Back to Journals » OncoTargets and Therapy » Volume 13
Yes-Associated Protein Contributes to Cell Proliferation and Migration of Gastric Cancer via Activation of Gli1
Authors Han T, Cheng Z, Xu M, Wang X, Wu J, Fang X
Received 6 June 2020
Accepted for publication 4 September 2020
Published 27 October 2020 Volume 2020:13 Pages 10867—10876
DOI https://doi.org/10.2147/OTT.S266449
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Ting Han,1 Zhengwu Cheng,1 Menglin Xu,2 Xiaoming Wang,3 Jian Wu,1 Xiaosan Fang3
1Department of Gastrointestinal Surgery, The First Affiliated Hospital of Wannan Medical College, Wuhu 241000, People’s Republic of China; 2Department of Oncology, The First Affiliated Hospital of Wannan Medical College, Wuhu 241000, People’s Republic of China; 3Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wannan Medical College, Wuhu 241000, People’s Republic of China
Correspondence: Xiaosan Fang
Department of Hepatobiliary Surgery, The First Affiliated Hospital of Wannan Medical College, No. 2 Zheshan West Road, Jinghu District, Wuhu 241000, Anhui Province, People’s Republic of China
Email [email protected]
Objective: In the present study, we aimed to explore the potential oncogenic property and the internal mechanism of yes-associated protein (YAP) in gastric cancer (GC).
Materials and Methods: YAP protein levels were evaluated in human GC tissues and paired normal tissues using immunohistochemistry (IHC). The role of YAP in regulating GC cell proliferation and migration was verified by genetic manipulation in vitro. Western blot analysis was used to determine the molecular signaling to explain the mechanism of the observed YAP effects in GC.
Results: Nuclear YAP protein expression was upregulated in GC tissues, and high nuclear YAP level was significantly correlated with lymph node metastasis (LNM) and tumor node metastasis (TNM) stage in patients suffered from GC. YAP knockdown inhibited GC cell proliferation, migration and epithelial–mesenchymal transition (EMT) progress in vitro, whereas YAP elevation did the opposite. YAP regulated glioma-associated oncogene-1 (Gli1) expression independent of smoothened homolog (SMO). YAP modulated protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) signaling pathway in GC cells.
Conclusion: YAP enhanced GC cell proliferation and migration potentially via its regulation of Gli1 expression through the non-classical Hedgehog pathway, indicating suppression of YAP/Gli1 signaling axis may highlight a new entry point for combination therapy of GC.
Keywords: YAP, proliferation, migration, Hedgehog pathway, gastric cancer
Introduction
Gastric cancer (GC) is the one of the leading causes of cancer-related mortality in the world, with high incidence and high-grade malignancy, which imposes a considerable global health burden.1,2 Most gastric cancers are diagnosed at advanced or metastatic stages, resulting in low 5-year survival rate.2 Hence, it is of utmost importance to identify sensitive biomarkers for early diagnosis of GC and to explore effective therapeutic targets to prevent the metastasis of GC.
Yes-associated protein (YAP) is a downstream effector of the Hippo pathway and can interact with the TEAD and other transcriptional factors to promote the transcription of genes mediating organ size and tissue homeostasis.3,4 In addition, YAP has recently gained much attention in tumor growth and metastasis and is identified as a candidate oncogene.3–5 YAP protein levels were reported to be frequently overexpressed in multiple types of malignancies including hepatocellular carcinoma,6,7 esophageal cancer,8 breast cancer,9 ovarian cancer,10 prostate cancer,11 colorectal cancer,12,13 pancreatic cancer14 and GC.15 In cancers, overexpression of YAP has been reported to be involved in tumor cell proliferation, metastasis and epithelial–mesenchymal transition (EMT).11,12,16 Recent studies showed that YAP was highly expressed in GC and its overexpression exhibits oncogenic property in GC,16–19 but the precise function and internal mechanisms of YAP in GC remain to be further explored.
The Hedgehog signal pathway is considered to be crucially involved in the development and progression of numerous types of malignant tumors, including GC.20,21 The canonical Hedgehog pathway involves G-coupled receptor-like signal transducer Smoothened homolog (SMO), suppressor of fused homolog (SUFU) and the zinc-finger transcription factors glioma-associated oncogene (Gli).22 The ligands bind and inactivate the Hedgehog receptor, protein patched homolog 1 (PTCH1), leading to the release of the SMO; Released SMO then activates Gli by blocking their inhibitory partner SUFU.23 Besides, the Gli transcription factors, mainly Gli1, can also be activated by other molecules and signaling independently of SMO, including AKT, Mitogen-activated protein kinase (MAPK)/Extracellular signal-regulated kinase (ERK) and mTOR/Ribosomal protein S6 kinase 1 (S6K1), which is termed non-canonical Hedgehog signaling pathway.23,24 Although the classical pathway has been well illustrated, how Gli1 is regulated by a SMO-independent way is still equivocal.
Herein, we compared the nuclear YAP expression in GC tissues and non-cancerous tissues and investigated the association between nuclear YAP expression and the clinicopathological features of patients with GC. We then performed in vitro experiments to assess the effects of YAP on GC cell proliferation, migration and EMT progress. Moreover, we determined its effects on regulation of Gli1 and AKT/mTOR signaling pathway. Our present study suggests that YAP-mediated Gli1 activation which may be implicated by AKT/mTOR pathway contributes to GC cell proliferation and migration, with potential usefulness for new strategies to GC therapy.
Materials and Methods
GC Tissues and Cell Lines
From January 2016 to January 2018, 68 pairs of primary GC tumors and surrounding normal tissues were obtained from patients who underwent surgical resection in the First Affiliated Hospital of Wannan Medical College. The clinicopathological features of these patients are shown in Table 1. All patients underwent radical resection without either chemotherapy or radiotherapy before surgery. Written informed consent was obtained from all patients. This study was conducted in compliance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Wannan Medical College.
 |
Table 1 Relationships Between YAP Expression and Clinicopathological Features in 68 Patients with GC |
The human GC cell lines SGC7901, MGC803, MKN45 and AGS were purchased from the Cell Bank of the Chinese Academy of Sciences, Shanghai, China. All the cells were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% penicillin/streptomycin (P/S; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cells were incubated at 37°C in a humidified atmosphere containing 5% CO2.
Immunohistochemistry (IHC)
IHC for YAP was performed according to the manufacturer’s instructions using rabbit anti-human YAP at 1:200 (#14074, Cell Signaling Technology (CST)). The final staining score was determined by color intensity and positive cell rate, ranging 0–12 which was described in our previous study.25 Patients were classified into two groups: scores 0–4 were considered as none or low, while 5–12 were considered as high expression.
Protein Extraction and Western Blot Analysis
Whole protein extracts were lysed using RIPA lysis buffer (Beyotime, Beijing, China) supplemented with protease inhibitors (Roche, CA, USA) according to manufacturer’s protocol. The proteins were separated by SDS-PAGE and transferred onto PVDF membranes, which were then blocked with TBS buffer containing 5% skim milk for 1 h at room temperature. After incubation with primary and secondary antibodies, the proteins were visualized by chemiluminescence. Antibodies used in this study are as follows: anti-YAP (dilution 1:1000; #14074, CST), anti-p-AKT (Ser473) (dilution 1:1000; #4058, CST), anti-AKT (dilution 1:1000; #9272, CST), anti-p-mTOR (dilution 1:1000; #9205, CST), anti-mTOR (dilution 1:1000; #2983, CST), anti-E-cadherin (dilution 1:500; #3195, CST), anti-Vimentin (dilution 1:500; #5741, CST), anti-Gli1 (dilution 1:1000; #ab15179, Abcam), anti-SMO (dilution 1:1000; #ab38686, Abcam) and anti-glyceraldehyde 3-phosphate dehydrogenase (dilution 1:5000; GAPDH, #AG019, Beyotime).
Construction of Stable Cell Lines
GC cell lines stably expressing YAP-specific short hairpin RNA (shRNA) and a plasmid encoding human YAP were constructed to silence and upregulate the expression of YAP, respectively, using a lentivirus technique (GenePharma, Shanghai, China). Lentiviral transduction was performed according to the manufacturer’s instructions. The human YAP shRNA target sequences are 5′-GGU CAG AGA UAC UUC UUA AAU-3′.
Transfection of Small Interfering RNA (siRNA)
Transfection of human Gli1-specific siRNA was performed using LipofectamineTM RNAiMax (Invitrogen) at a final concentration of 20 nM, as described in our previous study.25 The siRNA against human Gli1 (5ʹ-CUCCACAGGCAUACAGGAU-3ʹ) was synthesized by GenePharma (Shanghai, China). A scrambled siRNA (5ʹ-UUCUCCGAACGUGUCACGUTT-3ʹ) was used as a negative control.
Colony Formation Assay and Migration Assay
In total, about 1000 cells were placed in 6-well plates. After incubating for 7–10 days, the cells were fixed with 4% paraformaldehyde (Beyotime, Beijing, China), stained with 0.1% crystal violet (Beyotime, Beijing, China), and the images were captured by a digital camera (Nikon Corporation; Tokyo, Japan).
Transwell migration assay was used in this study. Detailed protocol was described in our previous study.25
Statistical Analysis
Data are presented as mean ± standard error of the mean (SEM). Student’s t-test (paired or unpaired, two-tailed) and chi-square test were used in this study. Results with P < 0.05 were considered as statistically significant.
Results
Upregulation of Nuclear YAP Protein Expression in Human GC Tissues
Previous studies have described that YAP was highly expressed in GC and its overexpression exhibits oncogenic property in GC.15–17 To further verify the oncogenic role of YAP in GC, we first conducted IHC analysis to measure the level of YAP protein in 68 pairs of GC tissues and surrounding normal tissues. Since the nuclear localization is critical in determining the function of YAP,4 we only scored the nuclear staining of YAP. Results revealed that marked nuclear immunoreactivity was seen in 71.1% (50/68) of the GC tissues, whereas only 36.8% (25/68) of paired normal tissues presented high nuclear staining of YAP (Figure 1A and B). The difference was statistically significant (P < 0.001, Figure 1B). Besides, IHC analysis also showed that nuclear YAP expression in GC patients suffered from LNM was further upregulated compared to those without LNM (P < 0.001, Figure 1A and C).
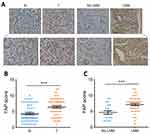 |
Figure 1 High YAP protein expression in human GC. |
Next, chi-square test was used to analyze the correlation between nuclear YAP expression and clinicopathological features in 68 patients with GC. Results showed that high nuclear YAP expression was significantly associated with LNM (P = 0.001, Table 1) and TNM stage (P = 0.012, Table 1), but was not associated with age, gender, tumor size, tumor location or depth of invasion (P > 0.05, Table 1).
These above data revealed that nuclear YAP is upregulated in human GC tissues and may function as an oncogene in the development of GC.
YAP Knockdown and Overexpression Efficiency in GC Cells
To investigate the potential oncogenic effects of YAP in GC cells, we first conducted Western blot analysis to detect the level of YAP protein expression in four human GC cell lines (SGC7901, MGC803, MKN45, AGS). Results showed high level of YAP protein expression in MGC803 and AGS cell lines, while there was lower YAP protein expression in SGC7901 cells and nearly no YAP expression in MKN45 cells (Figure 2A).
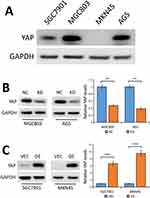 |
Figure 2 YAP protein levels in human GC cell lines. |
We then stably knocked down YAP expression using a lentivirus vector-based shRNA technique in MGC803 and AGS cell lines and the knockdown efficiency was tested by Western blot analysis. Results showed that YAP protein expression was markedly reduced in MGC803 and AGS cells stably transfected with shRNA against YAP (KD) compared to cells transfected with control-shRNA (NC) (P<0.01, Figure 2B). Next, we stably transfected SGC7901 and MKN45 cells with a plasmid encoding human YAP and YAP protein expression was statistically enhanced in cells stably transfected with human YAP (OE) compared with cells transfected with empty vector (VEC) (P<0.001, Figure 2C).
YAP Enhances GC Cell Proliferation and Migration in vitro and Regulates EMT Progress
Since YAP expression is elevated in GC tissues and correlates with disease stages, we speculated that YAP may contribute to the development of GC. As suspected, depletion of YAP weakened the ability of AGS cells to form foci as determined by the colony formation assays (P<0.01, Figure 3A). On the contrary, ectopic expression of YAP promoted the proliferation of MKN45 cells (P<0.01, Figure 3B). In vitro Transwell assays were conducted to assess the effect of YAP on the migration of AGS and MKN45 cells. As shown in Figure 3C, silencing YAP significantly impaired the migration ability of AGS cells (P<0.01). Inversely, YAP overexpression markedly increased the migration ability in MKN45 cells (P<0.01, Figure 3D).
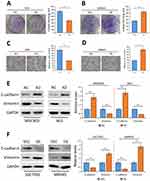 |
Figure 3 YAP promotes GC cell proliferation and migration in vitro. |
Considering that YAP contributes to GC metastasis and EMT is an early event in the metastasis of cancer,26,27 we speculated that YAP may be involved in regulating EMT progress in GC. As expected, results of Western blotting showed that silencing YAP significantly enhanced the protein expression of the epithelial marker E-cadherin and decreased Vimentin protein expression, a mesenchymal marker, in both MGC803 and AGS cells (Figure 3E). Conversely, elevation of YAP exerted the opposite effect on EMT markers (Figure 3F).
YAP Regulates GC Cell Proliferation and Migration via SMO-Independent Gli1 Activation
Given the evidence that aberrant Gli1 expression in Hedgehog pathway contributes to the development of GC,20,21 we asked whether YAP plays a part in modulating Gli1 expression on the development and progression of GC. Results from Western blot assays revealed that YAP knockdown in MGC803 and AGS cells significantly attenuated Gli1 protein level (Figure 4A) while YAP elevation notably enhanced Gli1 protein expression in SGC7901 and MKN45 cells (Figure 4B), indicating that YAP regulates Gli1 expression in GC cells.
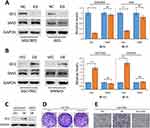 |
Figure 4 YAP regulates Gli1 expression in a SMO-independent manner. |
In canonical mammalian Hedgehog signaling, SMO is the central signal transducer, which activates Gli1 through blocking its inhibitory partner, SUFU.23 Surprisingly, the effect of YAP knockdown or overexpression on SMO protein level was minimal in GC cells (Figure 4A and B), suggesting the possible involvement of other molecules or signaling regulated by YAP which is responsible for the activation of Gli1.
To further explore whether Gli1 is implicated in YAP regulation of GC cell proliferation and migration, we used Gli1-specific siRNA (siGli1) to silence Gli1 expression in SGC7901 cells with YAP overexpression. Colony formation assays and migration assays showed that the increased proliferation and migration ability induced by YAP overexpression was partially reversed when cells lacked Gli1 (Figure 4D and E), indicating that YAP regulates GC cell proliferation and migration via regulating Gli1.
YAP Regulates AKT/mTOR Pathway in GC Cells
Considering that YAP modulates AKT/mTOR pathway to regulate cell size and tissue growth in MCF10A cells28 and hepatocellular carcinoma cells,29 and an activated AKT/mTOR pathway directly activates Gli1 independent of SMO,23 we then asked whether AKT/mTOR pathway is implicated in YAP-mediated Gli1 activation. Results from Western blot analysis showed that YAP depletion in MGC803 and AGS cells significantly reduced phosphorylated AKT (p-AKT) and phosphorylated mTOR (p-mTOR) protein levels, but not their total levels (Figure 5A). In contrast, YAP elevation notably resulted in an activation of AKT accompanied by an increase of mTOR phosphorylation in SGC7901 and MKN45 cells (Figure 5B). Collectively, our results indicated that YAP acts as a regulator on AKT/mTOR pathway in GC cells, which may be responsible for the activation of Gli1.
 |
Figure 5 YAP modulates AKT/mTOR pathway. |
Discussion
YAP, as a downstream effector of the Hippo pathway, has recently been proposed as a candidate oncogene.3–5 Ectopic YAP expression was reported in several types of cancers,6–15 and its overexpression was implicated in promoting tumor cell proliferation, metastasis and EMT.11,12,16 In GC, YAP was also showed to be upregulated and involved in the development and progression of GC,16–19 but the internal mechanism of YAP-mediated regulation of cell proliferation and migration in GC is not well understood and remains to be further explored.
In the present study, we confirmed that nuclear YAP was highly expressed in GC tissues compared to the adjacent normal tissues. We also evidenced that nuclear YAP expression level in GC patients with LNM was higher than those without LNM. Moreover, clinical data analyses revealed that high nuclear YAP expression was significantly correlated with LNM and TNM stage in patients suffered from GC. Our in vitro experiments provided further evidence that silencing YAP significantly weakened the proliferation and migration ability of GC cells, while YAP elevation did the opposite, suggesting the oncogenic property of YAP in GC. In view of the vital role of EMT progress in tumor metastasis,26,27 we then investigated the effect of YAP on EMT in GC cells. Results from Western blot showed that YAP promoted EMT progress evidenced by the fact that YAP depletion increased E-cadherin and decreased Vimentin protein expression while YAP overexpression had the opposite effect on EMT markers in GC cells.
Gli1, as an important downstream effector of the Hedgehog signal pathway, has been considered to be crucially involved in the development and progression of GC.20,21 Our results suggest that YAP played a role in modulating Gli1 expression evidenced by several observations. Knockdown of YAP markedly diminished Gli1 protein expression in cultured GC cells. Inversely, YAP elevation notably increased Gli1 protein expression in GC cells. The regulation of Gli1 by YAP is unlikely due to canonical Hedgehog signaling since the central signal transducer SMO, which activates Gli1 through blocking its inhibitory partner SUFU, was not affected by YAP knockdown or overexpression, suggesting the possible involvement of other molecules or signaling which is responsible for the YAP-mediated Gli1 activation.
Given the evidence that YAP modulates AKT/mTOR pathway to regulate cell size and tissue growth in MCF10A cells28 and in hepatocellular carcinoma cells,29 and an activated AKT/mTOR pathway directly activates Gli1 independent of SMO,23 we speculated that AKT/mTOR pathway may be involved in YAP-mediated Gli1 activation. In accordance with the previous studies,27,28 we provided first evidence that in GC cells YAP acts as a regulator on AKT/mTOR pathway, which may be responsible for the activation of Gli1.
The crosstalk of Hippo-YAP pathway and Hedgehog pathway has been reported previously. YAP is amplified and up-regulated in hedgehog-associated medulloblastomas and induces expression of Gli2, which translocates to the nucleus and then regulates Gli1 transcription.30 Another study demonstrated that Hedgehog signaling regulates YAP activity and verified YAP as a downstream effector of the Hedgehog pathway in regenerating mouse Liver.31 In the present study, we found that YAP regulated Gli1 expression in a SMO-independent manner and AKT/mTOR pathway may be implicated in YAP-mediated Gli1 activation in view of the positive regulation of AKT/mTOR signaling pathway by YAP in GC cells.
Taken together, our in vitro experiments not only indicated that nuclear YAP was highly expressed in GC tissues but also emphasized its role in regulating GC cell proliferation, migration and EMT. Moreover, we revealed a critical mechanism for YAP in regulation of GC cell proliferation and migration via its participation in modulating Gli1 expression through the non-classical Hedgehog signaling pathway. Considering the interreaction between Hippo-YAP pathway and Hedgehog pathway, suppression of YAP/Gli1 signaling axis may highlight a new entry point for combination therapy of GC.
Funding
This study was supported by the Nature and Science Fund from Wannan Medical College, CN (Grant Nos. WK2019F07, WK2019F09).
Disclosure
All authors declare that they have no conflict of interest.
References
1. Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260. doi:10.1111/apt.12814
2. Van Cutsem E, Sagaert X, Topal B, et al. Gastric cancer. Lancet. 2016;388:2654–2664. doi:10.1016/S0140-6736(16)30354-3
3. Qiao Y, Li T, Zheng S, et al. The Hippo pathway as a drug target in gastric cancer. Cancer Lett. 2018;420:14–25. doi:10.1016/j.canlet.2018.01.062
4. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015;15(2):73–79. doi:10.1038/nrc3876
5. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell. 2016;29:783–803. doi:10.1016/j.ccell.2016.05.005
6. Tschaharganeh DF, Chen X, Latzko P, et al. Yes-associated protein upregulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144(7):1530–1542. doi:10.1053/j.gastro.2013.02.009
7. Zhang S, Zhou D. Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol. 2019;61:64–71. doi:10.1016/j.ceb.2019.07.006
8. Song S, Honjo S, Jin J, et al. The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21(11):2580–2590. doi:10.1158/1078-0432.CCR-14-2191
9. Tufail R, Jorda M, Zhao W, et al. Loss of yes associated protein (YAP) expression is associated with estrogen and progesterone receptors negativity in invasive breast carcinomas. Breast Cancer Res Treat. 2011;131:743–750. doi:10.1007/s10549-011-1435-0
10. Cho SY, Kim K, Park MS, et al. Expression of yes-associated protein 1 and its clinical significance in ovarian serous cystadenocarcinoma. Oncol Rep. 2017;37(5):2620–2632. doi:10.3892/or.2017.5517
11. Jin X, Zhao W, Zhou P, et al. YAP knockdown inhibits proliferation and induces apoptosis of human prostate cancer DU145 cells. Mol Med Rep. 2018;17(3):3783–3788.
12. Ling HH, Kuo CC, Lin BX, et al. Elevation of YAP promotes the epithelial-mesenchymal transition and tumor aggressiveness in colorectal cancer. Exp Cell Res. 2017;350(1):218–225. doi:10.1016/j.yexcr.2016.11.024
13. Zhang S, Wei Q, Yang Y, et al. Loss of yes-associated protein represents an aggressive subtype of colorectal cancer. J Cancer. 2019;10(3):689–696. doi:10.7150/jca.28333
14. Yoo W, Lee J, Jun E, et al. The YAP1-NMU axis is associated with pancreatic cancer progression and poor outcome: identification of a novel diagnostic biomarker and therapeutic target. Cancers (Basel). 2019;11(10):1477. doi:10.3390/cancers11101477
15. Shao DD, Xue W, Krall EB, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158(1):171–184. doi:10.1016/j.cell.2014.06.004
16. Kang W, Tong JH, Chan AW, et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17(8):2130–2139. doi:10.1158/1078-0432.CCR-10-2467
17. Sun D, Li X, He Y, et al. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget. 2016;7(49):81062–81076. doi:10.18632/oncotarget.13188
18. Choi W, Kim J, Park J, et al. YAP/TAZ initiates gastric tumorigenesis via upregulation of MYC. Cancer Res. 2018;78(12):3306–3320.
19. Liu H, Mei D, Xu P, et al. YAP promotes gastric cancer cell survival and migration/invasion via the ERK/endoplasmic reticulum stress pathway. Oncol Lett. 2019;18(6):6752–6758.
20. Taipale J, Beachy PA. The Hedgehog and Wnt signalling pathways in cancer. Nature. 2011;411:349–354. doi:10.1038/35077219
21. Fukaya M, Isohata N, Nakanishi Y, et al. Hedgehog signal activation in gastric pit cell and in diffuse-type gastric cancer. Gastroenterology. 2006;131:14–29. doi:10.1053/j.gastro.2006.05.008
22. Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11(7):493–501. doi:10.1038/nrc3079
23. Wang Y, Ding Q, Yen CJ, et al. The crosstalk of mTOR/S6K1 and Hedgehog pathways. Cancer Cell. 2012;21(3):374–387. doi:10.1016/j.ccr.2011.12.028
24. Teperino R, Aberger F, Esterbauer H, et al. Canonical and non-canonical Hedgehog signalling and the control of metabolism. Semin Cell Dev Biol. 2014;33:81–92. doi:10.1016/j.semcdb.2014.05.007
25. Cheng Z, Shao X, Xu M, et al. Rab1A promotes proliferation and migration abilities via regulation of the HER2/AKT-independent mTOR/S6K1 pathway in colorectal cancer. Oncol Rep. 2019;41(5):2717–2728.
26. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal. 2014;7(344):re8.
27. Thiery JP, Acloque H, Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
28. Tumaneng K, Schlegelmilch K, Russell RC, et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14(12):1322–1329. doi:10.1038/ncb2615
29. Tian Y, Tang B, Wang C, et al. Metformin mediates resensitivity to 5-fluorouracil in hepatocellular carcinoma via the suppression of YAP. Oncotarget. 2016;7(29):46230–46241. doi:10.18632/oncotarget.10079
30. Fernandez-L A, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi:10.1101/gad.1824509
31. Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64(1):232–244. doi:10.1002/hep.28542
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
