chamber is pressurized, the microvalve closes the small hole of the nozzle, preventing ejection. The form of droplet generation is determined by the applied pressure and the duration of valve closure. Micro-valve bioprinting can maintain the viability of various proteins and cells by adjusting parameters such as pneumatic pressure, nozzle geometry, cell concentration, and bioink composition.30
Photocuring-Based Printing
Photocuring-based printing harnesses the potential of photosensitive materials to undergo photopolymerization, facilitating the formation of structures layer by layer. Photocuring-based 3D printing, based on photopolymerization technology, utilizes photosensitive liquid resin as the material and solidifies the resin under light exposure. After printing, the model can be easily and quickly separated from the resin. Due to the high precision and rapid polymerization rate of photocuring-based technology, models can be printed quickly.31 This cutting-edge technology exhibits impressive attributes, boasting high printing resolution and speed, while circumventing issues like nozzle clogging or shear stress that could impact cell viability. However, in biomedical applications of bioprinting, good biocompatibility is the most important property of materials. Most photosensitive resin materials have cell toxicity due to unreacted double bonds and residual photoinitiators.32 Therefore, currently, photocuring-based 3D printing technology and materials are mainly used in the field of temporary substitutes, such as dental restoration, orthodontics, dental surgery, models, molds, etc. While there is some potential for other research directions such as tumors, it is not highly suitable.
Bio-Ink in 3D Bioprinting
Bio-ink is an important part of 3D printing. It serves as the essential precursor for biological 3D printing, functioning as a cell carrier that contains bioactive components and biomaterial cell formulations. It is imperative for bio-ink to possess excellent formability while safeguarding cell integrity during printing and providing a supportive microenvironment for cell growth post-printing.33 Bioprinting’s bio-ink, a biomaterial solution, is extensively synthesized from self-assembling polymers. These organic biomaterials with long chains and high water content create a hydrated tissue-like environment that fosters vital cell functions, including adhesion, proliferation, and differentiation, promoting tissue regeneration. Currently, widely used natural polymers in bioprinting include alginate, chitosan, agarose, hyaluronic acid, collagen, and decellularized extracellular matrix (dECM), while synthetic polymers encompass poly(ε-caprolactone) and poly(vinyl) polymers (Figure 2). The selection of the appropriate bio-ink is critical when constructing 3D printing models, as it significantly influences the final shape and performance characteristics of the printed structure, including physical properties, rheological behavior, cross-linking mechanisms, mechanical attributes, and biological compatibility.34
 |
Figure 2 The cells components and types of biomaterials in bio-ink. Notes: This figure is drawn by Figdraw. |
Tumor Microenvironment (TME)
In recent years, there has been extensive research exploring tumor heterogeneity, leading to the development of highly effective therapeutic strategies. As tumors progress, the heterogeneity within the TME becomes evident as cells and non-cellular components undergo further differentiation and maturation. The TME is composed of immune cells, stromal cells, extracellular matrix (ECM), as well as blood and lymphatic vessel networks. Immune cells include T lymphocytes, B lymphocytes, tumor-associated macrophages (TAMs), dendritic cells (DCs), natural killer (NK) cells, neutrophils, and myeloid-derived suppressor cells (MDSCs). Stromal cells encompass cancer-associated fibroblasts (CAFs), pericytes, and mesenchymal stromal cells. Additionally, the ECM and secreted molecules, such as growth factors, cytokines, chemokines, and extracellular vesicles (EVs), play pivotal roles in communication between these components and their interactions with heterogeneous cancer cells. During tumor development, the TME undergoes complex biological events involving multiple components, while tumor metabolism contributes to hypoxia, oxidative stress, and acidosis, reshaping the TME.35 The TME profoundly influences abnormal tissue functions, playing a pivotal role in the progression of malignant tumors36 (Figure 3).
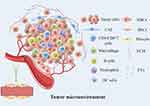 |
Figure 3 Basic components of tumor microenvironment. Notes: This figure is drawn by Figdraw. |
Immune Cells in TME
The immune cell composition within the TME comprises a diverse array of tumor-associated macrophages (TAMs), CD8+T cells, CD4+helper T cells, regulatory T cells (Tregs), B lymphocytes, neutrophils, monocytes, and other components. As the tumor advances, the immune microenvironment undergoes significant changes characterized by a gradual decrease in cytotoxic CD8+ T cells and natural killer (NK) cells, coupled with an increase in dysfunctional CD8+ T cells, immunosuppressive CD4+ T cells, Tregs, and regulatory B cells. Moreover, TAMs, which are macrophages related to the tumor, exhibit dynamic reshaping. The interactions between TAMs and cancer cells are intricate and crucial, playing indispensable roles in tumor progression, angiogenesis, and autophagy due to their heterogeneity and remarkable plasticity.37,38 Mounting evidence suggests that neutrophils (TANs), akin to TAMs, are abundant myeloid cells within the TME. A multitude of critical tumor-derived mediators mobilize and activate both TANs and TAMs, leading to a profound reshaping of the TME.39,40 This, in turn, gives rise to distinct subtypes of M1 and M2 TAMs, as well as N1 and N2 neutrophils. M1 macrophages are typically acknowledged as tumor-killing macrophages, exerting anti-tumor and immune-promoting effects. In contrast, M2 macrophages exhibit immune-suppressive properties, promoting tissue repair and tumor development. Throughout different stages of tumor development, both M1 and M2 TAMs can coexist, with M1 predominantly present in the early stages and M2 becoming more prevalent in later stages. As the tumor progresses, M1 polarization gradually shifts towards M2, and an increased number of M2 TAMs are associated with a poor prognosis. These two types of macrophages possess distinct markers, metabolic characteristics, and gene expression profiles. M1 macrophages secrete pro-inflammatory cytokines such as IL-12, tumor necrosis factor (TNF)-α, CXCL-10, and interferon (IFN)-γ, while expressing high levels of iNOS. On the other hand, M2 macrophages secrete anti-inflammatory cytokines like IL-10, IL-13, and IL-4, and express abundant arginase-1, CD206, and other markers. Neutrophils polarize towards N1 in response to IFN-β, IL-1β, IL-8, and TNF-α. N1 neutrophils exhibit a short lifespan, mature phenotype, high cytotoxicity, and strong immune activity, promoting CD8+ T cell activation. Conversely, under the influence of TGF-β, IL-8, IL-6, IL-17, and other factors, neutrophils polarize towards N2, characterized by a long lifespan, immature phenotype, low cytotoxicity, and promoting tumor growth, invasion, metastasis, angiogenesis, and immune suppression.41–44
Stromal Cells
Stromal cells reside in the interstitial spaces surrounding various organ parenchyma. They provide essential structural support and secrete growth factors to uphold tissue integrity. For instance, endothelial cells (BECs) and pericytes ensure vascular integrity and facilitate the delivery of oxygen and nutrients to the tissues.45 Cancer-associated fibroblasts (CAFs), characterized by the expression of proteins such as vimentin, alpha-smooth muscle actin (αSMA), and fibroblast activation protein (FAP), continuously remodel the ECM in response to mechanical pressures within the connective tissue. Additionally, they promote tumor cell growth and metastasis through diverse mechanisms. Growing evidence indicates that CAFs play a pivotal role in the effectiveness of anti-tumor therapies.46 Mesenchymal stem cells (MSCs) can be recruited by tumors and act as a source of fibroblasts and pericytes within the TME. Functioning as immune-regulatory cells, studies have suggested that MSCs may contribute to suppressing effective anti-tumor immunity.47
Extracellular Matrix (ECM)
ECM is a dynamic, three-dimensional non-cellular structure that undergoes constant remodeling to regulate tissue homeostasis and coordinate various cellular processes, including cancer dissemination and metastasis, thereby influencing tumor cell growth, survival, adhesion, and migration within TME.48,49 Comprising over 300 different proteins collectively known as the core matrisome, the ECM includes collagen, proteoglycans (PGs), glycoproteins, and other components.50 Ongoing advancements in proteomic techniques and extensive research have progressively revealed the specific roles of these proteins. For example, collagen, proteoglycans (PGs), and glycosaminoglycans (GAGs), elastin and elastic fibers, laminins, fibronectin, and other protein/glycoprotein constituents contribute to the complex ECM structure.51 The ECM serves as a reservoir for secreted molecules and facilitates intercellular communication through cell adhesion and migration interactions. Remodeling of the ECM by proteases releases previously sequestered molecules, leading to the local accumulation of released mediators. Moreover, cancer and TME cells directly engage with the surrounding ECM through receptors, such as integrins and CD44, thereby contributing to intricate signaling networks that play a significant role in cancer development and progression.52
Blood and Lymphatic Vessel Networks
Tumor progression is often accompanied by a continuous increase in tissue vascularization. This process is mediated by soluble factors such as vascular endothelial growth factor A (VEGFA), which promotes the proliferation of blood endothelial cells (BECs) and angiogenesis, as well as VEGFC and VEGFD, which induce proliferation, activation, and lymphatic vessel generation of lymphatic endothelial cells (LECs).45,53 Drugs that inhibit angiogenesis, such as bevacizumab (Avastin), have been proven to be effective in exerting anti-tumor effects.54 Similarly, the interaction between the ECM and the lymphatic system, along with the biophysical characteristics of the matrix, also influence tumor formation, growth, and metastasis. Therapeutic strategies focusing on the interactions between the lymphatic system and other components of the TME have also garnered attention from researchers.55
Applications of 3D Printing in Tumor Microenvironment Research
3D bioprinting technology holds tremendous potential in TME research. By creating intricate three-dimensional tumor models, this technology provides more realistic and reliable tools to advance our comprehension of tumor biology and disease progression mechanisms.56,57 In the realm of TME research, by utilizing 3D bioprinting technology, it is possible to construct tumor models with precision and simulate the intricate microenvironment of tumors. These models can encompass various cell types, ECM, and vascular networks, thereby providing a more realistic and accurate platform for tumor research. The applications of 3D bioprinting technology encompass diverse fields. Firstly, it offers a more precise and dependable platform for drug screening and toxicity testing. Through the printing of models featuring tumor characteristics, researchers can evaluate the anticancer activity of drugs against tumor cells and study their impact on normal cells, thereby establishing a stronger foundation for drug development and personalized treatments. Secondly, 3D bioprinting technology is instrumental in investigating the mechanisms behind tumor development and metastasis. By printing intricate tumor models, researchers can simulate the growth, diffusion, and invasion processes of tumor cells, leading to a more comprehensive understanding of tumor development and metastasis mechanisms. This section will delve deeper into the research progress of 3D bioprinting technology in various types of tumors (Figure 4).
 |
Figure 4 Common applications of 3D bioprinting technology in cancer research. Notes: This figure is drawn by Figdraw. |
Breast Cancer
3D bioprinting technology has shown tremendous potential in breast cancer research. By creating more realistic breast cancer models, it allows for in-depth investigations into metastasis, cell-cell interactions, and drug resistance, which promotes the development of new drugs and personalized therapies, providing more precise and effective treatment options for breast cancer patients.In one study, Zhou et al utilized a desktop stereolithography 3D bioprinter to manufacture a series of bone matrices consisting of osteogenic cells or MSCs encapsulated within methacrylated gelatin (GelMA) hydrogels and nanocrystalline hydroxyapatite (nHA). These matrices were then seeded with breast cancer cells to develop a co-cultured bioprinted breast cancer model, simulating sternal metastasis.58 Additionally, in the research conducted by Torsten Blunk, the printing capability of adipose-derived stromal/stem cells (ASC) spheroids using an extrusion-based 3D bioprinting system was studied. The ability of these spheroids to differentiate into adipose microtissues within the printed structure was observed. Optimizing spherical vitality and uniform distribution was achieved using hyaluronic acid-based bioinks. The differentiation of adipogenesis in the printed spheroids was confirmed through lipid accumulation, expression of adipogenic marker genes, and characterization of adipose ECM components. Following this, breast cancer cells (MDA-MB-231) were printed onto the adipose tissue structure. After co-culturing for nine days, a reduction in lipid content and ECM remodeling induced by cancer cells, along with increased expression of fibronectin, type I collagen, and type VI collagen, was observed.59 Similarly, a 3D bioprinted breast cancer-adipose cell model was created using a combination of alginate and gelatin as bioinks. The ratio of alginate to gelatin was 3:2, and 5% alginate was used to prepare the ideal bioink.60 In summary, 3D bioprinted breast cancer-stromal cells and breast cancer-adipose tissue models comprehensively capture critical aspects of cell-cell and cell-matrix interactions within the complex tumor-stromal microenvironment.
Moreover, the incorporation of dECM from pig liver allowed for the development of a metastatic tumor model where 4T1 mouse breast cancer cells were seeded on the scaffold, providing a platform for in vivo simulation.61 3D bioprinting plays a significant role in breast cancer drug resistance research as well. Using an extrusion-based 3D bioprinting system with dual hydrogel bioinks, researchers co-cultured adipose-derived mesenchymal stem cells/matrix cells (ADMSC) with human epidermal growth factor receptor 2-positive primary breast cancer cells (21PT) to investigate their response to doxorubicin (DOX). The 3D bioprinted breast cancer models were compared to 2D cell cultures, demonstrating that these models better replicate in vivo conditions.62 Furthermore, Anna Sebestyén et al utilized alginate-based hydrogel bioinks for 3D bioprinting and long-term cultivation of tumor cells in vitro. The model was used to compare the therapeutic effects of lapatinib, doxycycline, and doxorubicin as monotherapy and combination therapy on tumors, and it was confirmed that this 3D bioprinted breast cancer model showed drug sensitivity and a similar mTOR/metabolism protein expression profile, comparable to the previously established 3D cell culture system and traditional 2D cell culture, offering prospects for reducing animal experiments and improving the success rate of clinical trials.63 Furthermore, Song et al achieved the 3D growth of drug-resistant breast cancer spheroids in alginate-gelatin medium through 3D cell printing. During the 3D cultivation, the phenotype of drug-resistant breast cancer spheroids, characterized by high CD44/low CD24/high ALDH1 expression, was maintained. Overexpression of GRP78 and ABCG2 was clearly observed in the drug-resistant spheroids. This 3D bioprinting system is cost-effective, rapid, and capable of simple one-step in situ quantitative assessment of drug-resistant cancer spheroids for anti-cancer drug screening, making it an effective strategy for characterizing drug-resistant models in vitro64 (Table 1).
 |
Table 1 The Role of 3D Bioprinting in the Study of Breast Cancer |
Hepatocellular Carcinoma
Hepatocellular Carcinoma (HCC) is the most prevalent form of primary liver cancer, originating from malignant tumors in the liver. It often develops on the foundation of pre-existing chronic liver diseases, such as hepatitis B or C, alcoholic liver disease, fatty liver, and cirrhosis.65 Cirrhosis is a chronic progressive liver disease characterized by the scarring and fibrosis of liver tissue. As cirrhosis progresses, normal liver cells are gradually replaced by fibrous tissue, resulting in impaired liver function.66 To study the behavior of different liver cancer cells in specific fibrotic environments, it is crucial to replicate the clinically relevant mechanical properties of cirrhotic liver tissue. This can be achieved through specific techniques and materials that mimic the degree of fibrosis and tissue characteristics of cirrhosis, providing a better understanding of the interactions and behavior of liver cancer cells with fibrotic liver tissue. Chen et al successfully prepared dECM from the liver and combined it with methacrylated gelatin (GelMA) as a bioink. They utilized light-based rapid 3D bioprinting to create liver dECM patterns with customizable mechanical properties, thus reconstructing the clinically relevant mechanical characteristics of cirrhotic tissue to study the behavior of different liver cancer cells in specific fibrotic environments.67
Concerning drug screening for liver cancer, Mao et al established a 3D bioprinted liver cancer model using HepG2 cells and gelatin/alginate. They conducted comprehensive comparisons between this model and 2D cultured cells, revealing that using the 3D model for drug research may yield drug efficacy results closer to in vivo conditions.68 Notably, in 2021, Mao et al achieved a groundbreaking advancement by employing primary hepatocellular carcinoma cells for in vitro 3D bioprinting. The 3D bioprinting system mixed separated primary hepatocellular carcinoma cells with gelatin and sodium alginate to form a bioink for printing, constructing multiple personalized models for individualized drug screening of various liver cancer targeted drugs. Compared to PDX and Organoid models, the 3D bioprinted primary hepatocellular carcinoma model significantly shortened the construction time (a few hours vs 2–3 months), exhibited uniform and controllable shape and density, retained the parental characteristics of liver cancer, demonstrated high reliability and stability in the construction process, and did not rely on the inherent proliferative capacity of primary tumors. This approach offers great advantages and potential, successfully conducting meaningful drug sensitivity tests.69 In addition to classical 3D bioprinted models, Sun et al proposed a 3DPF model that combines 3D printing, microfluidics, and co-culture techniques. They used 3D cell printing to prepare liver cancer cell clusters and maintained uniformity in cluster size during 3D fabrication. The microfluidic chip provided a higher level of biomimetic microenvironment for the cells inside the chip compared to regular 3D bioprinted models. This model was primarily used for the pharmacological study of Metuzumab70(Table 2).
 |
Table 2 The Role of 3D Bioprinting in the Study of HCC and CAA |
Cholangiocarcinoma
Cholangiocarcinoma (CCA) is the most common malignant tumor of the bile duct and the second most prevalent primary liver malignancy, after hepatocellular carcinoma (HCC).73 Sun et al extracted primary tumor cells from cholangiocarcinoma patients and expanded them in culture. They used a bioink composed of gelatin, sodium alginate, and Matrigel to create a 3D bioprinted model. This model exhibited a survival rate of over 90% and maintained continuous cell proliferation and clonogenicity. In comparison to 2D cultured models, the 3D bioprinted model showed significantly upregulated tumorigenic phenotypes, including malignancy, stemness, fibrosis, invasion, and metastasis abilities. The enhanced drug resistance further confirmed its characteristics resembling stem cells.71 This 3D bioprinted primary ICC cell model possesses a rich population of CSCs, demonstrating great potential in personalized treatment and tumor development research. However, a single tumor cell 3D bioprinted model is still unable to fully construct the TME, as it requires the involvement of other stromal cells. Mao et al also used 3D bioprinting to construct a model of stromal cell influence on tumor cells, incorporating human umbilical vein endothelial cells (HUVEC), cancer-associated fibroblasts (CCC-HPF-1), and induced M2 macrophages.72 With technological advancements, more stromal cell types will gradually be integrated into 3D bioprinted models to study their impact on tumor cells (Table 2).
Colorectal Cancer
Colorectal cancer (CRC) is a malignant tumor that originates from the colon or rectum. It typically arises from the epithelial cells of the colon or rectum, forming tumors within the intestines.74,75 3D bioprinting can be used for CRC model preparation and customized treatment plan design, offering innovative possibilities for the diagnosis and treatment of colorectal cancer. Tan et al were the first to utilize a collagen-PCL scaffold along with three types of cells - CRC cells, cancer-associated fibroblasts (CAF), and tumor-associated endothelial cells (TEC) - to construct a 3D bioprinted model of colorectal cancer. This model allowed direct cell-cell interactions and tissue network formation. The 3D tumor model displayed physiological characteristics similar to those found in vivo, exhibiting high drug resistance, and was compatible with long-term monitoring and functional assessment.76 Tumor tissue consists of a small subset of cancer stem cells (CSCs) and a majority of differentiated non-CSCs. CSCs possess self-renewal and multi-lineage differentiation potentials and play crucial roles in cancer initiation, invasion, metastasis, and drug resistance.77,78 Xiong et al proposed an efficient induction and enrichment method for CRC CSCs using a hybrid hydrogel of GelMA and nanoclay, which demonstrated excellent printability, porosity, and mechanical properties close to in vivo tumors. This 3D bioprinting approach efficiently promoted the formation of CRC cell spheroids and induced/enriched CSCs through activation of the Wnt/β-catenin signaling pathway. Compared to traditional CSC enrichment models, the GelMA-nanoclay hydrogel enriched CSC microspheres displayed not only uniform morphology and higher quantity but also stronger stemness and increased sensitivity to drugs targeting CSCs, making it a superior model for CSC isolation, enrichment, and drug screening.79 In the TME, abnormal glycosylation events occur on ECM proteins and cell surface receptors, closely associated with malignant biological behaviors such as proliferation, angiogenesis, and immune escape.80,81 Laura Russo et al developed a 3D bioprinted model of colorectal cancer (CRC) using crosslinked hyaluronic acid and gelatin functionalized with three signal polysaccharides with characteristic 3’-sialylgalactose, 6’-sialylated lactose, and 2’-fucosylated lactose. The use of azide-functionalized gelatin and hyaluronic acid, along with 4arm-PEG-dibenzo-cyclooctyne, produced a biocompatible hydrogel, which was 3D bioprinted with HT-29 cells and CRC tumor cells from patients. The glycosylated hydrogel demonstrated good 3D printability, biocompatibility, and stability over time.82
Radiotherapy and chemotherapy play crucial roles in the treatment of colorectal cancer. Radiotherapy employs high-energy X-rays or other particle beams to directly irradiate colorectal cancer lesions, damaging the DNA structure and function of cancer cells to inhibit their growth and division. Chemotherapy uses chemical drugs to kill or inhibit the proliferation of cancer cells. For colorectal cancer, chemotherapy is commonly used in combination with radiotherapy before or after surgery to eliminate residual cancer cells, reduce tumor volume, or control disease recurrence. Commonly used chemotherapy drugs include fluorouracil, oxaliplatin, platinum compounds, among others.74,75,83 3D bioprinting plays a crucial role in constructing in vitro platforms for the screening of therapeutic drugs used in radiotherapy and chemotherapy for colorectal cancer. Both the teams of Adam W Perriman and Victoria Sarafian utilized established CRC cell lines for 3D bioprinting to assess the treatment resistance to common chemotherapeutic drugs (5-fluorouracil, oxaliplatin, and irinotecan) and radiation therapy. Compared to 2D culture, the 3D bioprinted cell culture model displayed stronger resistance to radiotherapy and chemotherapy.84,85 Building upon this, Mao et al constructed a multicellular 3D model that included SW480 cells, tumor-associated macrophages, and endothelial cells. In comparison to the single-cell 3D bioprinted model, the multicellular model exhibited stronger drug resistance to chemotherapy drugs (5-FU, oxaliplatin, irinotecan).86 This model more accurately simulated the TME and laid the foundation for further investigation into the mechanisms of tumor chemoresistance through intercellular interactions (Table 3).
 |
Table 3 The Role of 3D Bioprinting in the Study of CRC |
In the field of tumor stem cells, GelMA-Nanoclay hydrogel fabricated through 3D bioprinting is utilized to induce CRC CSCs and promote spheroid formation. GelMA-Nanoclay hydrogel exhibits high porosity, suitable mechanical properties, and excellent cytocompatibility towards CRC cells. Moreover, the hydrogel induces and enriches CRC CSCs that possess enhanced in vitro self-renewal capacity and in vivo tumorigenic potential. Furthermore, investigation into the underlying regulatory mechanisms reveals that the GelMA-Nanoclay hydrogel stimulates the activation of Wnt/β-catenin protein signaling, which contributes to the induction and enrichment of CSCs.79
Cervical Cancer
Cervical cancer is a malignant tumor that originates from cervical epithelial cells. It is often associated with Human Papillomavirus (HPV) infection, and persistent infection with high-risk HPV can lead to abnormal cervical cells and eventually develop into cervical cancer, making it one of the most common cancers in women.87 The Hela cell line, derived from cervical cancer cells, is one of the earliest successfully cultured cell lines in vitro. A 3D bioprinted model of Hela cells was created using gelatin/alginate/fibrinogen hydrogel as a bioink. Compared to cells cultured in 2D, Hela cells in the 3D bioprinted model exhibited increased proliferation rate, higher MMP protein expression, and enhanced chemoresistance.88 TGF-β is considered a major inducer of various tumor EMT processes. In a 3D bioprinted cervical cancer model with the addition of TGF-β, HeLa cells underwent a transformation into fibroblast-like spindle shapes, and a downregulation of the epithelial marker E-cadherin, along with an upregulation of mesenchymal markers (such as snail, vimentin, and N-cadherin), was observed. This indicates that the 3D bioprinted cervical cancer model can be used to study TGF-β-mediated EMT.89 Oxygen concentration is one of the critical factors for cell survival and a key participant in cell biology under different conditions. By using nano-electrodes as minimally invasive SECM probes, the concentration gradient existing within spheroids grown in the 3D bioprinted matrix can be quantified. Furthermore, SECM enables the assessment of molecular penetration and diffusion in 3D environments at high temporal resolution (ie, milliseconds). This analysis provides insights into the diffusion process within the 3D bioprinted cervical tumor structure, which will guide the design of 3D bioprinted models for simulating the diffusion of anticancer compounds in tumor masses90(Table 4).
 |
Table 4 The Role of 3D Bioprinting in the Study of Cervical Cancer |
Lung Cancer
Lung cancer is a malignant tumor that originates from lung tissue and is the second most common cancer globally, with the highest fatality rate among malignant tumors.91 A significant amount of research has explored the mechanisms of lung cancer occurrence, progression, and drug resistance from the perspective of the TME through 2D cell culture. However, 3D models seem more appropriate for simulating the TME of lung cancer. Sodium alginate-gelatin (SA-GL) hydrogel can be used to bioprint co-cultures of NSCLC patient-derived xenograft cells and lung cancer-associated fibroblasts (CAFs). The combination of these cells with the hydrogel showed high printability and cell viability. By demonstrating the overexpression of vimentin, α-SMA, and the loss of E-cadherin in the model, which promotes EMT, it was shown that this 3D lung cancer model can effectively simulate the interactions between tumor cells and the matrix. Therefore, studies have suggested that the SA-GL hydrogel-based bioprinting model of co-cultures of NSCLC patient-derived xenograft cells and lung CAFs can be used for high-throughput drug screening and other preclinical applications.92,93 Wang et al proposed that the gelatin-sodium alginate system can reduce severe damage to lung cancer cells during the 3D printing process caused by temperature and pressure changes.94 R. Utama et al described an enabling technology consisting of a customized on-demand droplet 3D bioprinter capable of high-throughput printing on a 96-well spheroid plate. Researchers embedded NSCLC cell line H460 within the matrix to form matrix-embedded multicellular spheroids. Compared to manually prepared spheroids, the 3D printed matrix-embedded spheroids demonstrated better simulation of the hypoxic characteristics of the TME, as shown by HIF1-α immunolabeling and fluorescence-activated cell sorting (FACS) results.95 Aragaw Gebeyehu et al improved bioprinting ink using a non-xenogeneic VitroGel system. Both ink H4 and RGD-modified ink H4-RGD could be printed at extrusion pressures of 25–40 kPa, displaying 90% cell viability, shear-thinning, and rapid shear recovery properties, allowing them to be extrusion bioprinted without UV curing or temperature regulation. 3D bioprinted NSCLC patient-derived xenograft cells (PDCs) exhibited rapid spheroid growth with a diameter of approximately 500 µm and formed a TME within 7 days. This study further verified that the 3D printed spheroids exhibited higher resistance to docetaxel, doxorubicin, and erlotinib. The flowability, shape fidelity, scaffold stability, and biocompatibility of H4-RGD indicated that this hydrogel could be used for 3D cell bioprinting and the development of in vitro TMEs for screening various anticancer drugs.96 In research on in vitro organoid models of lung cancer patients with different underlying diseases, Choi et al used lung decellularized extracellular matrix (LudECM) hydrogel from pig lung sources to construct a model combining lung cancer and idiopathic pulmonary fibrosis. In the model with fibrosis-associated lung cancer, the expression of genes related to cancer cell proliferation, invasion, metastasis, and drug resistance, such as JAK2, FGFR1, and IL-6R, was universally enhanced. In the drug resistance assessment results, the model exhibited more significant changes in resistance to sensitizing targeted anticancer drugs. This model partially reproduced the drug responsiveness of the vascularized lung cancer model with fibrosis, helping to determine appropriate treatment methods for lung cancer patients with fibrosis97 (Table 5).
 |
Table 5 The Role of 3D Bioprinting in the Study of Lung Cancer, Melanoma and Glioblastoma |
Melanoma
Melanoma is a malignant tumor that originates from skin melanocytes and is primarily caused by exposure to ultraviolet radiation.103 Bioprinting models that simulate the complex structure of the skin have been proposed in the literature, laying the groundwork for bioprinting melanoma.104,105 Jeong et al introduced an extrusion-based bioprinting technique to create collagen scaffolds, which demonstrated higher maintenance and viability of cryopreserved patient-derived melanoma explants (PDME) compared to 2D culture. The 3D printed collagen scaffold retained the expression of melanoma biomarkers MITF, Mel A, and S100 in cryopreserved PDME for up to 21 days, making this model more suitable for studying the 3D structure and TME of melanoma98 (Table 5).
Glioblastoma
Glioblastoma (GBM) is the most common and aggressive primary brain cancer in adults.106 Single-cultured three-dimensional GBM models are beneficial for studying the interactions and mechanisms between tumors and the TME. Many studies have integrated glioma stem cells (GSCs) into these bioprinted models for further investigation. GSCs in these bioprinted models exhibit higher cell viability and stable cell proliferation, and they also show characteristics related to stemness, tumor angiogenesis-related gene expression, and vascular potential in vitro, providing potential research prospects for studying GBM tumor angiogenesis.99,100 Digital Light Processing (DLP) bioprinting is a rapid projection-based stereolithography technique capable of manufacturing millimeter to centimeter-sized structures in a matter of seconds to minutes.107 Tang et al utilized a DLP 3D bioprinting system and photocrosslinkable native ECM derivatives to create a biomimetic 3D cancer microenvironment for high-grade brain tumor glioblastoma. This 3D tetra-culture brain tumor model includes patient-derived GSCs, macrophages, astrocytes, and neural stem cells (NSCs) in the hydrogel. Through next-generation sequencing, this model recapitulates the transcriptional states present in patient-derived glioblastoma tissues. Moreover, this model preferentially polarizes macrophages to the M2 phenotype, consistent with M2 polarization observed in glioblastoma tumors.101
The blood-brain barrier (BBB) is a dynamic and complex interface between the blood and the central nervous system (CNS) and also a limiting factor for the efficacy of glioblastoma chemotherapy. 3D printing technology can be used to simulate BBB models and further provide potential approaches for glioblastoma treatment. Marino et al proposed a 1:1 biomimetic hybrid BBB model using two-photon lithography. They fabricated several tube-like structures with diameters of 10 μm, equivalent to brain microvessels, between the inlet and outlet and used them as scaffolds for co-culturing endothelial-like bEnd.3 cells and U87 glioblastoma cells. This construct demonstrated tight junctions and good performance in hindering dextran diffusion compared to a cell-free microfluidic system and exhibited satisfactory transendothelial electrical resistance (TEER), making it a realistic in vitro model suitable for studying the interaction between nanomaterials, drugs, and the BBB.102 Wang et al developed a microfluidic BBB model that can simulate BBB characteristics over a long period and allows reliable in vitro drug permeability studies under perfusion conditions. This model consisted of three layers of 3D-printed chambers and cell inserts containing brain microvascular endothelial cells (BMECs) and astrocytes derived from human-induced pluripotent stem cells (hiPSCs) cultured on both sides of a porous membrane. This BBB-on-a-chip model achieved significant barrier integrity, with the highest TEER value exceeding 4000Ω·cm2, within the range of in vivo values. Additionally, in the permeability study of large molecules (FITC-dextran) and model drugs (caffeine, cimetidine, and doxorubicin), the permeability coefficients measured by this model were comparable to in vivo values, providing a promising platform for future BBB simulation studies108 (Table 5).
Summary and Outlooks
Bioprinting is an advanced technology for preparing cell-laden hydrogels, which has become a powerful manufacturing tool for creating complex microscale and macroscale biomedical systems. Numerous studies have demonstrated the feasibility of bioprinting to simulate the TME. 3D bioprinting technology enables the controlled patterning of bioinks to form intricate TME structures, offering advantages such as microscale precision, high throughput, and cell deposition capabilities.
As previously mentioned, a wide range of biomaterials can be utilized in bioprinting, allowing for the simulation of ECM’s various ultramicroscopic structures to better mimic the in vivo environment. Additionally, bioprinting relies on bioinks, which typically consist of biomaterials (eg, hydrogels), cells, or cell aggregates, alone or in combination. When simulating different TMEs, natural materials (such as alginate and gelatin) and synthetic polymers (eg, PCL, PEG, Pluronic) have been proven feasible as bioink materials. Extensive research is ongoing to optimize bioink materials and formulations to achieve high-fidelity bioprinting. Moreover, compared to traditional 2D culture models, several existing bioprinting strategies allow for the deconstruction and reconstitution of TME through computer-aided design, resulting in 3D bioprinted models that better resemble the characteristics of the in vivo environment, including similar genomics and proteomics. Furthermore, bioprinting technology has made it possible to simulate vascular networks in TME, a feature that traditional 2D models lack. This advancement facilitates more practical research on drug responses and resistance in tumors and provides a foundation for subsequent preclinical drug experiments and commercial drug screening platforms. Similarly, due to the rapidity of 3D bioprinting, precise testing of drug responses and resistance for specific patients can be achieved in clinical settings, closely mimicking the tumor conditions inside the human body and simulating interactions between various components within the TME after drug administration. This approach eliminates the time and economic costs associated with animal experiments, leading to the acquisition of real data for drug development and usage in large samples. Currently, there are still some adjustments and unresolved issues in 3D bioprinting for simulating the TME, which should receive more attention in subsequent research. Firstly, there is a lack of standardized protocols for specific tumor types in current studies. Establishing standardized 3D printing model protocols, such as uniform criteria for cell viability and genetic characteristics under different experimental conditions, would facilitate reproducibility across different laboratories and enhance the credibility of preclinical and commercial research based on standardized models.
Regarding bioprinting technology, numerous studies have improved bioinks and biomaterials, but there is a need for more appropriate materials to simulate the spatiotemporal aspects of TME and genetic information. While bioinks can partially mediate cellular organoid-like behavior, they still fall short in fully replicating the complete composition of organ-specific ECM. Utilizing decellularized ECM (dECM) derived from the desired tissue, containing all the natural compounds and growth factors specific to the tissue, as mentioned earlier, could offer a solution to this issue. Faramarzi et al used platelet-rich plasma (PRP) combined with hydrogels and incorporated it into 3D constructs, making PRP a patient-specific source of autologous growth factors, thus enhancing vascularization, stem cell recruitment, and tissue regeneration in the bioprinted models.109 This patient-specific bioink is more suitable for clinical translation of bioprinting technology. On the other hand, the construction of blood vessel networks in current 3D bioprinting models is still in its early stages, as most bioprinted vascular models struggle to replicate the physiological functions of natural blood vessels in human organs. Different tumors from different organ sources have specific requirements for vascular formation. Therefore, for constructing corresponding vascular tissues in different tumor types, a combination of multiple cell types with evolving polymer bioinks should be employed to mimic the key structural components of natural blood vessels as closely as possible, providing a more credible foundation for subsequent research on drug therapies simulated through vascular networks. In the clinical translation of 3D bioprinting to simulate TME, there are also many challenges. Analyzing the complex components and ultrastructure of TME, as well as isolating diverse cellular and genetic components, all limit the efficiency of 3D bioprinting for simulating TME. Moreover, these processes require experienced researchers to ensure the maintenance of fine structure while avoiding adverse events such as hypoxia and necrosis. All of these factors restrict the clinical translation of 3D bioprinted TME models, calling for further advancements in the field. Building upon this foundation, 4D printing, as an emerging manufacturing technology, adds a fourth dimension - time - to traditional 3D printing. In 4D printing, objects are printed with materials that can autonomously deform, dynamically change shape, and exhibit functionalities in response to external stimuli such as temperature, humidity, light, etc. This capacity for self-transformation bestows printed objects with greater flexibility and adaptability.110,111
In summary, the research on 3D bioprinting to simulate the TME has undergone rapid development in the past decade, with a strong commitment to clinical translation and addressing key challenges in regenerative medicine, precise tumor treatment, and personalized therapy. Although most 3D bioprinted models are still in the early stages of research and not yet ready for clinical applications, an increasing number of studies are now focusing on issues such as tissue vascularization, multicellular design, and high-resolution bioprinting, demonstrating highly encouraging results. Advancements in biomaterial research, standardization of model construction, and the integration of 3D printing technology with computer and laser techniques are expected to further enhance the 3D bioprinted TME models. These continuously optimized in vitro tumor models are being utilized for testing new generations of anticancer drugs and immunotherapies, gradually leading to a revolution in precision medicine.
Ethics Approval and Consent to Participate
The authors declare that their participation in writing this review as well as its publication is voluntary.
Acknowledgments
Thank you very much for the illustrations provided by Figdraw.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from Natural Science Foundation of Liaoning Province (2021-BS-094), Shenyang Science and Technology Plan Project (20-205-4-043).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Berthiaume F, Maguire TJ, Yarmush ML. Tissue engineering and regenerative medicine: history, progress, and challenges. Annual Rev Chem Biomol Engin. 2011;2:403–430. doi:10.1146/annurev-chembioeng-061010-114257
2. Klebe RJ. Cytoscribing: a method for micropositioning cells and the construction of two- and three-dimensional synthetic tissues. Experim Cell Res. 1988;179:362–373. doi:10.1016/0014-4827(88)90275-3
3. Matai I, Kaur G, Seyedsalehi A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi:10.1016/j.biomaterials.2019.119536
4. Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Engin. 2020;4:370–380. doi:10.1038/s41551-019-0471-7
5. Correia Carreira S, Begum R, Perriman AW. 3D bioprinting: the emergence of programmable biodesign. Advan Health Mater. 2020;9:e1900554. doi:10.1002/adhm.201900554
6. Sharma R, Restan Perez M, da Silva VA, et al. 3D bioprinting complex models of cancer. Biomat Sci. 2023;11:3414–3430. doi:10.1039/D2BM02060B
7. Wang Z, Xiang L, Lin F, Tang Y, Cui W. 3D bioprinting of emulating homeostasis regulation for regenerative medicine applications. J Control Release. 2023;353:147–165. doi:10.1016/j.jconrel.2022.11.035
8. Chae S, Cho DW. Biomaterial-based 3D bioprinting strategy for orthopedic tissue engineering. Acta biomaterialia. 2023;156:4–20. doi:10.1016/j.actbio.2022.08.004
9. McMillan A, McMillan N, Gupta N, Kanotra SP, Salem AK. 3D bioprinting in otolaryngology: a review. Advan Health Mater. 2023;12:e2203268. doi:10.1002/adhm.202203268
10. Pontiggia L, Van Hengel IA, Klar A, et al. Bioprinting and plastic compression of large pigmented and vascularized human dermo-epidermal skin substitutes by means of a new robotic platform. J Tissue Eng. 2022;13:20417314221088513. doi:10.1177/20417314221088513
11. Santos-Beato P, Midha S, Pitsillides AA, Miller A, Torii R, Kalaskar DM. Biofabrication of the osteochondral unit and its applications: current and future directions for 3D bioprinting. J Tissue Eng. 2022;13:20417314221133480. doi:10.1177/20417314221133480
12. Stocco E, Porzionato A, De Rose E, Barbon S, De Caro R, Macchi V. Meniscus regeneration by 3D printing technologies: current advances and future perspectives. J Tissue Eng. 2022;13:20417314211065860. doi:10.1177/20417314211065860
13. De S, Singh N. Advancements in three dimensional in-vitro cell culture models. Chem Record. 2022;22:e202200058. doi:10.1002/tcr.202200058
14. Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trend Biotechnol. 2015;33:504–513. doi:10.1016/j.tibtech.2015.06.007
15. Lam EHY, Yu F, Zhu S, Wang Z. 3D bioprinting for next-generation personalized medicine. Int J Mol Sci. 2023;24:6357. doi:10.3390/ijms24076357
16. Lin M, Tang M, Duan W, Xia S, Liu W, Wang Q. 3D bioprinting for tumor metastasis research. ACS Biomat Sci Engin. 2023;9:3116–3133. doi:10.1021/acsbiomaterials.3c00239
17. Gao G, Kim BS, Jang J, Cho DW. Recent strategies in extrusion-based three-dimensional cell printing toward organ biofabrication. ACS Biomat Sci Enginee. 2019;5:1150–1169. doi:10.1021/acsbiomaterials.8b00691
18. Cui X, Li J, Hartanto Y, et al. Advances in extrusion 3D bioprinting: a focus on multicomponent hydrogel-based bioinks. Advan Health Mater. 2020;9:e1901648. doi:10.1002/adhm.201901648
19. Skylar-Scott MA, Mueller J, Visser CW, Lewis JA. Voxelated soft matter via multimaterial multinozzle 3D printing. Nature. 2019;575:330–335. doi:10.1038/s41586-019-1736-8
20. Gu Z, Fu J, Lin H, He Y. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci. 2020;15:529–557. doi:10.1016/j.ajps.2019.11.003
21. Naghieh S, Chen X. Printability-A key issue in extrusion-based bioprinting. J Pharm Anal. 2021;11:564–579. doi:10.1016/j.jpha.2021.02.001
22. Vijayavenkataraman S, Yan WC, Lu WF, Wang CH, Fuh JYH. 3D bioprinting of tissues and organs for regenerative medicine. Advan Drug Deliv Rev. 2018;132:296–332. doi:10.1016/j.addr.2018.07.004
23. Xu C, Chai W, Huang Y, Markwald RR. Scaffold-free inkjet printing of three-dimensional zigzag cellular tubes. Biotechnol Bioenginee. 2012;109:3152–3160. doi:10.1002/bit.24591
24. Faulkner-Jones A, Greenhough S, King JA, Gardner J, Courtney A, Shu W. Development of a valve-based cell printer for the formation of human embryonic stem cell spheroid aggregates. Biofabrication. 2013;5:015013. doi:10.1088/1758-5082/5/1/015013
25. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–785. doi:10.1038/nbt.2958
26. Tasoglu S, Demirci U. Bioprinting for stem cell research. Trend Biotechnol. 2013;31:10–19. doi:10.1016/j.tibtech.2012.10.005
27. Li X, Liu B, Pei B, et al. Inkjet bioprinting of biomaterials. Chem Rev. 2020;120:10793–10833. doi:10.1021/acs.chemrev.0c00008
28. Gudapati H, Dey M, Ozbolat I. A comprehensive review on droplet-based bioprinting: past, present and future. Biomaterials. 2016;102:20–42. doi:10.1016/j.biomaterials.2016.06.012
29. Jentsch S, Nasehi R, Kuckelkorn C, Gundert B, Aveic S, Fischer H. Multiscale 3D bioprinting by nozzle-free acoustic droplet ejection. Small Methods. 2021;5:e2000971. doi:10.1002/smtd.202000971
30. Faulkner-Jones A, Fyfe C, Cornelissen DJ, et al. Bioprinting of human pluripotent stem cells and their directed differentiation into hepatocyte-like cells for the generation of mini-livers in 3D. Biofabrication. 2015;7:044102. doi:10.1088/1758-5090/7/4/044102
31. Bakhshi H, Kuang G, Wieland F, Meyer W. Photo-curing kinetics of 3D-printing photo-inks based on urethane-acrylates. Polymers. 2022;14. doi:10.3390/polym14152974
32. Quan H, Zhang T, Xu H, Luo S, Nie J, Zhu X. Photo-curing 3D printing technique and its challenges. Bioact Mater. 2020;5:110–115. doi:10.1016/j.bioactmat.2019.12.003
33. Xiaorui L, Fuyin Z, Xudong W, et al. 1Biomaterial inks for extrusion-based 3D bioprinting: property, classification, modification, and selection. Internat J Bioprint. 2023;9:649. doi:10.18063/ijb.v9i2.649
34. Hsu L, Jiang X. ‘Living’ Inks for 3D Bioprinting. Trend Biotechnol. 2019;37:795–796. doi:10.1016/j.tibtech.2019.04.014
35. Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its microenvironment: a synergistic interplay. Semin Can Biol. 2013;23:522–532. doi:10.1016/j.semcancer.2013.08.007
36. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi:10.1016/j.ccr.2012.02.022
37. Cheng K, Cai N, Zhu J, Yang X, Liang H, Zhang W. Tumor-associated macrophages in liver cancer: from mechanisms to therapy. Cancer Communications. 2022;42:1112–1140. doi:10.1002/cac2.12345
38. DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369–382. doi:10.1038/s41577-019-0127-6
39. Güç E, Pollard JW. Redefining macrophage and neutrophil biology in the metastatic cascade. Immunity. 2021;54:885–902. doi:10.1016/j.immuni.2021.03.022
40. Pittet MJ, Michielin O, Migliorini D. Author Correction: clinical relevance of tumour-associated macrophages. Nat Rev Clin Oncol. 2022;19:424. doi:10.1038/s41571-022-00632-2
41. Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: ”N1” versus ”N2. TAN, Cancer Cell. 2009;16:183–194. doi:10.1016/j.ccr.2009.06.017
42. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy, Nature reviews. Cancer. 2020;20:485–503.
43. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76. doi:10.1186/s13045-019-0760-3
44. Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trend Immunol. 2002;23:549–555. doi:10.1016/S1471-4906(02)02302-5
45. Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi:10.1016/j.ccr.2005.01.017
46. Biffi G, Tuveson DA. Diversity and biology of cancer-associated fibroblasts. Physiol Rev. 2021;101:147–176. doi:10.1152/physrev.00048.2019
47. Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications, Cellular and molecular life sciences. CMLS. 2019;76:3323–3348. doi:10.1007/s00018-019-03125-1
48. Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi:10.1126/science.1176009
49. Karamanos NK, Piperigkou Z, Passi A, Götte M, Rousselle P, Vlodavsky I. Extracellular matrix-based cancer targeting. Trend Molecul Med. 2021;27:1000–1013. doi:10.1016/j.molmed.2021.07.009
50. Hynes RO, Naba A. Overview of the matrisome--an inventory of extracellular matrix constituents and functions. Cold Spring Harbor Perspect Biol. 2012;4:a004903. doi:10.1101/cshperspect.a004903
51. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Advan Drug Deliv Rev. 2016;97:4–27. doi:10.1016/j.addr.2015.11.001
52. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat Commun. 2020;11:5120. doi:10.1038/s41467-020-18794-x
53. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176:1248–1264. doi:10.1016/j.cell.2019.01.021
54. Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the eastern cooperative oncology group study E3200. J Clin Oncol. 2007;25:1539–1544. doi:10.1200/JCO.2006.09.6305
55. Wiig H, Keskin D, Kalluri R. Interaction between the extracellular matrix and lymphatics: consequences for lymphangiogenesis and lymphatic function. Matrix Biol. 2010;29(8):645–656. doi:10.1016/j.matbio.2010.08.001
56. Germain N, Dhayer M, Dekiouk S, Marchetti P. Current advances in 3D bioprinting for cancer modeling and personalized medicine. Inter J Molec Sci. 2022;23:3432. doi:10.3390/ijms23073432
57. Shukla P, Yeleswarapu S, Heinrich MA, Prakash J, Pati F. Mimicking tumor microenvironment by 3D bioprinting: 3D cancer modeling. Biofabrication. 2022;14:032002. doi:10.1088/1758-5090/ac6d11
58. Zhou X, Zhu W, Nowicki M, et al. 3D bioprinting a cell-laden bone matrix for breast cancer metastasis study. ACS Appl Mater Interf. 2016;8:30017–30026. doi:10.1021/acsami.6b10673
59. Horder H, Guaza Lasheras M, Grummel N, et al. Bioprinting and differentiation of adipose-derived stromal cell spheroids for a 3D breast cancer-adipose tissue model. Cells. 2021;10:803. doi:10.3390/cells10040803
60. Chaji S, Al-Saleh J, Gomillion CT. Bioprinted three-dimensional cell-laden hydrogels to evaluate adipocyte-breast cancer cell interactions. Gels. 2020;6:6. doi:10.3390/gels6010006
61. Xu J, Yang S, Su Y, et al. A 3D bioprinted tumor model fabricated with gelatin/sodium alginate/decellularized extracellular matrix bioink. Internat J Bioprin. 2023;9:630. doi:10.18063/ijb.v9i1.630
62. Wang Y, Shi W, Kuss M, et al. 3D bioprinting of breast cancer models for drug resistance study. ACS Biomater Sci Enginee. 2018;4:4401–4411. doi:10.1021/acsbiomaterials.8b01277
63. Dankó T, Petővári G, Raffay R, et al. Characterisation of 3D bioprinted human breast cancer model for in vitro drug and metabolic targeting. Int J Mol Sci. 2022;23:7444. doi:10.3390/ijms23137444
64. Hong S, Song JM. 3D bioprinted drug-resistant breast cancer spheroids for quantitative in situ evaluation of drug resistance. Acta Biomater. 2022;138:228–239. doi:10.1016/j.actbio.2021.10.031
65. Müller M, Bird TG, Nault JC. The landscape of gene mutations in cirrhosis and hepatocellular carcinoma. J Hepatol. 2020;72:990–1002. doi:10.1016/j.jhep.2020.01.019
66. Friedman SL, Pinzani M. Hepatic fibrosis 2022: unmet needs and a blueprint for the future. Hepatology. 2022;75:473–488. doi:10.1002/hep.32285
67. Ma X, Yu C, Wang P, et al. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018;185:310–321. doi:10.1016/j.biomaterials.2018.09.026
68. Sun L, Yang H, Wang Y, et al. Application of a 3D bioprinted hepatocellular carcinoma cell model in antitumor drug research. Front Oncol. 2020;10:878. doi:10.3389/fonc.2020.00878
69. Xie F, Sun L, Pang Y, et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials. 2021;265:120416. doi:10.1016/j.biomaterials.2020.120416
70. Li Y, Zhang T, Pang Y, Li L, Chen ZN, Sun W. 3D bioprinting of hepatoma cells and application with microfluidics for pharmacodynamic test of Metuzumab. Biofabrication. 2019;11:034102. doi:10.1088/1758-5090/ab256c
71. Mao S, He J, Zhao Y, et al. Bioprinting of patient-derived in vitro intrahepatic cholangiocarcinoma tumor model: establishment, evaluation and anti-cancer drug testing. Biofabrication. 2020;12:045014. doi:10.1088/1758-5090/aba0c3
72. Han QF, Li WJ, Hu KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21:207. doi:10.1186/s12943-022-01671-0
73. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95–111. doi:10.1038/nrclinonc.2017.157
74. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer. A Review. JAMA. 2021;325:669–685. doi:10.1001/jama.2021.0106
75. Kuipers EJ, Grady WM, Lieberman D, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi:10.1038/nrdp.2015.65
76. Chen H, Cheng Y, Wang X, et al. 3D printed in vitro tumor tissue model of colorectal cancer. Theranostics. 2020;10:12127–12143. doi:10.7150/thno.52450
77. Batlle E, Clevers H. Cancer stem cells revisited. Nature Medicine. 2017;23:1124–1134. doi:10.1038/nm.4409
78. Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi:10.1038/nrclinonc.2017.44
79. Zhang Y, Wang Z, Hu Q, et al. 3D Bioprinted GelMA-nanoclay hydrogels induce colorectal cancer stem cells through activating wnt/β-catenin signaling. Small. 2022;18:e2200364. doi:10.1002/smll.202200364
80. Oliveira-Ferrer L, Legler K, Milde-Langosch K. Role of protein glycosylation in cancer metastasis. Semin Can Biol. 2017;44:141–152. doi:10.1016/j.semcancer.2017.03.002
81. Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi:10.1038/nrc3982
82. Cadamuro F, Marongiu L, Marino M, et al. 3D bioprinted colorectal cancer models based on hyaluronic acid and signalling glycans. Carbohyd Polym. 2023;302:120395. doi:10.1016/j.carbpol.2022.120395
83. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–1480. doi:10.1016/S0140-6736(19)32319-0
84. Johnson PA, Menegatti S, Chambers AC, et al. A rapid high throughput bioprinted colorectal cancer spheroid platform for in vitro drug- and radiation-response. Biofabrication. 2022;15:1.
85. Sbirkov Y, Molander D, Milet C, et al. A colorectal cancer 3D bioprinting workflow as a platform for disease modeling and chemotherapeutic screening. Front Bioenginee Biotechnol. 2021;9:755563. doi:10.3389/fbioe.2021.755563
86. Wang P, Sun L, Li C, et al. Study on drug screening multicellular model for colorectal cancer constructed by three-dimensional bioprinting technology. Internat J Bioprint. 2023;9:694. doi:10.18063/ijb.694
87. Burd EM. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003;16:1–17. doi:10.1128/CMR.16.1.1-17.2003
88. Zhao Y, Yao R, Ouyang L, et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6:035001. doi:10.1088/1758-5082/6/3/035001
89. Pang Y, Mao SS, Yao R, et al. TGF-β induced epithelial-mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication. 2018;10:044102. doi:10.1088/1758-5090/aadbde
90. Becconi M, De Zio S, Falciani F, Santamaria M, Malferrari M, Rapino S. Nano-electrochemical characterization of a 3D bioprinted cervical tumor model. Cancers. 2023;15. doi:10.3390/cancers15041327
91. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi:10.3322/caac.21660
92. Arai K, Eguchi T, Rahman MM, et al. A novel high-throughput 3D screening system for EMT Inhibitors: a pilot screening discovered the EMT inhibitory activity of CDK2 inhibitor SU9516. PLoS One. 2016;11:e0162394. doi:10.1371/journal.pone.0162394
93. Mondal A, Gebeyehu A, Miranda M, et al. Characterization and printability of sodium alginate -gelatin hydrogel for bioprinting NSCLC co-culture. Scientific Reports. 2019;9:19914. doi:10.1038/s41598-019-55034-9
94. Wang X, Zhang X, Dai X, et al. Tumor-like lung cancer model based on 3D bioprinting, 3. Biotech. 2018;8:501.
95. Utama RH, Atapattu L, O’Mahony AP, et al. A 3D bioprinter specifically designed for the high-throughput production of matrix-embedded multicellular spheroids. iScience. 2020;23:101621. doi:10.1016/j.isci.2020.101621
96. Gebeyehu A, Surapaneni SK, Huang J, et al. Polysaccharide hydrogel based 3D printed tumor models for chemotherapeutic drug screening. Scientific Reports. 2021;11:372. doi:10.1038/s41598-020-79325-8
97. Choi YM, Lee H, Ann M, Song M, Rheey J, Jang J. 3D bioprinted vascularized lung cancer organoid models with underlying disease capable of more precise drug evaluation. Biofabrication. 2023;15. doi:10.1088/1758-5090/acd95f
98. Jeong YM, Bang C, Park M, et al. 3D-printed collagen scaffolds promote maintenance of cryopreserved patients-derived melanoma explants. Cells. 2021;10:589. doi:10.3390/cells10030589
99. Wang X, Li X, Dai X, et al. Bioprinting of glioma stem cells improves their endotheliogenic potential, Colloids and surfaces. B, Biointerfaces. 2018;171:629–637. doi:10.1016/j.colsurfb.2018.08.006
100. Dai X, Ma C, Lan Q, Xu T. 3D bioprinted glioma stem cells for brain tumor model and applications of drug susceptibility. Biofabrication. 2016;8:045005.
101. Tang M, Xie Q, Gimple RC, et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020;30:833–853. doi:10.1038/s41422-020-0338-1
102. Marino A, Tricinci O, Battaglini M, et al. A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small. 2018;14:1.
103. Sanchez JA, Robinson WA. Malignant melanoma. Annu Rev Med. 1993;44:335–342. doi:10.1146/annurev.me.44.020193.002003
104. Albanna M, Binder KW, Murphy SV, et al. In situ bioprinting of autologous skin cells accelerates wound healing of extensive excisional full-thickness wounds. Scientific Reports. 2019;9:1856. doi:10.1038/s41598-018-38366-w
105. Weng T, Zhang W, Xia Y, et al. 3D bioprinting for skin tissue engineering: current status and perspectives. J Tissue Eng. 2021;12:20417314211028574. doi:10.1177/20417314211028574
106. Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United states in 2012–2016. Neuro Oncol. 2019;21:v1–v100. doi:10.1093/neuonc/noz150
107. Goodarzi Hosseinabadi H, Dogan E, Miri AK, Ionov L. Digital light processing bioprinting advances for microtissue models. ACS Biomater Sci Enginee. 2022;8:1381–1395. doi:10.1021/acsbiomaterials.1c01509
108. Wang YI, Abaci HE, Shuler ML. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioenginee. 2017;114:184–194. doi:10.1002/bit.26045
109. Faramarzi N, Yazdi IK, Nabavinia M, et al. Patient-specific bioinks for 3D bioprinting of tissue engineering scaffolds. Advan Health Mater. 2018;7:e1701347. doi:10.1002/adhm.201701347
110. Mahmud MAP, Tat T, Xiao X, Adhikary P, Chen J. Advances in 4D-printed physiological monitoring sensors. Exploration. 2021;1:20210033. doi:10.1002/EXP.20210033
111. Zhou W, Qiao Z, Nazarzadeh Zare E, et al. 4D-printed dynamic materials in biomedical applications: chemistry, challenges, and their future perspectives in the clinical sector. J Med Chem. 2020;63:8003–8024. doi:10.1021/acs.jmedchem.9b02115
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
