Back to Journals » ClinicoEconomics and Outcomes Research » Volume 8
Relative cost-effectiveness of using an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow's milk allergy in Poland
Authors Guest J , Weidlich D, Kaczmarski M, Jarocka-Cyrta E, Kobelska-Dubiel N, Krauze A, Sakowska-Maliszewska I, Zawadzka-Krajewska A
Received 3 February 2016
Accepted for publication 16 March 2016
Published 28 June 2016 Volume 2016:8 Pages 307—316
DOI https://doi.org/10.2147/CEOR.S105748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Giorgio Colombo
Julian F Guest,1,2 Diana Weidlich,1 Maciej Kaczmarski,3 Elzbieta Jarocka-Cyrta,4 Natalia Kobelska-Dubiel,5 Agnieszka Krauze,6 Iwona Sakowska-Maliszewska,7 Anna Zawadzka-Krajewska8
1Catalyst Health Economics Consultants, Northwood, Middlesex, 2Faculty of Life Sciences and Medicine, King’s College, London, UK; 3Department of Pediatrics, Gastroenterology, and Allergology, Medical University of Bialystok, Białystok, 4Uniwersytet Warmińsko‑Mazurski, Wydział Nauk Medycznych, Katedra Pediatrii Klinicznej, Olsztyn, 5Department of Pediatric Gastroenterology and Metabolic Diseases, Poznań University of Medical Sciences, Poznań, 6Klinika Pneumonologii i Alergologii Wieku Dziecięcego, Warsaw, 7Poradnia Gastroenterologiczna Centrum Pediatrii, Sosnowiec, 8Department of Pediatric Pneumonology and Allergology, Medical University of Warsaw, Warsaw, Poland
Objective: To estimate the cost-effectiveness of using an extensively hydrolyzed casein formula (eHCF) containing the probiotic Lactobacillus rhamnosus GG (eHCF + LGG; Nutramigen LGG) as an initial treatment for cow’s milk allergy compared with eHCF alone and amino acid formulas (AAF) in Poland from the perspective of the Polish National Health Fund (Narodowy Fundusz Zdrowia [NFZ]) and parents.
Methods: Decision modeling was used to estimate the probability of cow’s milk allergic infants developing tolerance to cow’s milk by 18 months. The model also estimated the cost to the NFZ and parents (Polish Zloty [PLN] at 2013–2014 prices) for managing infants over 18 months after starting one of the formulas as well as the relative cost-effectiveness of each of the formulas.
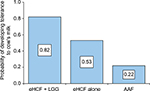
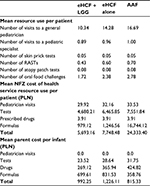
Results: The probability of developing tolerance to cow’s milk by 18 months was higher among infants who were fed eHCF + LGG (0.82) compared with those fed eHCF alone (0.53) or an AAF (0.22). An infant who is initially managed with eHCF + LGG is expected to consume fewer health care resources than infants managed with the other formulas. Hence, the estimated total health care cost incurred by the NFZ for initially feeding infants with eHCF + LGG (PLN 5,693) was less than that of feeding infants with eHCF alone (PLN 7,749) or an AAF (PLN 24,333). However, the total cost incurred by parents for initially feeding infants with an AAF (PLN 815) was marginally less than that of feeding with eHCF + LGG (PLN 993), which was less than that of feeding with eHCF alone (PLN 1,226).
Conclusion: Using eHCF + LGG instead of eHCF alone or an AAF for first-line management of newly diagnosed infants with cow’s milk allergy affords a cost-effective use of NFZ-funded resources, since it improves outcome for less cost. Whether eHCF + LGG would be viewed as being cost-effective by parents is dependent on their willingness to pay an additional cost for additional tolerance acquisition to cow’s milk.
Keywords: amino acid formula, cost-effectiveness, cow’s milk allergy, extensively hydrolyzed formula, Lactobacillus Rhamnosus GG, Poland
Introduction
Cow’s milk allergy (CMA) is an immune-mediated allergic response to milk proteins.1 It is one of the most common childhood food allergies in the developed world, with the highest prevalence during the first year of life. The estimated incidence of this allergy ranges between 0.02 and 0.03 in infants.2 Most children will acquire tolerance to cow’s milk proteins within the first 5 years of life,3 although recent evidence suggests that the natural history of this allergy is changing, with an increasing persistence until later ages.4,5 Strict exclusion of cows’ milk protein from a child’s diet (or maternal diet for exclusively breastfed babies) is currently the safest strategy for managing affected children, and for infants this necessitates substitution of a standard infant formula with a hypoallergenic formula.6
Probiotic bacteria are living microorganisms that exert beneficial effects on the health of the host.7 It has been postulated that beneficial probiotics from the human intestinal microflora8 could restore immune system homeostasis in children with CMA. Findings from studies examining the possible effects of the probiotic Lactobacillus rhamnosus GG (LGG) in pediatric allergic disorders support the use of LGG in the dietary management of cow’s milk allergic infants.9 The mechanism of the beneficial effects is multiple, ranging from modulation of intestinal microflora composition to a direct effect on intestinal mucosa structure and function, and on local and systemic immune response.9
In an open, nonrandomized, observational study in cow’s milk allergic infants in Italy, use of an extensively hydrolyzed casein formula with added LGG (eHCF + LGG; Nutramigen LGG) accelerated the development of tolerance to cow’s milk when compared with eHCF alone or amino acid formulas (AAF).10 Otherwise healthy cow’s milk allergic infants (n=260; mean age at recruitment of 5.92 months; 64% males; mean body weight 6.66 kg; 43% with immunoglobulin E (IgE)-mediated allergy) were prescribed a formula by a family pediatrician or general physician. Fifteen to 30 days after starting a formula, the infants were referred to a tertiary pediatric allergy center for a double-blind, placebo-controlled food challenge (DBPCFC) to confirm the diagnosis of CMA. At 12 months after starting a formula significantly more infants in the eHCF + LGG group developed oral tolerance to cow’s milk (78.9%; P<0.05) compared to those fed with eHCF alone (43.6%) or an AAF (18.2%).10 Tolerance was confirmed following the results of a full anamnestic and clinical evaluation, skin prick test, atopy patch test, and oral food challenge. All food challenges were performed in a DBPCFC manner. Clinical acquisition of tolerance was defined by the presence of a negative DBPCFC over a 7-day post-challenge observation period. Infants with negative DBPCFC were reevaluated after 6 months to check the persistence of tolerance to cow’s milk.10 Data from this study (kindly provided by the study’s authors) were used to construct decision models to estimate the relative cost-effectiveness of using eHCF + LGG as a first-line formula for managing cow’s milk allergic infants in Italy11 and Spain.12
The comparative health economic impact of eHCF + LGG, eHCF, and AAF in Poland is unknown. Hence, the objective of the current study was to amend the Italian model11 to estimate the cost-effectiveness of using eHCF + LGG as a first-line formula for CMA compared with eHCF and AAF in Poland, from the perspective of the Polish National Health Fund (Narodowy Fundusz Zdrowia [NFZ]) and parents.
Methods
Economic model
The Italian decision model depicting the management of cow’s milk allergic infants was adapted to reflect the structure of the Polish health care system and the context in which CMA is managed in this country. Similarly, patients’ pathways and resource use were adapted using estimates derived from a sample of Polish pediatricians with experience of managing CMA. The period of the model was up to 18 months or when an infant developed tolerance to cow’s milk, if that occurred earlier.
Model inputs: clinical outcomes
The model was populated with data from an observational study (as previously described).10,11 The percentages of infants who developed oral tolerance to cow’s milk after being fed a formula were used to populate the model with the probability of infants developing tolerance to cow’s milk at different time points, as previously described for our Italian model.11
Model inputs: resource use
The model was populated with estimates of health care resource use pertaining to the management of infants with CMA in Poland, which were derived from interviews with a sample of pediatricians.
Twenty-three pediatricians were asked to participate in the study, of whom 15 agreed and eight declined. The sample comprised six general pediatricians, four pediatric gastroenterologists, and five pediatric allergists. The clinicians were asked about their management of CMA using a structured questionnaire.
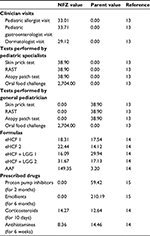
The general pediatricians who participated in this study each saw a mean of <10 infants with suspected CMA per month, with a mean age at presentation of ∼4 months (range: 3–6 months). According to these pediatricians, 25% would have IgE-mediated allergy and the other 75% would be non-IgE allergic. Twenty percent of all these infants would be referred to a pediatric gastroenterologist and 25% to a pediatric allergist for further investigations and confirmation of diagnosis. The pediatric specialists who participated in this study each saw a mean of 15–20 infants with CMA per month, with a mean age at presentation of ∼5 months (range: 2–9 months). Half of the infants referred to a pediatric allergist would have IgE-mediated allergy, and 80% of infants referred to a pediatric gastroenterologist would have non-IgE-mediated allergy. More than 90% of infants would be prescribed a formula at the initial visit to a pediatrician and the remainder at the second visit. In addition, 70% of infants would be prescribed an emollient for 6–12 months, 20% an antihistamine for 6 weeks, 10% a proton pump inhibitor for 2 months, and 2% a corticosteroid for 7–10 days.
Pediatricians prescribe formula based on an infants’ age and weight. Hence, up to 3 months of age, it would be ∼150 mL/kg/day (500–1,000 mL/day), decreasing to ∼120 mL/kg/day (800–900 mL/day) at 6 months of age. Between 7 and 9 months of age, infants would receive ∼600 mL/day, decreasing to ∼400 mL/day at >1 year of age. Infants enter the model at a mean age of <6 months. Hence, it was estimated that infants would be prescribed: 48×400 g cans of formula in the first 6 months of the model, 36×400 g cans of formula in the next 6 months of the model, and 36×400 g cans of formula after twelve months.
Statistical analyses
Using analysis of covariance (ANCOVA), differences in tolerance acquisition between formulas were adjusted for any differences in the following baseline variables: age, sex, presenting symptoms, and baseline values of the diagnostic tests. Covariates that had a P-value ≥0.05 were excluded from the ANCOVA model. The only covariates that remained were prick test result at baseline (P=0.006), respiratory symptoms at baseline (P=0.03), and atopy test results at baseline (P=0.01). All statistical analyses were performed using IBM SPSS Statistics (v21.0; IBM Corporation, Armonk, NY, USA).
Model outputs
The primary measure of clinical effectiveness was the probability of infants developing tolerance to cow’s milk by 18 months.
Unit costs in Polish Zloty (PLN) at 2013–2014 prices (Table 1)13–15 were assigned to the estimates of resource use in the model. The cost of seeing a general pediatrician was excluded from the analysis, as these clinicians are paid on a capitation basis based on the number of children in their catchment population, irrespective of the number of times they see a child.16 In Poland, parents of affected infants pay a proportion of the cost of prescriptions of nutritional formulas, as shown in Table 1. Additionally, parents pay a proportion of the cost of prescribed drugs and tests if performed by a general pediatrician (Table 1). Hence, the model was used to estimate the cost of health care resource use funded by the NFZ and the cost incurred by parents over 18 months from the start of a formula.
The model was used to estimate the cost-effectiveness of using one formula compared with another in terms of the incremental cost per additional infant who developed tolerance to cow’s milk by 18 months in Poland. This was calculated as the difference between the expected costs of two dietetic strategies divided by the difference between the expected outcomes of the two strategies in terms of the probability of developing tolerance to cow’s milk. If one of the formulas improved the probability of developing tolerance to cow’s milk for less cost, it was considered to be the dominant (cost-effective) dietetic strategy.
Sensitivity analyses
To assess uncertainty within the model, probabilistic sensitivity analyses were undertaken (10,000 iterations of the model) by simultaneously varying the probabilities, clinical outcomes, resource use values, and unit costs within the model. A beta distribution was used to represent uncertainty in probability values by assuming a 5% standard deviation around the mean values. Clinical outcomes and resource use estimates were varied randomly according to a log-normal distribution by assuming a 10% standard deviation around the mean values. Unit costs were varied randomly according to a gamma distribution by assuming a 10% standard deviation around the mean values. The outputs from these analyses were used to estimate the probability of being cost-effective at different thresholds of cost per additional infant who developed tolerance to cow’s milk by 18 months.
In addition, deterministic sensitivity analyses were performed to identify how the incremental cost-effectiveness of one dietetic strategy over the other would change by varying different parameters in the model. The budget impact and resource implications of starting infants with eHCF + LGG compared with current practice was also estimated for the annual cohort of newly diagnosed infants with CMA in Poland.
Results
Probability of developing tolerance to cow’s milk
The probability of developing tolerance to cow’s milk was higher among infants who were initially fed with eHCF + LGG (Figure 1) compared to eHCF alone and AAF.
Health care resource use and corresponding costs
An infant who was initially managed with eHCF + LGG was estimated to consume fewer health care resources than infants managed with the other formulas (Table 2). Hence, initially feeding infants with eHCF + LGG instead of the other formulas is expected to free up health care resources for alternative use by other patients. Consequently, the total health care cost incurred by the NFZ of initially feeding infants with eHCF + LGG was estimated to be less than that of feeding infants with eHCF alone or an AAF (Table 2).
Nevertheless, the total cost incurred by parents of initially feeding infants with an AAF was marginally less than that of feeding with eHCF + LGG, which was less than that of feeding with eHCF alone (Table 2). This is because parents pay a smaller proportion of the cost of AAF than for the other formulas (Table 1).
Cost-effectiveness analyses
From the NFZ’s perspective
Of the three formulas, use of eHCF + LGG yielded a greater probability of developing tolerance to cow’s milk and a lower 18 months cost to the NFZ (Table 3). Hence, starting feeding with this formula was found to be the dominant strategy (Table 3). Also, initial feeding with eHCF alone was found to be a dominant strategy when compared to starting feeding with an AAF (Table 3).
When the model was stratified according to IgE status, the probability of developing tolerance to cow’s milk was higher among those infants with non-IgE-mediated CMA compared to those with IgE-mediated allergy (Table 4). Additionally, the use of eHCF + LGG resulted in a lower 18 months cost and a greater probability of developing tolerance than the other two formulas among infants with both IgE-mediated and non-IgE-mediated CMA (Table 4). Hence, starting feeding with this formula was found to be the dominant strategy (Table 4). Also, initial feeding with eHCF was found to be a dominant strategy when compared to starting feeding with an AAF for both IgE-mediated and non-IgE-mediated infants (Table 4.
From the parents’ perspective
The use of eHCF + LGG resulted in a greater probability of developing tolerance to cow’s milk than the other two formulas and a lower 18 months cost when compared to eHCF alone (Table 3). Hence, initial feeding with eHCF + LGG was found to be a dominant strategy when compared to starting feeding with eHCF alone. When compared with AAF, eHCF + LGG resulted in a greater probability of developing tolerance to cow’s milk, but an additional cost of PLN 177 over 18 months. Hence, the additional cost for each additional infant acquiring tolerance to cow’s milk with eHCF + LGG compared to AAF was PLN 295. Similarly, the additional cost for each additional infant acquiring tolerance to cow’s milk with eHCF alone compared to AAF was PLN 1,326.
Sensitivity analyses
Probabilistic sensitivity analyses were performed to estimate the distribution of expected NFZ costs (Figure 2) and parental costs (Figure 3) over 18 months from starting a formula and probability of developing tolerance to cow’s milk by 18 months.
Using the distributions shown in Figures 2 and 3, the probability of each formula being cost-effective to the NFZ and parents at different cost-effectiveness thresholds was estimated (Figures 4 and 5).
Figure 4 shows that, from the NFZ’s perspective, the probability of eHCF + LGG being cost-effective was greater than with other formulas. The analyses also suggest that eHCF + LGG affords the greatest value for money to the NFZ, followed by eHCF alone and AAF, in that order, for managing cow’s milk allergic infants. Hence, eHCF + LGG is ranked as the preferred formula and AAF the last formula of choice.
From the parents’ perspective (Figure 5), the probability of eHCF + LGG being cost-effective was greater than eHCF alone. However, the analyses also suggest that the probability of AAF being cost-effective was greater than eHCF + LGG up to a threshold value of PLN 320, after which the probability of eHCF + LGG being cost-effective was greater than AAF. Hence, the ranking of the formula in terms of parents’ preferences is dependent on parents’ willingness to pay an additional cost for additional tolerance acquisition to cow’s milk.
Table 5 summarizes the sensitivity of the results to changes in the model’s inputs. In particular, the results were very sensitive to changes in the number of diagnostic tests. The results were also marginally sensitive to changing the proportion of IgE-mediated allergic infants within a cohort and the inclusion/exclusion of the probability of developing tolerance to cow’s milk after 6 and 12 months. However, changes in the model’s inputs are unlikely to change the ranking of dietetic choices, although if the number of prescribed drugs was increased by 50%, the cost to parents of an infant being fed an eHCF + LGG would fall below that of feeding with an AAF. The relative cost-effectiveness of the three formulas was not sensitive to changes in any other model input.
Budget impact and resource implications to the NFZ from using eHCF + LGG
There are an estimated 0.37 million live births in Poland per annum,17 and the incidence of CMA is reported to be 0.025.2 Hence, there are an estimated 9,360 new CMA-affected infants per annum in Poland. Using the distribution of formula use estimated from the interviewees and the pediatric authors, the current management of all 9,360 newly diagnosed infants was estimated to result in 62% of the cohort developing tolerance to cow’s milk by 18 months, 124,000 visits to general pediatricians, 8,900 visits to pediatric specialists, 31,900 diagnostic tests, and a cost to the NFZ of PLN 85.2 million. If 95% of these infants were initially managed with eHCF + LGG and 5% with an AAF, it is expected that 81% of the cohort would develop tolerance to cow’s milk by 18 months and there would be 24,000 fewer visits to general pediatricians, 400 fewer visits to pediatric specialists, 3,100 fewer diagnostic tests, and a cost reduction to the NFZ of PLN 9.0 million.
Discussion
To the authors’ knowledge, this was the first study to assess the cost-effectiveness of using alternative dietetic formulas for managing cow’s milk allergic infants in Poland. The basis of the analysis was the only comparative analysis of eHCF + LGG with other formulas that was available.10 The advantage of this study is that the dietary effect was measured under controlled conditions. However, infants were not randomized to their formula, sample sizes were small in absolute terms and unbalanced between the groups, and resource use was not recorded.10 The authors of the observational study made every attempt to account for baseline differences between the groups and overcome the nonrandomized study design.10 Nevertheless, before building the economic model, differences in developing tolerance to cow’s milk between treatments were adjusted for any heterogeneity in baseline variables using ANCOVA. However, the possibility that some differences have not been accounted for cannot be excluded. The inherent variability and uncertainty of using data from this small and unequal sample of patients was addressed to some extent by our extensive sensitivity analyses. The results from the observational study10 are consistent with another study, which showed that the addition of LGG to eHCF resulted in a higher rate of developing tolerance after 12 months of feeding.18
The relative cost-effectiveness of eHCF + LGG in Poland from the perspective of the NFZ is consistent with the findings from our recent studies in Italy11 and Spain,12 which also found that initial use of eHCF + LGG as a first-line management for CMA was cost-effective when compared with eHCF alone and AAF in both IgE-allergic and non-IgE-allergic infants. Additionally, our real-world evidence study in the US found that more cow’s milk allergic infants, who were initially managed with eHCF + LGG in clinical practice, were successfully managed compared with those who were fed with eHCF alone or AAF.19 The US analysis also found that initial dietary management with eHCF + LGG instead of eHCF alone or AAF affords a more cost-effective use of health care resources.19 There were no other published studies assessing the health economic impact of alternative formulas for the management of CMA, except our previous UK study,20 which also used real-world evidence, and found that eHCF alone affords a cost-effective use of health care resources in clinical practice when compared with AAF.
In Spain, the National Health Service reimburses the cost of prescriptions for nutritional formulas. Hence, parents of cow’s milk allergic infants do not incur prescription costs for the formulas.12 In Italy, parents of affected infants generally pay the whole cost of prescriptions for nutritional formulas, unless there is evidence of anaphylaxis or comorbidities such as malnutrition. Hence, eHCF + LGG was found to be the preferred dietetic choice for the parents of affected infants in Italy, as it improved outcome for less cost.11 In Poland, parents of cow’s milk allergic infants pay a varying contribution toward the cost of prescribed nutrition, depending on the formula. Consequently, the ranking of the formulas in terms of parents’ preferences is dependent on their willingness to pay an additional cost for additional tolerance acquisition to cow’s milk.
The decision model used for this analysis was based on Italian observational data. Hence, the model may not necessarily reflect clinical outcomes associated with managing a large cohort of infants in clinical practice in Poland. Accordingly, the results should be viewed with some caution until more data become available, which can be used to update the model, particularly the findings from a randomized controlled study measuring the cost-effectiveness of tolerance development in children receiving a probiotic-containing formula compared with other formulas.
The study has several other limitations. The model was informed with assumptions about treatment patterns from the pediatric authors and interviewed pediatricians, who are based at one of 15 centers. Hence, the levels of health care resource use incorporated into the model may not be representative of the whole of Poland. There was insufficient published evidence to enable us to extrapolate the model beyond 18 months. Therefore, the analysis estimated the cost-effectiveness of managing infants up to 18 months and does not consider the potential impact of managing infants who remain allergic beyond that period. Notwithstanding this, an estimated 73% of children are expected to outgrow their CMA in Poland after a mean of 16.4±0.8 months on an elimination diet.21 Moreover, milk-specific IgE and a history of paternal bronchial asthma and/or rhinitis were associated with persistence of CMA in Poland.21
Infants in the observational study10 were well matched, but those with comorbidities were excluded. Hence, the decision model used resource estimates for the “average infant” and does not consider the impact of other factors that may affect the results, such as comorbidities, underlying disease severity, and pathology of the underlying disease. Additionally, the analysis does not consider the suitability of infants to receive different formulas. The model only analyzed direct health care costs borne by the NFZ and treatment costs incurred by the parents. Indirect costs incurred by society as a result of employed parents taking time off work were excluded. Also excluded are changes in quality of life and improvements in the general well-being of sufferers and their parents, as well as parents’ preferences. Consequently, this study may have underestimated the relative cost-effectiveness of eHCF + LGG.
Despite these limitations, the decision model showed that, over the first 18 months, proportionally more infants fed with eHCF + LGG are likely to develop tolerance to cow’s milk than those fed with the other formulas. Consequently, they cost the Polish health service less to manage. This was expected, as the infants who develop tolerance to cow’s milk would no longer require any management or feeding with a hypoallergenic formula. Accordingly, treating 95% of the annual cohort of 9,360 newly diagnosed CMA-affected infants in Poland with eHCF + LGG instead of the current mix of formulas could increase the percentage of infants developing tolerance to cow’s milk from 62% to 81% and free up 24,400 visits to pediatricians and reduce health service costs by up to PLN 9.0 million. Clearly, initial use of eHCF + LGG has the potential to release health care resources for alternative use within the system.
Conclusion
In conclusion, within the study’s limitations, first-line management of newly diagnosed infants with CMA with eHCF + LGG instead of eHCF alone or AAF affords a cost-effective use of NFZ-funded resources, as it improves outcome for less cost. Whether eHCF + LGG would be viewed as being cost-effective by parents is dependent on their willingness to pay an additional cost for additional tolerance acquisition to cow’s milk. A randomized controlled study showing faster tolerance development in children receiving a probiotic-containing formula is required before this conclusion can be confirmed.
Acknowledgments
This study was supported with an unrestricted research grant from Mead Johnson Nutrition (Poland) Sp. z o.o., Warszawa, Poland. However, Mead Johnson Nutrition had no influence on the following 1) the study design; 2) the collection, analysis, and interpretation of data; 3) the writing of the manuscript; and 4) the decision to submit the manuscript for publication. The views expressed in this article are those of the authors and not necessarily those of Mead Johnson Nutrition.
The authors also wish to thank the following general pediatricians for their contributions to this study: Drs Monika Gołȩbiewska, Marek Ruszczynski, and Barbara Surowska, general pediatricians, Warsaw; Dr Małgorzata Skowron, general pediatrician, Gdansk; Dr Jolanta Słabczyńska-Kątnik, general pediatrician, Pulawe, Poland. The authors also wish to thank the following pediatric specialists for their contributions to this study: Dr Jacek Brodzicki, gastroenterologist, Gdańsk; Dr Maria Kotowska, gastroenterologist, Warszawa; Dr Emil Florkiewicz, allergist, Sieradz; Dr Anna Maron, allergist, Wrocław, Poland.
Disclosure
The authors report no conflicts of interest in this work.
References
Allen KJ, Koplin JJ. The epidemiology of IgE-mediated food allergy and anaphylaxis. Immun Allergy Clin North Am. 2012;32(1):35–50. | ||
Apps JR, Beattie RM. Cow’s milk allergy in children. Cont Med Educ. 2009;339:b2275. | ||
Høst A, Halken S, Jacobsen HP, Christensen AE, Herskind AM, Plesner K. Clinical course of cow’s milk protein allergy/intolerance and atopic diseases in childhood. Pediatr Allergy Immunol. 2002;13(Suppl 15):23–28. | ||
Skripak JM, Matsui EC, Mudd K, Wood RA. The natural history of IgE-mediated cow’s milk allergy. J Allergy Clin Immunol. 2007;120(5):1172–1177. | ||
Levy Y, Segal N, Garty B, Danon YL. Lessons from the clinical course of IgE-mediated cow milk allergy in Israel. Pediatr Allergy Immunol. 2007;18(7):589–593. | ||
Kneepkens CM, Meijer Y. Clinical practice. Diagnosis and treatment of cow’s milk allergy. Eur J Pediatr. 2009;168(8):891–896. | ||
Bodera P, Chcialowski A. Immunomodulatory effect of probiotic bacteria. Recent Pat Inflamm Allergy Drug Discov. 2009;3(1):58–64. | ||
Hill C, Guarner F, Reid G, et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. | ||
Berni Canani R, Di Costanzo M, Pezzella V, et al. The potential therapeutic efficacy of Lactobacillus GG in children with food allergies. Pharmaceuticals. 2012;5(6):655–664. | ||
Berni Canani R, Nocerino R, Terrin G, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163(3):771–777. | ||
Guest JF, Panca M, Ovcinnikova O, Nocerino R. Relative cost-effectiveness of an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow’s milk allergy in Italy. Clinicoecon Outcomes Res. 2015;7:325–336. | ||
Guest JF, Weidlich D, Mascuñan Díaz JI, et al. Relative cost-effectiveness of using an extensively hydrolyzed casein formula containing the probiotic Lactobacillus rhamnosus GG in managing infants with cow’s milk allergy in Spain. Clinicoecon Outcomes Res. 2015;7:583–591. | ||
Zarządzenie Nr 79/2014/DSOZ. Prezesa Narodowego Funduszu Zdrowia Z Dnia 5 Grudnia 2014 R. | ||
Reimbursement list 03/2015. Polish Ministry of Health, Poland. Available from: http://dziennikmz.mz.gov.pl/actdetails.html?year=2014&act=80. | ||
DOZ/Gemini Pharmacy. Available from: http://www.doz.pl and http://www.aptekagemini.pl. | ||
Zarządzenie Nr 86/2014/DSOZ. Prezesa Narodowego Funduszu Zdrowia Z Dnia 17 Grudnia 2014 R. | ||
Internal Affairs Ministry, Poland. Available from: https://msw.gov.pl/pl/aktualnosci/12844,Najwiecej-dzieci-urodzilo-sie-na-Mazowszu-wynika-z-danych-MSW-za-2014-r.html. | ||
Berni Canani R, Nocerino R, Terrin G, et al. Effect of Lactobacillus GG on tolerance acquisition in infants with cow’s milk allergy: a randomized trial. J Allergy Clin Immunol. 2012;129(2):580–582. 582.e1–5. | ||
Ovcinnikova O, Panca M, Guest JF. Cost-effectiveness of using an extensively hydrolyzed casein formula plus the probiotic Lactobacillus rhamnosus GG compared to an extensively hydrolyzed formula alone or an amino acid formula as first-line dietary management for cow’s milk allergy in the US. Clinicoecon Outcomes Res. 2015;7:145–152. | ||
Taylor RR, Sladkevicius E, Panca M, Lack G, Guest JF. Cost-effectiveness of using an extensively hydrolysed formula compared to an amino acid formula as first-line treatment for cow milk allergy in the UK. Pediatr Allergy Immunol. 2012;23(3):240–249. | ||
Kaczmarski M, Wasilewska J, Cudowska B, Semeniuk J, Klukowski M, Matuszewska E. The natural history of cow’s milk allergy in north-eastern Poland. Adv Med Sci. 2013;58(1):22–30. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.