Back to Journals » Journal of Experimental Pharmacology » Volume 12
Zingiber officinale Ethanolic Extract Attenuated Reserpine-Induced Depression-Like Condition and Associated Hippocampal Aberrations in Experimental Wistar Rats
Authors Olanrewaju JA , Owolabi JO , Awodein IP , Enya JI , Adelodun ST, Olatunji SY, Fabiyi SO
Received 7 August 2020
Accepted for publication 12 October 2020
Published 2 November 2020 Volume 2020:12 Pages 439—446
DOI https://doi.org/10.2147/JEP.S275260
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Edythe London
John Afees Olanrewaju,1,2 Joshua Oladele Owolabi,1,3 Ifedamola Patience Awodein,1 Joseph Igbo Enya,2 Stephen Taiye Adelodun,1 Sunday Yinka Olatunji,1 Sunday Oluwaseyi Fabiyi1
1Department of Anatomy, Ben Carson School of Medicine, Babcock University, Ilishan-Remo, Ogun State, Nigeria; 2Department of Anatomy, Faculty of Basic Medical Sciences, University of Ilorin, Ilorin, Nigeria; 3Department of Anatomy, Division of Basic Medical Sciences, University of Global Health Equity, Butaro, Rwanda
Correspondence: Joshua Oladele Owolabi Email [email protected]
Background: Repeated and regimented treatment with reserpine causes depression-like condition characterized by persistent mood disorder, feelings of severe despondency and dejection, thus altering the hippocampal morphology. Our study compared a well-known antidepressant (fluoxetine), with the potential of Zingiber officinale to ameliorate reserpine-induced depression and the associated hippocampal cornu ammonis 1 (CA1) neuronal cell damage.
Methods: Forty-eight male Wistar rats, weighing 130– 160 g, were randomly assigned to 6 groups (n=8), housed in plastic cages under natural light and dark cycles at room temperature with access to feed and water ad libitum. Group-A (control) received distilled water. Group-B and Group-C orally received 400 mg/kg of Zingiber officinale and 10 mg/kg of fluoxetine, respectively, for 7 days, while Group-D intraperitoneally received 0.2 mg/kg of reserpine for 14 days. Group-E and Group-F intraperitoneally received 0.2 mg/kg of reserpine for 14 days followed by 400 mg/kg of Zingiber officinale and 10 mg/kg of fluoxetine respectively for 7 days. All animals were sacrificed by cervical dislocation at the end of experiment, and the brains hippocampi were dissected, excised and processed for various analyses including histology [H&E], histochemistry of GFAP expression by astrocytes and specific gene expressions including p53 gene, glutathione reductase (GSR), glutathione peroxidase and catalase (CAT).
Results: Reserpine significantly depleted the expression of P53 and glutathione reductase (GSR) genes while significantly increasing the expression of glutathione peroxidase 1 (GPx-1) gene (P≤ 0.05). Also, a marked increase in the expression of catalase (CAT) gene was observed. Furthermore, histoarchitecture (photomicrographs) of hippocampus CA1 region showed disruption in the arrangement of pyramidal neurons and alterations in their morphologies when animals were treated with reserpine (Group D). There was also accompanying increased astrocyte densities within the CA1 region following reserpine treatment. These features indicated deleterious effects of reserpine. Both Zingiber officinale and fluoxetine treatments ameliorated these effects.
Conclusion: These findings showed structural and molecular alterations associated with reserpine-induced depression. Also, Zingiber officinale was effective to provide ameliorative and protective effects against the neurotoxic effects of reserpine in the hippocampus, making it a potential candidate for treating depression and its associated neurodegenerative diseases.
Keywords: reserpine-induced depression, Zingiber officinale (ginger), neurotoxicity, hippocampus
Introduction
The absence of positive effect and a range of associated emotional, physical, cognitive and behavioral symptoms characterize depression, which is a mental health problem.1 It is a chronic illness and it affects mood, thought, physical health and behavior of any individual and has been estimated to affect over 21% of the world’s population.2 In most cases, it is very problematic trying to differentiate mood changes between depression occurring normally and what is reported as clinically significant degrees of depression, hence it is appropriate to consider the symptoms of depression as occurring.3,4
Depression ranges from mild, temporary episodes of sadness to severe persistent depression, and clinical depression also known as major depression or major depressive disorder is the more severe form of depression.5 A 2007 World Health Organization (WHO) research of over 200,000 adult’s participants across the world disclosed that depression produced the greatest reduction in the health and wellbeing of affected individuals when compared with chronic diseases such as arthritis and diabetes.6 Thus, depression globally is a significant public health challenge which is often comorbid with other chronic health problem and can worsen their outcomes.6
Serotonin (5-HT), dopamine (DA) and noradrenaline (NA) which are monoamine neurotransmitters, play critical roles in brain functions regulation such as motor control, social behaviour, cognition, sleep, appetite, and anxiety both in animals and humans.7–9 Reserpine, also known as raudixin, serpalan and serpasil, has been used for the control of high blood pressure, likewise the relief of psychotic symptoms.10 It is also highly significant in promoting the thought of a biogenic amine hypothesis of depression.11 Reserpine-propitiate reduction of monoamine (5-HT, DA and NA) neurotransmitters in the synapses is evidence that depletion of serotonin, dopamine and noradrenaline neurotransmitters causes depression in humans.11,12
However, natural medicinal plants could be important sources of novel antidepressant drugs and Zingiber officinale (ginger) on the other hand is a medicinal plant in a tuber form that is consumed as a delicacy, medicine, or herb. It has been suggested by locals in certain places that it has antidepressant properties. This is yet to be scientifically established. It also has several other medical uses. It has been reported to increase the motility of the gastrointestinal tract experimentally, and have analgesic, sedative, antipyretic and antibacterial properties. In this study, we studied the potential of the ethanolic extract of Zingiber officinale in attenuating reserpine-induced depression-like condition and its effects on the hippocampus of adult male Wistar rats.
Materials and Methods
Ethical Statement
All procedures and experiment in this study were done according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2010),13 and the study protocol was approved by Babcock University Health Research Ethics Committee (BUHREC) with approval number BUHREC 818/18.
Animals Care and Management
Forty-eight (48) healthy adult male Wistar rats (Rattus novegicus) weighing between 130 and 160 g (age 10 weeks) were procured from the animal house, Babcock University, Ilisan-Remo, Ogun State, Nigeria. The rats were randomly assigned to six (6) groups of eight rats each, housed in plastic cages with net covers for adequate ventilation, under natural light and dark cycles at room temperature with access to fed and water ad libitum. Also, the rats were allowed to acclimatize in the environment for seven days before the research commenced.
Reserpine and Fluoxetine Procurement
Reserpine and Fluoxetine used in this research were obtained from Sigma Aldrich, USA. Dried grinded Zingiber officinale was procured from Agege, Lagos, Nigeria.
Zingiber officinale Extraction
The dried Zingiber officinale was soaked in absolute ethanol for twenty-four hours.14 The lumps of the powdered Zingiber officinale were then sieved after twenty-four hours, using different sizes of strainer in order to discard the graded particles and separate the ethanol from the Zingiber officinale shaft. The ethanol is then allowed to properly settle for a day. Thus, the extract was transferred into a beaker and kept in a water bath within the temperature of about 40°c, allowing the ethanol to evaporate leaving the clumps of Zingiber officinale behind. These clumps were then removed from the beaker and properly dried before use.
Experimental Design and Drug Administration
This study was carried out using Forty-eight (48) rat randomly divided into six groups (n = 8), labeled A, B, C, D, E and F. Group-A (control) received distilled water, Group-B and Group-C orally received 400 mg/kg of Zingiber officinale and 10 mg/kg of fluoxetine, respectively, for 7 days, while Group-D intraperitoneally received 0.2 mg/kg of reserpine for 14 days. Group-E and Group-F intraperitoneally received 0.2 mg/kg of reserpine for 14 days followed by 400 mg/kg of Zingiber officinale and 10 mg/kg of fluoxetine respectively for 7 days. Administration of Zingiber officinale and fluoxetine was carried out orally using an oral cannula while that of reserpine was carried out intraperitoneally using an insulin syringe.
Animal Sacrifice and Tissue Processing
Experimental rats were sacrificed by cervical dislocation, 24 hours after the last administration of reserpine, fluoxetine and Zingiber officinale. From each group, two rats were perfused with 10% formal saline and the brain tissues were later fixed in 10% formalin for histological and immunohistological investigations while the others were not perfused and used for relative gene expression; p53 gene, glutathione reductase (GSR) gene, glutathione peroxidase 1 (GPx-1) gene and catalase (CAT) gene.
Histological and Immunohistochemical Examination
Four (4) days after fixation, thin slice coronal section of the hippocampus was obtained at the level of the optic chiasma, processed for rapid routine tissue processing and were stained for Haematoxylin and Eosin (H&E) in order to demonstrate the general histological appearance.15 The hippocampus was immunohistochemically stained for glia fibrillary acidic proteins (GFAP) for the demonstrations of astrocytic reactions.16 Histological and immunohistological slides were viewed using LEICA DM 750 microscope connected to a digital camera and a computer.
P53, GSR, GPx-1 and CAT Gene Expression Analysis
Gene expression analysis was done according to Omotuyi, et al.17 Thus, RNA was isolated from the thin slice coronal section of the hippocampus using TRIzol Reagent (ThermoFisher Scientific). DNA contaminant was removed following DNAse I treatment (ThermoFisher Scientific) following manufacturer’s protocol. Purified DNA-free RNA was converted to cDNA immediately using ProtoScript®First Strand cDNA Synthesis Kit (NEB). PCR amplification was done using OneTaq® 2X Master Mix (NEB).
Statistical Analysis
Study results were presented as Mean±SEM, analyzed using One-way ANOVA test for multiple comparison. GraphPad Prism® software (Version 5) was the statistical tool used in analysing data and the P-values ≤ 0.05 was considered statistically significant.
Results
Results for Relative Gene Expression
In Figure 1A, a significant decrease was seen in the relative expressions of p53 gene across all treated groups when compared to control (Group A). Also, p53 gene expression in Group F was significantly depleted when compared with Group B and Group C. The expressions of GSR gene as seen in Figure 1B also showed a significant depletion in Group D, Group E, and Group F when compared with Group A and Group B. Also, a marked reduction was seen in Group C when compared with Group A and Group B. The relative expressions of GPx-1 gene showed a significant increase in Group B, Group C, Group D and Group E as well as a marked increase in Group-E when compared with Group A. Group F showed an increase and a significant decrease when compared to Group A and Group B, respectively (see Figure 1C). Furthermore, a marked increase in relative expressions of CAT gene, in Group B and Group D when compared with the control (Group A). Group F significantly depleted when compared to Group B and Group D, as well as Group E and Group F obviously depleted when compared to control (see Figure 1D). The effects of Zingiber officinale on the expressions of the selected genes in the hippocampus of experimental animals mimicked the effects of the orthodox fluoxetine which is indicative of the former’s potential to ameliorate depression-related hippocampal changes.
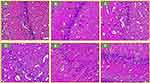
Histological Demonstration and Immunohistochemical GFAP Expression of Pyramidal Neurons in the Cornu Ammonis 1 (CA1) Region of the Hippocampus
Histological staining of tissues using Haematoxylin and Eosin, helped in studying tissue and cells within the hippocampal CA1 area and changes that are associated with potential or established pathological alterations. In this study, Figure 2 shows the general histoarchitecture of the CA1 region of the hippocampus. The control (Group-A) exhibited distinct neuronal cells and well-arranged pattern of the pyramidal cell with long axons in the CA1 region, similar finding was seen in the Group B and Group C. These observations collectively showed that the treatments in Group C and D were not deleterious, hence safe. Group D showed dispersion in the arrangement of pyramidal neurons, and degenerating features in the hippocampus CA1 region, suggesting a deleterious effect of reserpine. Also, the administration and therapeutic treatments using Zingiber officinale and (Group E) fluoxetine (Group F) showed evidence of ameliorative effects including well defined and arranged pyramidal cells with axons in the CA1 region, very similar to those of control.
Furthermore, the GFAP immunohistochemistry method was used in this study to demonstrate astrocyte morphology and distribution in the CA1 region of the hippocampus. The GFAP expressing astrocytes within the hippocampal CA1 region of the control rats (Group-A) and the rats treated with Zingiber officinale (Group B) and fluoxetine (Group-C) appeared relatively normal (blue arrow). However, slightly enhanced astrocytic GFAP expression was observable (red arrow) in the CA1 region of reserpine (Group D) treated rats. Therapeutic treatment using Zingiber officinale and (Group E) fluoxetine (Group F) showed evidence of amelioration that were attributable to the treatments with respect to relatively normal astrocytic processes and cellular distribution which were very similar to Group A.
Discussion
Reduced hippocampal volumes have been reported both in animal and human subjects with depression. As a result, many researchers have focused on the development of drugs, supplements and other substances from medicinal herbs and plants that may enhance cognitive activities, especially executive functions and long-term memory, under diseases or normal condition.18–20 Lim et al,21 reported that Zingiber officinale could facilitate learning task performances and executive function in both scopolamine-induced models and normal animal. Thus, this current study examined whether Zingiber officinale can ameliorate reserpine-induced depression and hippocampal damage.
Biomarker P53 (TP53 or tumor protein) initiates DNA repair and functions as a tumor suppressor gene that codes for a protein that regulates the cell cycle.22,23 Thus, we documented in this study a significantly depleted expression of the P53 gene following reserpine treatment which indicated an enhanced tendency of tumorigenesis or cellular trauma. Similarly, a significant reduction was observed in GSR expression following reserpine treatment. GSR gene encodes a member of the class-I pyridine nucleotide-disulfide oxidoreductase family,24 which is an essential enzyme of cellular antioxidant defense, and it also reduces oxidized glutathione disulfide to the sulfhydryl form Glutathione (an important cellular antioxidant).25,26 Hence, inhibition of GSR in an organism could account for the activation of reactive oxygen species. Under normal conditions, Gpx-1 plays a significant role in minimizing oxidative damage and its main biological role is to protect the organism from oxidative stress damage, which makes it a very important antioxidant for a healthy wellbeing.27,28
In this study, there was a significant increase in the expression of GPx-1 following reserpine treatment, like wise in Zingiber officinale and fluoxetine treated rat when compared to control. Various studies have reported that overexpression of Gpx-1 delays cell growth without affecting viability or decreasing resistance to hydrogen peroxide-induced oxidative stress.29,30 Thus, supplementation with antioxidants which act as free radical scavengers seems to be indispensable for sustaining oligodendroglial functions. Literature data from various biological and clinical studies have reported Zingiber officinale to have analgesic, anti-inflammatory, anti-hyperglycemic, and neuroprotective action.21,31 The therapeutic treatment with Zingiber officinale (Group E) showed a mark reduction in GPx-1 gene expression, which was very similar when compared to control.
In furtherance, CAT is an essential antioxidant which to a considerable extent, attenuates oxidative stress by destroying cellular hydrogen peroxide to produce H2O and O2.32,33 There was a slight increase which was not significant in the expression of CAT in reserpine treated rats’ hippocampi when compared to control group. Hence, it is postulated that CAT malfunction correlates with the pathogenesis of numerous age-related deteriorating diseases such as Alzheimer’s disease, Parkinson’s disease, diabetes mellitus, hypertension, anemia, bipolar disorder and cancer.33,34 Therapeutic treatment with Zingiber officinale (Group-E) ameliorated this deficit associated with reserpine administration (see Figure 1D).
With specific reference to histological features of the hippocampus, the characteristic cell of the hippocampal Cornu Ammonis (CA) is the pyramidal cell, and the cell of the CA1 region in this study exhibited dispersion in arrangement, vacuolation and degenerating features following reserpine treatment only. Alterations in cellular morphology of this manner may lead to loss of signal processing, neuronal timing and synaptic efficacy in the hippocampus, and are often associated with long-term memory deteriorative conditions such as depression and Alzheimer’s diseases.35 Features that are typical of degeneration and inflammation were absent in the control- Group A, Group B and Group C. As seen in Figure 2, the control (Group A), Group B and Group C showed distinct pyramidal neurons and well-arranged pattern of the pyramidal cell with long axons which suggest normal synaptic connections, unlike Group D which showed signs of morphological alterations of certain cells and general disruption of the pattern of spatial cellular distribution in the CA1. These signs of potential vacuolation and degeneration of pyramidal neurons as seen in the Group D were absent in the Group E and the Group F that received Zingiber officinale and fluoxetine respectively as therapeutic treatments for reserpine-induced depression. These observations would, therefore, provide evidence in support of the attenuating the potentials of Zingiber officinale against neuroinflammation and neuronal degeneration caused by reserpine administration which is attributable to its antioxidant content.
Astrocytes consist of the largest glial population, occupying the spaces between neurons and playing important roles in regulating the extracellular space and restricting the spread of released neurotransmitter molecules.36,37 The current study showed (Figure 3), normal astrocytes, expressing GFAP in the control (Group A), Group B and Group C. These hippocampi were characterized by clearly expressed astrocytes, with regular sized and numerous processes, which form an array of network within the neurophil. This would further show that Zingiber officinale would not ordinarily induce neuroinflammation in the hippocampus, hence safe for the tissue. To further buttress this point, neuronal morphology in these groups appeared normal, with several of them observed around the branched astrocytic processes. On the contrary, increased expression of glial fibrillary acidic protein (GFAP) account for astrogliosis or astrocyte reactivity which manifests as cell swelling, hypertrophy of cellular process and hyperplasia. Interestingly, CA1 morphology of rat treated with reserpine (Group-D) showed increased astrocytic densities with reactive astroglia and hypertrophic cells, supposedly as a result of neuronal injury which might lead to astrogliosis. However, such-enhanced expression of GFAP-in-astrocytes were absent in the Group E and Group F that received Zingiber officinale and fluoxetine respectively as therapeutic treatment against reserpine-induced depression. This indicated the neuroprotective effects of Zingiber officinale against reserpine-induced hippocampal neurotoxicity, which is attributable to its antioxidant potentials. These findings are, therefore, in line with several other studies in humans and animals that have shown similar evidences that Zingiber officinale could ameliorate numerous deleterious effects of certain agents.18–20
Conclusion
The results of this study showed the structural and molecular alterations that are associated with reserpine-induced depression. Zingiber officinale was capable of offering natural therapeutic remedy to neurotoxic effects of reserpine, making its active ingredients potential candidates for treating depression and its associated neuroinflammation and neurodegenerative effects on the brain and its hippocampus with a level of potency that favourably compares with the orthodox medicinal uses of fluoxetine.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Keyes CLM. The mental health continuum: from languishing to flourishing in life. J Health Soc Behav. 2002;43(2):207–222. doi:10.2307/3090197
2. Govindarajan VS, Connell DW. Ginger — chemistry, technology, and quality evaluation: part 1. Crit Rev Food Sci Nutr. 1983;17(1):1–96. doi:10.1080/10408398209527343
3. Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. J Abnorm Psychol. 2000;109(2):345. doi:10.1037/0021-843X.109.2.345
4. National Collaborating Centre for Mental Health (UK. Depression: The Treatment and Management of Depression in Adults (Updated Edition). British Psychological Society; 2010.
5. Divya Mary SM, Kirupa K, Vaishnavi G, et al. Effects of total body workout in women with mild-to-moderate clinical depression. Drug Invent Today. 2019;11(5).
6. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi:10.1016/S0140-6736(07)61415-9
7. August JT, Murad F, Anders MW, et al. Catecholamines: Bridging Basic Science with Clinical Medicine. Vol. 42. Academic Press; 1997.
8. Lillesaar C. The serotonergic system in fish. J Chem Neuroanat. 2011;41(4):294–308. doi:10.1016/j.jchemneu.2011.05.009
9. Kyzar E, Stewart AM, Landsman S, et al. Behavioral effects of bidirectional modulators of brain monoamines reserpine and d-amphetamine in zebrafish. Brain Res. 2013;1527:108–116. doi:10.1016/j.brainres.2013.06.033
10. Subramani R, Narayanasamy M, Feussner K-D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. Biotech. 2017;7(3):172. doi:10.1007/s13205-017-0848-9
11. Barchas J, Altemus M. Monoamine hypotheses of mood disorders. Basic Neurochem. 1999.
12. Hinz M, Stein A, Uncini T. Monoamine depletion by reuptake inhibitors. Drug Healthc Patient Saf. 2011;3:69. doi:10.2147/DHPS.S24798
13. National Research Council. Guide for the Care and Use of Laboratory Animals: Eight Edition. Washington, DC: The National Academies Press. 2010;1–198.
14. Santo Grace U, Sankari M. Antimicrobial activity of ethanolic extract of Zingiber Officinale-an in vitro Study. J Pharm Sci Res. 2017;9(9):1417.
15. Omotoso GO, Arietarhire L, Ukwubile I, Gbadamosi I. The protective effect of kolaviron on molecular, cellular and behavioral characterization of cerebellum in rat model of demyelinatiing diseases. Basic Clin Neurosci. 2018; 28:1–31 In press.
16. Olajide OJ, Ugbosanmi AT, Enaibe BU, et al. Cerebellar molecular and cellular characterization in rat models of alzheimer’s disease: neuroprotective mechanisms of garcinia biflavonoid complex. Ann Neurosci. 2017;24(1):32–45. doi:10.1159/000464421
17. Omotuyi OI, Nash O, Inyang OK, et al. Flavonoid-rich extract of Chromolaena odorata modulate circulating GLP-1 in Wistar rats: computational evaluation of TGR5 involvement. Biotech. 2018;8(2):124. doi:10.1007/s13205-018-1138-x
18. Jagetia G, Baliga M, Venkatesh P. Ginger (Zingiber officinale Rosc.), a dietary supplement, protects mice against radiation- induced lethality: mechanism of action. Cancer Biother Radiopharm. 2004;19(4):422–435. doi:10.1089/cbr.2004.19.422
19. Reddy YA, Chalamaiah M, Ramesh B, Balaji G, Indira P. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sci Technol. 2014;51(5):908–914. doi:10.1007/s13197-011-0568-9
20. Alshathly MR. Efficacy of ginger (Zingiber officinale) in ameliorating streptozotocin-induced diabetic liver injury in rats: histological and biochemical studies. J Microsc Ultrastruct. 2019;7(2):91. doi:10.4103/JMAU.JMAU_16_19
21. Lim S, Moon M, Oh H, Kim HG, Kim SY, Oh MS. Ginger improves cognitive function via NGF-induced ERK/CREB activation in the hippocampus of the mouse. J Nutr Biochem. 2014;25(10):1058–1065. doi:10.1016/j.jnutbio.2014.05.009
22. Shu K-X, Li B, Wu L-X. The p53 network: p53 and its downstream genes. Colloids Surf B Biointerfaces. 2007;55(1):10–18. doi:10.1016/j.colsurfb.2006.11.003
23. Cherdyntseva NV, Gervas PA, Litvyakov NV, et al. Age-related function of tumor suppressor gene TP53: contribution to cancer risk and progression. Exp Oncol. 2010.
24. O’grady GL, Best HA, Sztal TE, et al. Variants in the oxidoreductase PYROXD1 cause early-onset myopathy with internalized nuclei and myofibrillar disorganization. Am J Hum Genet. 2016;99(5):1086–1105. doi:10.1016/j.ajhg.2016.09.005
25. Wang Z, Lu B, Sun L, Yan X, Xu J. Identification of candidate genes or microRNAs associated with the lymph node metastasis of SCLC. Cancer Cell Int. 2018;18(1):161. doi:10.1186/s12935-018-0653-5
26. Manda G, Postolache C, Neagoe IV, Csolti A, Milanesi E, Dobre M. The expression profile of redox genes in human monocytes exposed in vitro to γ radiation. Radiat Phys Chem. 2020;170:108634. doi:10.1016/j.radphyschem.2019.108634
27. Esposito LA, Kokoszka JE, Waymire KG, Cottrell B, MacGregor GR, Wallace DC. Mitochondrial oxidative stress in mice lacking the glutathione peroxidase-1 gene. Free Radic Biol Med. 2000;28(5):754–766. doi:10.1016/S0891-5849(00)00161-1
28. Spanier G, Xu H, Xia N, et al. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4). J Physiol Pharmacol. 2009;60(Suppl 4):111–116.
29. Cheng W-H, Ho Y-S, Ross DA, Han Y, Combs GF
30. Faucher K, Rabinovitch-Chable H, Barriere G, Cook-Moreau J, Rigaud M. Overexpression of cytosolic glutathione peroxidase (GPX1) delays endothelial cell growth and increases resistance to toxic challenges. Biochimie. 2003;85(6):611–617. doi:10.1016/S0300-9084(03)00089-0
31. Jittiwat J, Wattanathorn J. Ginger pharmacopuncture improves cognitive impairment and oxidative stress following cerebral ischemia. J Acupunct Meridian Stud. 2012;5(6):295–300. doi:10.1016/j.jams.2012.09.003
32. Krishnamurthy P, Wadhwani A. Antioxidant enzymes and human health. Antioxid Enzyme. 2012;1–17.
33. Nandi A, Yan LJ, Jana CK, Das N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxid Med Cell Longev. 2019;2019.
34. Serra JA, Domínguez RO, Marschoff ER, Guareschi EM, Famulari AL, Boveris A. Systemic oxidative stress associated with the neurological diseases of aging. Neurochem Res. 2009;34(12):2122. doi:10.1007/s11064-009-9997-5
35. Ashaari Z, Hassanzadeh G, Mokhtari T, et al. Luteolin reduced the traumatic brain injury-induced memory impairments in rats: attenuating oxidative stress and dark neurons of Hippocampus. Acta Med Iran. 2018;56(9):570.
36. Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35.
37. Araque A, Navarrete M. Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci. 2010;365(1551):2375–2381. doi:10.1098/rstb.2009.0313
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.