Back to Journals » Drug Design, Development and Therapy » Volume 18
Zishen Yutai Pills Promote Angiogenesis at the Maternal-Fetal Interface in Recurrent Spontaneous Abortion Mice by Regulating miR-187/VEGF Axis
Authors Wang X, Hu H, Yu X, Liang C, Han Y , Chen H, Chu J
Received 19 September 2023
Accepted for publication 22 January 2024
Published 12 February 2024 Volume 2024:18 Pages 407—423
DOI https://doi.org/10.2147/DDDT.S436718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Xiaoli Wang,1,* Heng Hu,1,2,* Xinhui Yu,1 Chengcheng Liang,1 Yanquan Han,1 Hongxia Chen,1 Jijun Chu1
1The First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, Anhui, 230031, People’s Republic of China; 2Anhui University of Chinese Medicine, Hefei, Anhui, 230038, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongxia Chen; Jijun Chu, Department of Gynaecology, the First Affiliated Hospital of Anhui University of Chinese Medicine, Hefei, People’s Republic of China, Email [email protected]; [email protected]
Ethnopharmacological Relevance: Zishen Yutai pills (ZYP), a traditional Chinese patent medicine, was listed in China in 1981. It is composed of 15 traditional Chinese medicines and has the effects of regulating menstruation, helping pregnancy, and preventing abortion. In clinical practice, it is effective in preventing habitual and threatened miscarriages, and continuing to explore its mechanism of action is very meaningful research.
Aim of the Study: To explore the possible mechanism of ZYP promoting angiogenesis at the maternal-fetal interface in recurrent spontaneous abortion (RSA).
Materials and Methods: In vitro experiments, placental trophoblast cells (PTCs) were isolated from the placental tissue of RSA mice and divided into six groups: Control group, Model group, ZYP group, miR-187 inhibitor NC group, miR-18 7 inhibitor group, and miR-187 inhibitor+ZYP group. Cell viability and cell cycle were measured using CCK8 and flow cytometry, respectively. The expression levels of miR-187, VEGF, VEGF-R1, and VEGF-R2 were measured using RT-qPCR, WB, and IF staining. Animal experiments first establish an RSA mice model (CBA/J × DBA/2) and then randomly divide the mice into four groups (n=10): normal pregnancy group, RSA model group, ZYP group, and progesterone capsule group. Observed the changes in embryo absorption rate, pathological morphology of decidual tissue, and ultrastructure of vascular endothelial cells in each group of mice. RT-qPCR, WB, and IF staining methods were used to determine the expression of miR-187, VEGF, VEGF-R1, and VEGF-R2.
Results: In vitro, ZYP promoted the viability of PTCs and regulated their cell cycle, and ZYP down-regulated miR-187, up-regulated VEGF, VEGF-R1 and VEGF-R2 levels. miR-187 inhibitor showed the same effects, and further ZYP intervention enhanced the effects. In vivo, ZYP remarkably reduced embryo resorption rates, and improved the pathological morphology of decidual tissues and ultrastructure of vascular endothelial cells. Moreover, ZYP down-regulated miR-187, up-regulated VEGF, VEGF-R1 and VEGF-R2.
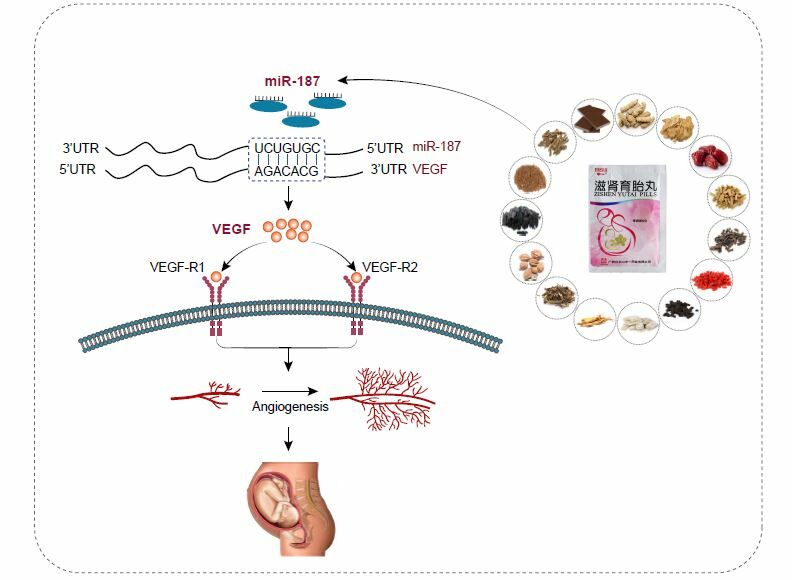
Conclusion: In summary, ZYP can regulate the expression of VEGF via miR-187, then promote the angiogenesis at the maternal-fetal interface, and playing a therapeutic role in RSA.
Keywords: recurrent spontaneous abortion, Zishen Yutai pills, miR-187, vascular endothelial growth factor, VEGF, angiogenesis
Graphical Abstract:
Introduction
Recurrent spontaneous abortion (RSA) refers to spontaneous abortion that occurs two or more times in succession with the same sexual partner before 28 weeks of pregnancy.1 An estimated 0.5%~2.5% of women in reproductive age are affected by RSA around the world.2 Apart from many known causes such as genetic mutations, anatomical abnormalities, immunological dysfunction and thrombotic state, the pathogenesis of approximately 50–60% RSA patients remains unknown.3 Notably, the normal angiogenesis of placental vellus at the maternal-fetal interface is an important factor to maintain normal pregnancy. On the other hand, disorders of angiogenesis are thought to cause RSA. Among numerous factors, the synthesis and secretion of vascular endothelial growth factor (VEGF) is of great importance for angiogenesis.4 VEGFR-1 has a dual effect, as it can intercept VEGF during embryonic development and inhibit angiogenesis. In adults, it plays a promoting role in angiogenesis through a kinase-dependent pathway. VEGF and VEGFR-2 binding can recruit hematopoietic progenitor cells and promote the migration of monocytes. The VEGF/VEGFR-1 and VEGFR-2 pathways have a promoting effect on inflammatory response and angiogenesis.5 VEGF-R1 and VEGF-R2 are high-affinity receptors of VEGF and coordinate angiogenesis after binding to VEGF.6 VEGF and its receptors play an important role in recurrent miscarriage.7 Therefore, to improve the effects of treatment, it is essential to understand the mechanism of angiogenesis at the maternal-fetal interface in RSA.
Zishen Yutai pills (ZYP), a Chinese patent medicine, was listed in China in 1981 and has been used clinically for more than 40 years. It was created on the basis of the famous prescription “Shoutai Pill” and has been used for clinical treatment of “deficiency of spleen and kidney” syndrome. Previously, index components and high performance liquid chromatography (HPLC) fingerprint analysis indicated that ZYP contained active ingredients such as loganic acid, chlorogenic acid, loganin, sweroside, asperosaponin Ⅵ and so on, which were thought to be very important for its efficacy.8,9 A lot of pharmacological and clinical studies have showen that ZYP has definite efficacy in the treatment of RSA,10 threatened abortion and premature ovarian failure,11,12 etc. Research also indicates that ZYP effectively improved the embryo loss rate and decidual angiogenesis of RSA mice by regulating the level of VEGF in serum.13 However, the mechanism of ZYP promoting the angiogenesis at the maternal-fetal interface in RSA has not been investigated clearly.
microRNAs (miRNAs), a group of endogenous non-coding RNAs with a length about 22 nucleotides, function mainly by regulating the expression of target genes.
MiR-187, a kind of miRNA that attracts much attention, has been extensively studied in malignant tumors,14 cerebral ischemia,15 osteoporosis16 and other diseases, but rare studied in RSA.16 Conducted a genome-wide miRNA analysis in villus and decidua of RSA patients in 2014, and found that the expression of miR-187 in villus of RSA patients was significantly higher than that of normal pregnant women. Our previous research found that miR-187 could directly target VEGF, so we speculate that miR-187 may participate in the development of RSA by regulating VEGF level. Interestingly, it was found that ZYP could upregulate VEGF expression in RSA mice. This makes us consider that the therapeutic effect of ZYP on RSA may be related to the fact that ZYP modulates the activity of miR-187, upregulates the expression of VEGF, and promotes angiogenesis at the maternal-fetal interface. Therefore, the aim of this study was to explore the mechanism of action of ZYP on angiogenesis at the maternal-fetal interface in RSA mice through in vivo and vitro experiments, with a particular focus on the miR-187/VEGF axis.
Materials and Methods
Animals
CBA/J female mice (6 to 7 weeks old, 19±2 g) were purchased from Beijing Huafukang Biotechnology Corporation, with Certificate No: SCXK (Beijing) 2019–0008. BALB/c, DBA/2 male mice (6 to 7 weeks old, 19±2 g) as well as Sprague-Dawley (SD) male rats (6 to 7 weeks old, 200±10 g) were obtained from Shanghai Slaughter Laboratory Animal Corporation, with Certificate No: SCXK (Shanghai) 2017–0005. The study complies with the Regulations on the Management of Experimental Animals approved by the State Council of the People’s Republic of China and has been approved by the Local Animal Ethics Committee of Anhui University of Chinese Medicine (AHUCM-rats-2,021,078 China; AHUCM-mouse- 2,021,079 China)
Drugs
ZYP (Batch number: National Drug Certification Z44020008) was provided by Guangzhou Baiyunshan Zhongyi Pharmaceutical Co. Ltd. (Guangzhou, Guangdong Province, China). ZYP is composed of fifteen traditional Chinese medicines (TCM), and the names and plant sources (or preparation methods) of these TCM are as follows: Cuscutae Semen (Dry mature seeds of Cuscuta australis R. Br. or Cuscuta chinensis Lam.), Atractylodis Macrocephalae Rhizoma (Dry rhizomes of Atractylodes macrocephala Koidz.), Jujubae Fructus (Dry and mature fruits of Ziziphus jujuba Mill.), Codonopsis Radix (Dry radix of Codonopsis pilosula (Franch.) Nannf, Codonopsis pilosula Nannf. var. modesta (Nannf.) L. T. Shen or Codonopsis tangshen Oliv.), Ginseng Radix et Rhizoma (Dry roots and rhizomes of Panax ginseng C. A. Mey.), Dipsaci Radix (Dry roots of Dipsacus asper Wall. ex Henry.), Eucommiae Cortex (Dry bark of Eucommia ulmoides Oliv), Taxilli Herba (Dry leafy stems of Taxillus chinensis (DC.) Danser.), Morindae Officinalis Radix (Dry roots of Morinda officinalis How.), Cornu Cervi Degelatinatum (The remaining bone residue after boiling deer antler glue for the antlers of Cervus nippon Temminck or Cervus elaphus Linnaeus.), Lycii Fructus (Dry and mature fruits of Lycium barbarum L.), Rehmanniae Radix (Dry root tubers of Rehjnannia glutinosa Libosch), Aisni Corii Colla (Solid glue made from dried or fresh skin of Equus asinus L. by boiling and concentrating), Polygoni Multiflori Radix (Dry root tubers Polygonum multiflorum Thunb), Amomi Fructus (Dry and mature fruits of Amomum villosum Lour, Amomum villosum Lour. var. xanthioides T. L. Wu et Senjen or Amomum longiligulare T. L. Wu.).17 Progesterone capsule (Batch number: National Drug Certification H20041902) was provided by Zhejiang Xianju Pharmaceutical Co. Ltd. (Taizhou, Zhejiang, China).
Cells Isolation, Culture and Transfection
According to Clark’s classical method (Clark et al 1980) to establish RSA model (CBA/J×DBA/2) and normal pregnancy model (CBA/J×BALB/c), two female mice were mated with one male mouse. The female mice were examined for vaginal plugs every morning and separated from the males if pregnant. The day of vaginal plug appearance was indicated as Day 0 of pregnancy. RSA pregnant mice and normal pregnant mice were established, 15 in each group, were euthanized by cervical dislocation on Day 14 of gestation without any treatment. Placental tissues were isolated from the uterus and stored in a −80 °C refrigerator.
The PTCs were isolated from placental tissues. Firstly, placental tissues were minced into small fragments and then digested with trypsin (Beyotime, Shanghai, China) for 30 min at 37 °C in a shaking water bath for three cycles. The digestion products were resuspended; then, supernatant was discarded and filtered through 200μm pores nylon cell strainers. The PTCs were collected by density gradient centrifugation using percoll solution (Solarbio, Beijing, China) and the macrophages further were removed by differential adhesion. Finally, the obtained PTCs were added with warm DMEM-P/S to gain PTCs suspension. PTCs were added with DMEM/F12 (1:1) of 10% fetal bovine serum and cultured in a 37 °C, 5% CO2 incubator. For miRNA transfection, the RSA mice PTCs obtained in the logarithmic growth phase were inoculated into 6-well plate with 105–106 cells in each well. Next day, the transfection of target gene was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, USA) following the instructions, the target gene including miR-187 inhibitor, miR-187 inhibitor negative control (miR-187 inhibitor NC), all purchased from Gene Pharma Co, Ltd, Shanghai, China.
Preparation of Drug-Containing Serum
SD male rats were divided into 2 groups randomly (10 per group): drug-containing serum and blank serum groups. Drug-containing serum group was gavaged with ZYP 2.5g/kg body weight/d (Equivalent to 10 times the median clinical dose for human. The dosage of ZYP for clinical adults is 15g/d, which is equivalent to the animal dosage of 0.25g/kg/d.), meanwhile, the same volume of distilled water was gavaged to blank serum group. Gavage volume was 0.2 mL/10 g, twice a day for 7 consecutive days. Two hours after the last administration, the abdominal aortic blood was collected and centrifuged at 3000 rpm/min for 20 min to obtain the upper serum. Then, the upper serum was inactivated and sterilized, and stored at −80 °C for further examination.
Selection of Optimal Concentration and Time of ZYP
The logarithmic growth phase PTCs obtained from RSA mice was inoculated into 96-well plate at a concentration of 1.0×105/mL (100μL per well). After adhering to the plate wall, the cells were cultured in DMEM containing 10% fetal bovine serum with 5% CO2 at 37 °C. The PTCs were divided into drug-containing serum and blank serum groups, 3 parallel wells were set in each group. In the drug-containing serum group, 100μL of serum with ZYP at a concentration of 1.25%, 2.5%, 5%, 10%, 20%, 40% was added, respectively, and an equal volume of blank serum at density consistent with 20% serum with ZYP-containing serum was added to the blank serum group. The cell proliferation was detected when the cells were incubated for 12, 24, 48 or 72 h. Then, 10μL of CCK8 solution (Bioss, USA) was added to each well, and cells were cultured continuously for 4 h, and the supernatant was carefully aspirated. Next, the blank well was set as zero, the optical density (OD) of each well was collected at 450 nm using a microplate reader.
Cell Groups
The optimal concentration and acting time of ZYP-containing serum were obtained by CCK8 assay, and according to the results, PTCs were randomly distributed to following 6 groups: AG. Control group (normal pregnant mice PTCs exposed to blank serum); BG. Model group (RSA pregnant mice PTCs exposed to blank serum); CG. ZYP group (RSA pregnant mice PTCs exposed to optimal concentration of ZYP-containing serum); DG. miR-187 inhibitor NC group (RSA pregnant mice PTCs transfected with miR-187 inhibitor NC exposed to blank serum); EG miR-187 inhibitor group (RSA pregnant mice PTCs transfected with miR-187 inhibitor exposed to blank serum); FG. miR-187 inhibitor+ZYP group (RSA pregnant mice PTCs transfected with miR-187 inhibitor exposed to optimal concentration of ZYP-containing serum). All PTCs were cultured for optimal acting time.
CCK8 Assay
According to the results of optimal concentration and time of ZYP, the cell viability of each group was measured using CCK8. In short, 100 μL optimal concentration of ZYP-containing serum or blank serum was added according to different groups and incubated cells for optimal acting time. Then, 10 μL CCK8 solution was added to each well and further cultured cells for 4 h. Finally, the OD value under 450 nm was detected using a microplate reader.
Flow Cytometry
The PTCs in different groups were inoculated into 96-well plate at a density of 1.0×106/mL. The cells were washed with pre-cooled PBS, digested by trypsin, centrifuged at 2000 rpm for 5 min to remove the supernatant. Then, the cells were resuspended in 0.5mL PBS, and 1 mL of pre cooled 70% ethanol was added at 4 °C for 2 hours. The cells were cleaned with PBS and centrifuged again. One hundred microliter RNase A and 500 μL propidium iodide (Biosharp, Beijing, China) were added and incubated at 37 °C for 30 min. Finally, cell cycle distribution was detected by using flow cytometry.
Groups and Drug Treatment
RSA model and normal pregnancy model were established. For in vivo experiment, the pregnancy model mices were allocated into 4 groups (10 in each group), 10 normal pregnant mices formed normal pregnancy group (NPG), and 30 RSA pregnant mices were distributed randomly into RSA group (RSAG), ZYP group (ZYPG) and progesterone capsule group (PCG). ZYP was intragastricly administrated with diluted solution containing ZYP at dose of 2.25g/kg/d and progesterone capsule with diluted solution containing progesterone capsule at dose of 30 mg/kg/d (the dosage is based on the clinical dose of 60 kg for adults and converted by the superficial area of the animal). The ZYP and progesterone capsule were dissolved in distilled water and configured into diluted solutions with concentrations of 0.11g/mL and 1.5mg/mL, respectively. Starting from the first day of pregnancy, intragastric administration with a volume of 0.2mL/10g was administered, while the NPG and the RSAG were gavaged with equal volume of distilled water. The dose was given once a day and for successive 14 days.
Embryo Resorption
On the 14th day of pregnancy, pregnant mice were euthanized with cervical dislocation, and the uterus was dissected to observe the embryo. Compared with the NPG mice, the uterus of aborted mice shows a “bamboo”-like change and the resorbed embryo is marked with smaller size and necrotic hemorrhagic appearance. The embryo resorption rate = resorbed embryos/(surviving embryos + resorbed embryos)×100%.
Sample Collection
After the observation of embryo resorption, the decidual tissues were peeled off from the uterine wall and rinsed with 0.9% saline. Then, the decidual tissues were split into three parts due to different purposes, one part was stored at −80 °C for real-time qPCR (RT-qPCR) and Western Blot (WB), and one part was quickly fixed in 4% paraformaldehyde for hematoxylin-eosin (HE) staining and Immunofluorescent (IF) staining, meanwhile the other part was cut into pieces at the size of 1 mm2 and fixed with 2.5% glutaraldehyde for transmission electron microscopy (TEM).
HE Staining
The decidual tissues we obtained were fixed in 4% paraformaldehyde and embedded in conventional paraffin. Then, continuous sections with a thickness of 4 μm were made. After that, the sections were stained with HE (Ebiogo, Hefei, China) and sealed with neutral resin. The pathological morphology of decidual tissue was observed under an optical microscope.
TEM Examination
The fresh samples we obtained for TEM were sent to Anhui Xinle Biology Co, LTD (Hefei, China) to examine the ultrastructure of vascular endothelial cells using a transmission electron microscope.
RT-qPCR
Total RNA was extracted from the PTCs or decidual tissue using Trizol reagent (Life Technologies, USA). The equal amounts of total RNA were reverse-transcribed into cDNA using PrimeScript™RT reagent Kit (TaKaRa, Dalian, China), and the RT-QPCR was performed using Novostart SYBR qPCR kit (Novoprotein, Shanghai, China) in accordance with the instructions. In addition, U6 was the internal control gene for miR-187 and β-actin was the internal reference for VEGF, VEGF-R1 and VEGF-R2. Specific information is shown in Table 1. The relative expression was calculated using the formula 2−ΔΔCt, and each group randomly selects 3 samples for the experiment.
 |
Table 1 The Sequence of Primers |
Western Blot
The PTCs or decidual tissue was treated with RIPA lysis solution (Beyotime, Shanghai, China) and centrifuged at 12,000 rpm for 10 min, and then the supernatant was collected. The total protein was separated by SDS-PAGE gel electrophoresis (both purchased from Solarbio, Beijing, China) with 70 V of electric voltage for 1 h and then directly transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, Massachusetts, USA). After the transferring process was finished, the membrane was immediately washed with western washing solution for 5 min and blocked with 5% skim milk powder for 2 h at room temperature. Next, the primary antibodies were added to the blocked membrane and incubated for overnight at 4 °C, including anti-VEGF (1:1000, bs-1313R, Bioss, USA), anti-VEGF-R1 (1:1500, bsm-52338R, Bioss, USA), anti-VEGF-R2 (1:1000, ab39256, Abcam, Britain) and anti-GAPDH (1:1000, TA-08, Zsbio, Beijing, China). Next day, the membrane was rinsed with PBS and added with secondary antibodies (Zsbio, Beijing, China) and then incubated at room temperature for 2 h before washing. Finally, the protein bands were generated using ECL luminescence kit (Thermo, Germany), and all bands were analyzed using the Image J software (Rawak Software Inc, Germany). The relative expression of the total target proteins was calculated by taking GAPDH as an internal reference. Three samples were randomly selected from each group.
IF Staining
IF staining of the cells, the PTCs were made onto slides, firstly fixed with 4% paraformaldehyde solution, and then blocked with goat serum for 20 min. After the blocking process was completed, cells were covered with the primary antibody dilutions, including anti-VEGF (1:50, bs-1313R, Bioss, USA), anti-VEGF-R1 (1:200, ab2350, Abcam, Britain) and anti-VEGF-R2 (1:200, BS1373, Bioworld, USA), and incubated at 37 °C for 60 min, then rinsed in PBS wash buffer. Next, secondary antibody (1:200, Ebiogo, Hefei, China) was added to the cells and cultured at 37 °C for 30 min, then rinsed in the same way. Finally, slides were sealed by ProLong Gold Antifade Reagent with DAPI (Ebiogo, Hefei, China) and scanned using digital slice scanner (3DHISTECH, Hungary).
IF staining of the tissues, the paraffin-embedded decidual tissues, with a thickness of 4μm, were deparaffinized and rehydrated firstly. Then, endogenous peroxidase activity was quenched, and antigen was retrieved in a microwave oven (Midea, China). The following steps are the same as IF staining of the cells. Randomly, select 3 samples from each group for the experiment.
Luciferase Activity Assay
The PTCs were transferred into 96-well plates and grown to 50–70% confluence, then the cells were co-transfected with wild-type (WT) or mutant (MUT) type of VEGF (both purchased from Hanbio Biotechnology Co, Ltd. Shanghai, China) and miR-187 mimics or negative control using Lipofectamine 2000. After 48h, luciferase activity was tested by Dual-Luciferase system (Promega, USA).
Statistical Analysis
All data were analyzed using the software SPSS 26.0. The experimental data were expressed as mean ± standard deviation (mean ± SD). One-way analysis of variance (ANOVA) was used for multiple groups comparison when data were normal distribution. Then, pairwise comparison between groups was further performed, where LSD test was used if variance was equal, and Tamhane Test if variance was uneven. Kruskal–Wallis test was adopted for that data did not conform to a normal distribution. Spearman test was used for correlation analysis. Differences were significant at P < 0.05.
Results
Optimal Concentration and Time of ZYP
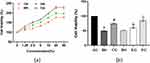
As shown in Figure 1a, the viability of PTCs reached their peak at 48 h with the prolongation of the action time of the ZYP-containing serum. There was a statistical difference compared with the 24 h dose groups (P<0.05). However, the cell viability of each dose group did not increase at 72 hours, and there was no significant difference compared to 48 hours (P>0.05). As the concentration of serum containing ZYP increases, cell viability reaches its highest level under the action of 20% concentration, which is statistically different from the effect of 10% concentration. Interestingly, under the action of 40% concentration of ZYP serum, the cell viability was lower than that of 20% concentration of ZYP serum. This may be related to the toxic effect of high concentration drug-containing serum on cells, leading to poor cell growth and an increase in apoptotic cells. Therefore, a 20% concentration of ZYP serum was selected for the experiment, with a cultivation time of 48 hours as the optimal serum concentration and cultivation time for subsequent experiments.
 |
Figure 5 Spearman correlation analysis showed that miR-187 has a negative correlationship with VEGF, VEGF-R1, VEGF-R2, with R values of −0.645, −0.649 and −0.653, respectively (all P < 0.01). |
 |
Figure 9 VEGF is a target gene of miR-187. (a) The target site between miR-187 and VEGF mRNA. (b) Results of Luciferase activity assay (***P<0.01). |
Cell Viability
The activity of PTCs was measured using CCK8 assay. The results (Figure 1b) were shown as follows: the cell viability in model group was significantly lower than that in control group. However, compared with the model group, the cell viability was significantly increased after the intervention of ZYP. In addition, when compared with the miR-187 inhibitor NC group, the cell viability was raised obviously in miR-187 inhibitor group, and the cell viability in miR-187 inhibitor + ZYP group was higher than that in miR-187 inhibitor group.
Cell Cycle
Flow cytometry was performed to detect the cell cycle changes of PTCs (Figure 2a). As shown in Figure 2b, in comparison with the control group, the proportion of G1 phase in model group was decreased, and the proportion of S+G2 was increased (P<0.05), but the result was reversed after the intervention of ZYP (P < 0.05). Meanwhile, when compared with the miR-187 inhibitor NC group, the proportions of miR-187 inhibitor group and miR-187 inhibitor + ZYP group in G1 phase were increased, and S+G2 were decreased (P<0.05), and there were significant differences between the two groups (P<0.05).
Effect of ZYP on Embryo Resorption
The embryo resorption rates of all groups were counted on day 14 of gestation. As listed in Table 2, the embryo resorption rate in RSA group was obviously higher than that in normal pregnancy group. However, ZYP group and progesterone capsule group showed lower resorption rates compared with RSA group.
 |
Table 2 Comparisons of Embryo Resorption Rates in Pregnant Mice |
The Pathological Morphology of Decidual Tissues
To observe the pathological morphology of decidual tissues, HE staining was performed. As shown in Figure 3a. In the normal pregnancy group, cells in the decidua tissue expressed regular morphology, clear boundaries and compact arrangement. Besides, cell nucleus inside was oval or almost round, and the cytoplasm was uniformly stained. Moreover, there are abundant blood vessels in decidual tissue, with intact wall and no obvious blood stasis. Compared with the normal pregnancy group, the decidual tissues in RSA group showed degenerative changes, decidual cells are characterized by reduced number, irregular shape and disordered arrangement, the nucleus were pyknotic and stained deeply, and the cytoplasm was edematous and even vacuolated. In addition, the number of blood vessels in decidual tissues was decreased as well. Whereas in ZYP group and progesterone capsule group, the morphology of decidual cells was improved, and the number of blood vessels was increased obviously compared with the RSA group.
The Ultrastructure of Vascular Endothelial Cells
The ultrastructure of vascular endothelial cell was observed by using TEM. Compared with the normal pregnancy group, the decidual tissues in RSA group showed as following: incomplete structures of vascular endothelial cells, blurred nuclear membrane, obviously reduced ribosomes and rough endoplasmic reticulum with degranulation, severely deformed mitochondria with most ridges and membranes were fused and obscured, and erythrocytes were found in blood vessels without thrombosis. However, compared with the RSA group, structures of vascular endothelial cells in ZYP group and progesterone capsule group were improved distinctly tending to the state of normal pregnancy group (Figure 3b).
Expression of miR-187, VEGF, VEGF-R1 and VEGF-R2 Gene and Protein
The results of RT-qPCR (Figure 4a) and Western blot (Figure 4b and c) in vitro study were shown in Figure 4. In comparison with the control group, model group was significantly increased in the expression of miR-187 gene, while apparently decreased in the gene and protein expression of VEGF, VEGF-R1 and VEGF-R2. However, the results in the ZYP group were totally in the opposite direction when compared with the model group. In addition, compared with the miR-187 inhibitor NC group, the miR-187 inhibitor group showed significant downregulation in miR-187 gene expression but obvious upregulation in VEGF, VEGF-R1 and VEGF-R2 gene and protein expression, these results indicated that PTCs of RSA mice were successfully transfected with miR-187 inhibitor, and also suggested that miR-187 could negatively regulate the expression of VEGF, VEGF-R1 and VEGF-R2. And the effects in the miR-187 inhibitor + ZYP group were enhanced when compared with the miR-187 inhibitor group.
Furthermore, Spearman correlation analysis was conducted between miR-187 and VEGF, VEGF-R1, and VEGF-R2 mRNA, and the results (Figure 5) showed that miR-187 has a negative correlationship with VEGF, VEGF-R1, VEGF-R2 mRNA, with R values of −0.645, −0.649 and −0.653, respectively.
The results of RT-qPCR (Figure 6a and b) and Western blot (Figure 6c and d) in vivo experiment were shown in Figure 6. Compared with the normal pregnancy group, miR-187 gene expression in RSA group was markly increased, while VEGF, VEGF-R1 and VEGF-R2 gene, and protein expression were significantly decreased. In comparison with the RSA group, ZYP group and progesterone capsule group were apparently reduced in miR-187 gene expression, but the expressions of VEGF, VEGF-R1 and VEGF-R2 gene, and protein were increased noticeably.
Expressions of VEGF, VEGF-R1 and VEGF-R2 Protein Verified by IF
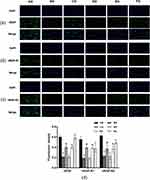
The results of IF staining verified the protein expressions of VEGF, VEGF-R1 and VEGF-R2, which were consistent with the results of Western blot experiment. For in vitro experiments, the positive expressions of VEGF, VEGF-R1 and VEGF-R2 in the control group were the strongest, while those in model group were obviously weakened, but the positive expressions in ZYP group were significantly increased when compared with the model group. In addition, compared with the miR-187 inhibitor NC group, the positive expressions of VEGF, VEGF-R1 and VEGF-R2 in the miR-187 inhibitor group were enhanced, furthermore, the positive expressions in miR-187 inhibitor + ZYP group were more remarkable than that in miR-187 inhibitor group (Figure 7).
For in vivo experiments, the positive expressions of VEGF, VEGF-R1 and VEGF-R2 in RSA group were visibly decreased compared with the normal pregnancy group; however, the positive expressions in ZYP group and progesterone capsule group were apparently increased than that in RSA group (Figure 8).
VEGF is a Target Gene of miR-187
As shown in Figure 9a, the possible target site between miR-187 and VEGF mRNA was predicted by using Bibiserv (https://bibiserv.cebitec.uni-bielefeld.de/). Furthermore, in order to verify the targeting relationship between miR-187 and VEGF, a luciferase assay was conducted, and the results revealed that, compared with the miR-187 mimics NC, miR-187 mimics significantly down-regulated luciferase expression of VEGF-WT. However, when VEGF was mutated, miR-187 mimics had no apparent effect on the luciferase expression of VEGF-MUT contrast to miR-187 mimics NC (Figure 9b). These results all suggested that VEGF is a target gene of miR-187.
Discussion
In this experiment, firstly, the PTCs model of RSA mice were obtained to study the effects of ZYP on it. This study revealed that ZYP down-regulated miR-187, up-regulated VEGF, VEGF-R1 and VEGF-R2 expression. Meanwhile, ZYP promoted the viability of PTCs and regulated their cell cycle. Then, using the miR-187 inhibitor, cells transfected with miR-187 inhibitor showed the same effects as above, and the effects were more remarkable after further ZYP intervention. In general, this study indicated that ZYP improved viability of PTCs by upregulating the expression of VEGF and its receptor via miR-187.
To further validate the above inference, RSA mice models were established for in vivo experiments. It is satisfied that the results of in vivo experiments were consistent with those of in vitro experiments. After treatment with ZYP, the embryo resorption rate of pregnant RSA mice was significantly reduced, and the pathological morphology of decidual tissues and ultrastructure of vascular endothelial cells were remarkably improved. In addition, the expression of miR-187 in decidual tissues was decreased, while VEGF, VEGF-R1 and VEGF-R2 were increased. Based on this, we conclude that ZYP can regulate the expression of VEGF via miR-187, promote the angiogenesis at the maternal-fetal interface, and thus play a therapeutic role in RSA.
In the process of pregnancy, about the third week after fertilization, the chorionic vasculature begins to generate, and the placental circulation is established. Adequate circulating perfusion is an important factor in maintaining normal embryonic development. If there is an imbalance in placental vascularization and insufficient blood supply, the embryo may stop developing and miscarriage may occur.18 Currently, studies have confirmed that impaired angiogenesis at the maternal-fetal interface is an important factor in the occurrence and development of RSA.19 Angiogenesis, a complex process regulated by pro-angiogenesis and anti-angiogenesis factors,20 in which VEGF is the pivotal substance that plays an important role.
VEGF has the ability to increase vascular permeability, promote vascular endothelial cell migration and proliferation, and ultimately accelerate neovascularization. However, the role of VEGF is played need to combine with its transmembrane receptors, such as VEGF-R1 and VEGF-R2, etc. If there is abnormal expression of VEGF or its receptors, it can lead to impaired angiogenesis. For example, diseases like cerebral ischemia and myocardial infarction both show reduced VEGF expression and impaired angiogenesis.21,22 Previous studies have shown that the expressions of VEGF, VEGF-R1 and VEGF-R2 in RSA were significantly decreased,23,24 which is consistent with our research results. Studies showed that asperosaponin VI, the main active extract of ZYP, enhanced angiogenesis of human umbilical vein endothelial cells in vitro through activating the HIF-1α/VEGF pathway; meanwhile, which could promote vascularization of regenerated tissue and wound healing in vivo.25 BSAT, a prescription similar to ZYP containing the same Chinese herbs such as Cuscutae Semen, Atractylodis Macrocephalae Rhizoma, Codonopsis Radix, Dipsaci Radix, Taxilli Herba, Rehmanniae Radix Praeparata, improved angiogenesis at maternal-fetal interface in RSA mice by modulating the VEGF/Ras/MAPK signaling pathway.26 Previous studies showed that ZYP increased the expression of VEGF in the endometrium of post-abortion rat model, promoted endometrial blood supply, and accelerated the recovery of endometrium.27 In the present experiment, both in vivo and in vitro experiments, ZYP increased the expression of VEGF, VEGF-R1 and VEGF-R2, improved the viability of PTCs, and promoted the angiogenesis of maternal-fetal interface.
miRNAs, a class of endogenous non-coding RNAs, which are involved in a variety of diseases. Studies showed that miRNAs are involved in the occurrence of RSA mainly through mechanisms that mediate angiogenesis,28 cell proliferation, apoptosis and immune tolerance,29,30 etc. miR-187, a kind of miRNAs mainly associated with cancer, has been deeply studied in many malignancies,14 cerebral ischemia and other diseases.15 Studies showed that high expression of miR-187 suppresses the proliferation of non-small-cell lung cancer cells and induces cell cycle arrest at G0/G1 phase.31 Another study found that miR-187 inhibitor can reduce the infarction size in middle cerebral artery occlusion/reperfusion rat model. These experiments suggested that miR-187 negatively regulates both cell proliferation and angiogenesis.
There are few studies on miR-187 in RSA. One study found that miR-187 is highly expressed in the villus tissue of patients with RSA, and overexpressed miR-187 suppresses the proliferation, migration and invasion of trophoblast cells by inhibiting the PI3K/AKT signaling pathway via BCL6.32 Here, we found that the expression of miR-187 in RSA mice was significantly increased, but it could be down-regulated obviously after ZYP intervention. At the same time, our study also found that miR-187 can directly target VEGF and play a negative regulatory role on it. This suggested that ZYP may play a therapeutic role on RSA by modulating the miR-187/VEGF axis and then further promoting angiogenesis at the maternal-fetal interface.
However, there are certain limitations in our study, due to the complex composition of ZYP, further research should determine which active ingredients can promote angiogenesis, which will help fully clarify the therapeutic mechanism of Zishen Yutai pills on RSA. The study on miR-187/VEGF axis in this experiment is still superficial, and further experiments are needed to deeply explore the mechanism of miR-187/VEGF axis, then further study to understand the role of ZYP on this mechanism.
Conclusion
In summary, this study firstly proposed the mechanism that miR-187 can target and regulate VEGF, by the mechanism the delivery of ZYP promoted the angiogenesis at maternal-fetal interface and improved embryo survival rates of RSA pregnant mice. The present study confirmed the effectiveness of ZYP and provided a novel treatment strategy for RSA patients.
Abbreviations
ANOVA, analysis of variance; CCK, cholecystokinin; CHM, Chinese herbal medicines; DBA, Data Base Administrator; DMEM, dulbecco modified eagle medium; HE, hematoxylin-eosin; HPLC, high performance liquid chromatography; IF, immunofluorescence; mean ± SD, mean ± standard deviation; miRNAs, microRNAs; NC, National Certificate; OD, optical density; PTCs, placental trophoblast cells; PVDF, polyvinylidene fluoride; RIPA, Radio immunoprecipitation assay; RSA, recurrent spontaneous abortion; RT-qPCR, real time qPCR; SD, Sprague-Dawley; TCM, traditional Chinese medicine; TEM, transmission electron microscopy; VEGF, vascular endothelial growth factor; WT, wild-type; WB, Western blot. ZYP, Zishen Yutai pills.
Consent for Publication
This manuscript has been approved by all authors, and there are no conflicts of interest in the submitted content.
Funding
The present work was financially supported by grants from Luo Yuankai-Zishen Yutaiwan-Research Fund for Young Scholars (20190805); Key Project of Natural Science Research of Anhui Provincial Higher Education Institutions (KJ2020A0408) and Clinical Research Fund of Anhui University of Chinese Medicine (2021yfylc09).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Writing Group of Chinese Expert Consensus on Diagnosis and Treatment of Spontaneous Abortion. Chinese expert consensus on diagnosis and treatment of spontaneous abortion (2020 edition). Chine J Practical Gynecol Obstetrics. 2020;36(11):1082–1090.
2. Christiansen OB. Special Issue Recurrent Pregnancy Loss: etiology, Diagnosis, and Therapy. J Clin Med. 2021;10(21):5040. doi:10.3390/jcm10215040
3. Roomandeh N, Saremi A, Arasteh J, et al. Comparing Serum Levels of Th17 and Treg Cytokines in Women with Unexplained Recurrent Spontaneous Abortion and Fertile Women. Iran J Immunol. 2018;15(1):59–67.
4. Dimova I, Rizov M, Giragosyan S, et al. Molecular pathogenesis of spontaneous abortions - Whole genome copy number analysis and expression of angiogenic factors. Taiwan J Obstet Gyne. 2020;59(1):99–104. doi:10.1016/j.tjog.2019.11.015
5. Farghaly TA, Al-Hasani WA, Abdulwahab HG. An updated patent review of VEGFR-2 inhibitors (2017-present) [J]. Expert Opin Ther Patents. 2021;31(11):989–1007. doi:10.1080/13543776.2021.1935872
6. Ozturk N, Gozukara I, Kamalak Z, et al. The importance of some angiogenic markers in spontaneous abortion. Clin Exp Obstet Gynecol. 2017;44(3):444–447. doi:10.12891/ceog3627.2017
7. Amirchaghmaghi E, Rezaei A, Moini A, et al. Gene expression analysis of VEGF and its receptors and assessment of its serum level in unexplained recurrent spontaneous abortion. Cell J. 2015;16(4):538–545. doi:10.22074/cellj.2015.498
8. Ma HW, Zou Q, Li C, et al. HPLC fingerprint of Zishen Yutai pills s and simultaneous determination of 5 index components. China J Chinese Materia Medica. 2018;43(14):2878–2883. doi:10.19540/j.cnki.cjcmm.2018.0090
9. Cao J, Lei T, Wu S, et al. Development of a comprehensive method combining UHPLC-CAD fingerprint, multi-components quantitative analysis for quality evaluation of Zishen Yutai pills s: a step towards quality control of Chinese patent medicine. J Pharmaceut Biomed. 2020;191:113570. doi:10.1016/j.jpba.2020.113570
10. Yang XN, Liao HS, Bao HQ, et al. Observation on the effect of Dydrogesterone combined with Zishen Yutai pills in the treatment of fetal preservation in patients with early unexplained recurrent spontaneous abortion. Chine J Family Planning Gynecotokol. 2021;13(09):63–67.
11. Xu XF, Gu L, Tu CY, et al. Study on the maternal-fetal immune tolerance effect mediated by human leukocyte antigen-G with Zishen-Yutai pills in the treatment of early threatened abortion: a randomized controlled trial. Chine J Family Planning Gynecotokol. 2021;41(12):1109–1116.
12. Feng Y, Chai X, Chen Y, et al. Network Pharmacology Approach for Predicting Targets of Zishen Yutai pills s on Premature Ovarian Insufficiency. Evid-Based Complementary Altern Med. 2021;8215454.
13. Chu JJ, Wang RX, Yu XY, et al. Effects of Zishen Yutai pills s on the expressions of regulatory factors in recurrent spontaneous abortion mice. Chinese Traditional Patent Med. 2018;40(4):777–782.
14. Peng W, Sha H, Sun X, et al. Role and mechanism of miR-187 in human cancer. Am J Transl Res. 2020;12(9):4873–4884.
15. Ren Z, Hu Y, Guo D, et al. Increased miR‑187‑3p expression after cerebral ischemia/reperfusion induces apoptosis via initiation of endoplasmic reticulum stress. Neurosci Lett. 2021;759:135947. doi:10.1016/j.neulet.2021.135947
16. Sun Y, Wang X, Chen G, et al. miRNA-187-5p Regulates Osteoblastic Differentiation of Bone Marrow Mesenchymal Stem Cells in Mice by Targeting ICAM1. Biomed Res Int. 2020;2020:6139469. doi:10.1155/2020/6139469
17. Dong F, Zhang Y, Xia F, et al. Genome-wide miRNA profiling of villus and decidua of recurrent spontaneous abortion patients. Reproduction. 2014;148(1):33–41. doi:10.1530/REP-14-0095
18. Fortis MF, Fraga LR, Boquett JA, et al. Angiogenesis and oxidative stress-related gene variants in recurrent pregnancy loss. Reprod Fert Dev. 2018;30(3):498–506. doi:10.1071/RD17117
19. Li M, Peng X, Qian J, et al. Galectin-9 regulates HTR8/SVneo function via JNK signaling. Reproduction. 2021;161(1):1–10. doi:10.1530/REP-19-0543
20. Shahgaldi S, Rezaei Kahmini F, Moazzeni SM. Mesenchymal stem cell therapy attenuates complement C3 deposition and improves the delicate equilibrium between angiogenic and anti-angiogenic factors in abortion-prone mice. Mol Immunol. 2022;141:246–256. doi:10.1016/j.molimm.2021.11.010
21. Sun Y, Jin K, Xie L, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111(12):1843–1851. doi:10.1172/JCI200317977
22. Wang H, Qiu L, Ma Y, et al. Naoxintong inhibits myocardial infarction injury by VEGF/eNOS signaling-mediated neovascularization. J Ethnopharmacol. 2017;209:13–23. doi:10.1016/j.jep.2017.06.040
23. Fang Y. STAT3 Signaling Pathway is Involved in the Pathogenesis of Early Spontaneous Abortion by Down Regulating CyclinD1 and VEGF [Master’s Thesis]. Hefei: Anhui Medical University; 2020.
24. Li YQ, Li WL, Yu XH, et al. Mechanisms of Traditional Chinese Medicine Bushenantai granules in promoting angiogenesis at the maternal-fetal interface of recurrent spontaneous abortion mice. J Traditional Chine Med. 2021;41(4):556–563.
25. Wang CG, Lou YT, Tong MJ, et al. Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1α/VEGF signaling. Acta Pharmacol Sin. 2018;39(3):393–404. doi:10.1038/aps.2017.161
26. Chang W. Effect of BuShen AnTai Granule on Expression of VEGF/Ras/MAPK Pathway in Placental Microvascular Endothelial Cells [Master’s Thesis]. Hefei: Anhui University Chinese Medicine.; 2021.
27. Li M, Ning N, Liu Y, et al. The potential of Zishen Yutai pills s to facilitate endometrial recovery and restore fertility after induced abortion in rats. Pharm Biol. 2021;59(1):1505–1516. doi:10.1080/13880209.2021.1993272
28. Zhu Y, Lu H, Huo Z, et al. MicroRNA-16 inhibits feto-maternal angiogenesis and causes recurrent spontaneous abortion by targeting vascular endothelial growth factor. Sci Rep. 2016;6(1):35536. doi:10.1038/srep35536
29. Liu HN, Tang XM, Wang XQ, et al. MiR-93 Inhibits Trophoblast Cell Proliferation and Promotes Cell Apoptosis by Targeting BCL2L2 in Recurrent Spontaneous Abortion. Reprod Sci. 2020;27(1):152–162. doi:10.1007/s43032-019-00003-w
30. Zhao L, Li J, Huang S. Patients with Unexplained Recurrent Spontaneous Abortion Show Decreased Levels of microRNA-146a-5p in the Deciduae. Ann Clin Lab Sci. 2018;48(2):177–182.
31. Liang Z, Xu J, Ma Z, et al. MiR-187 suppresses non-small-cell lung cancer cell proliferation by targeting FGF9. Bioengineered. 2020;11(1):70–80. doi:10.1080/21655979.2019.1706287
32. Chen X, Song QL, Ji R, et al. MiR-187 regulates the proliferation, migration and invasion of human trophoblast cells by repressing BCL6-mediated activation of PI3K/AKT signaling. Placenta. 2022;118:20–31. doi:10.1016/j.placenta.2022.01.001
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.