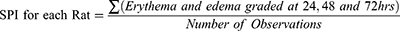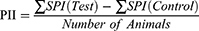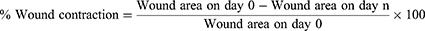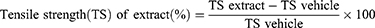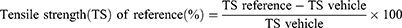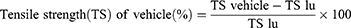Back to Journals » Journal of Experimental Pharmacology » Volume 14
Wound Healing Activity of 80% Methanolic Crude Extract and Solvent Fractions of the Leaves of Justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) in Mice
Authors G/giorgis SG, Ambikar D, Tsegaw A, Belayneh YM
Received 24 September 2021
Accepted for publication 19 April 2022
Published 13 May 2022 Volume 2022:14 Pages 167—183
DOI https://doi.org/10.2147/JEP.S340177
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Paola Rogliani
Shemelis Gebrewoled G/giorgis,1 Digambar Ambikar,2 Asegedech Tsegaw,2 Yaschilal Muche Belayneh3
1Department of Pharmacy, Dessie Health Science College, Dessie, Ethiopia; 2Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 3Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, Dessie, Ethiopia
Correspondence: Yaschilal Muche Belayneh, Department of Pharmacy, College of Medicine and Health Sciences, Wollo University, P.O. Box, 1145, Dessie, Ethiopia, Tel +251 918092466, Email [email protected]
Introduction: Justicia schimperiana has been used traditionally for the treatment of wound and skin burn, but there is no scientific evidence that supports the traditional claim.
Objective: To evaluate the wound healing activity of 80% methanol crude extract and solvent fractions of the leaves of Justicia schimperiana in mice.
Methods: Mice were used for wound healing study, while rats were used for acute dermal toxicity test. The 80% methanol crude extract and chloroform, ethyl acetate and aqueous fractions were formulated in ointments with 5% and 10% strength. Burn, excision and incision wound models were used to evaluate the effect of the crude extract, whereas the activity of the solvent fractions was evaluated using excision wound model. Parameters such as wound contraction, and period of epithelialization were studied in the excision and burn wound models, while tensile strength was measured in incision wound model.
Results: Treatment of wound with 80% methanol extract of Justicia schimperiana leaves using 5% (w/w) and 10% (w/w) ointment formulation induced significant (P< 0.05) improvement in wound contraction rate, epithelialization time and skin breaking strength in excision, incision and burn wound model, respectively as compared to negative control. The chloroform, ethyl acetate and aqueous fractions with 5% (w/w) and 10% (w/w) ointment formulation showed significant (p< 0.001) improvement in wound contraction and epithelialization time in excision wound model as compared to the negative control group.
Conclusion: This study has demonstrated that the 80% methanol crude extract and solvent fractions of Justicia schimperiana leaves possess wound healing activity.
Keywords: Justicia schimperiana, wound, wound healing
Introduction
Wounds are significant causes of morbidity and mortality worldwide. Studies showed that there are 10,000 deaths due to microbial infections for every million wound patients.1,2 Chronic wounds have a significant impact on the health and quality of life of patients and their families causing pain, loss of function and mobility, depression, distress and anxiety, embarrassment and social isolation, prolonged hospital stays and chronic morbidity or even death due to the low rate of complete healing.2
Current treatment strategies of wounds include debridement, irrigation, use of antiseptics, antibiotic and corticosteroid therapy and tissue grafts. However, these treatment approaches are associated with unwanted side effects like bleeding, tissue damage, contact dermatitis, delay in wound healing and potential bacterial resistance.3
Despite the tremendous advances in pharmaceutical drug industry, the availability of drugs capable of stimulating the process of wound repair is still limited, and only 1–3% of the drugs listed in western pharmacopoeias are intended for use on wounds.4 Furthermore, increased prevalence of resistant bacteria, high cost and shortage of new generation drugs has escalated infection-related morbidity and mortality particularly in developing countries.5 Hence, there is a great need for scientific investigation of medicinal plants to develop nontoxic and effective wound healing agents.
The leaves of Justicia schimperiana have been used traditionally for the treatment of wound in Libo Kemkem District, South Gondar Zone of the Amhara Region, northwestern Ethiopia.6 The crushed and powdered leaves of the plant are applied to the wounded area.6 However, the plant has not been explored scientifically for its wound healing activity. Additionally, different parts of Justicia schimperiana widely used in folk medicine of Ethiopia for various diseases including skin burns,7 skin lesion,8 rabies,6,9–12 arthritis,7,13 asthma,13 jaundice/ hepatitis,6,12,14–16 dysentery,15 diarrhea,6 diphtheria,17 common cold,6 headache,9,10 rheumatism,18 malaria,19, 9 venereal diseases,9 typhoid fever,19 tapeworm,6,20 anthrax,6,20 leg distension,21 gastritis,12 seizure,12 evil eye,6,22,23 external parasite6 and stomach-ache.6,24
The crude methanolic extract of Justica schimperiana leaves showed antibacterial activity against Neisseria gonorrhoea and Shigella flexneri.25 Additionally, the aqueous, methanol and chloroform extracts of different parts (leaf, bark, stem and root) of Justicia schimperiana have significant antibacterial activity against S. aureus, E. coli, B. cereus, P. mirabilis, K. pneumoniae and P. aeruginosa.26 The hydromethanol extract of the leaves has in vivo antioxidant27 and anti-inflammatory activates.28 The most common mechanisms behind phytochemical-mediated wound healing activity are antioxidant, anti-inflammatory and anti-microbial effects29 suggesting Justica schimperiana leaves may have wound healing activity.
Materials and Methods
Drugs and Chemicals
Wool fat (Uni-Chem chemical reagents, Serbia), hard paraffin (Lab tech chemicals, Ethiopia), white soft paraffin (Ethiopian pharmaceuticals manufacturing, SH.CO, Ethiopia), cecostearyl alcohol (Blulux Laboratories (P) Ltd., India), methanol absolute (AppliChem, Germany), Chloroform (Nice Chemicals Pvt. Ltd., India), ethyl acetate (Carlo Erba reagent S.A.S., France), 0.2% nitrofurazone skin ointment (shanghai general pharmaceutical CO., LTD, China), 1% silver sulfadiazine cream (Galentic pharm PVT. LTD, India), hydrochloric acid (Blulux Laboratories (P) Ltd., India), ethanol absolute (Blulux Laboratories (P) Ltd., India), ferric chloride (Blulux Laboratories (P) Ltd., India), ketamine hydrochloride injection (Neon laboratories limited, India), diazepam injection (Gland pharm limited, India), normal saline 0.9% (IV infusion BP Medsol pharmaceuticals), sodium hydroxide (Blulux Laboratories (P) Ltd., India), Wagner’s reagent (Research-Lab Fine Chem Industries, India), lead acetate (Guangdong Guanghua chemicals factories, China), sulphuric acid (HiMedia Laboratories Pvt. Ltd., India), dichloromethane (Blulux Laboratories (P) Ltd., India), halothane inhalation (Piramal enterprises limited, India), acetic anhydride, distilled water and bee wax were used. All the chemicals and reagents were analytical grade.
Instruments, Apparatus and Supplies
Sensitive digital weighing balance (Shinko Denshi CO., LTD, Vietnam), lyophilizer (Benchtop Freeze Dryer, India), rotary evaporator (Lab Scale, REV100-P, China), deep freezer, water bath, mortar and pestle, ointment slab, sharp sterilized scissors, surgical threads with curved needles, forceps, surgical scalpel blade, conical flask, beaker, Buchner funnel, adhesive plaster, gauze, elastic bandage, Whatman filter paper No.1, permanent marker, transparent polythene sheet and thermometer were used.
Collection of Plant Materials
The fresh leaves of Justicia schimperiana were collected from Libo Kemkem District, South Gondar Zone of the Amhara Region, northwest Ethiopia, 645 km away from Addis Ababa in January, 2018. The plant was identified and authenticated by Botanists at Department of Biology, College of Natural and Computational science, University of Gondar. The plant specimen (voucher number, SGG001) has been deposited there for future reference.
Experimental Animals
Healthy, adult Swiss albino mice of either sex (25–35 g, 8–10 weeks of age) or adult healthy, female Wistar rats (180–200 g, 3–4-month of age) were procured from the animal house of the Ethiopian Public Health Institute. Mice were used for wound healing study while rats were used for acute dermal toxicity test. The animals were housed in clean polypropylene cages with softwood shavings and chips as bedding in a room with a 12 h light/12 h dark cycle at room temperature and 55% humidity, and with free access to standard pellet rodent diet and water ad libitum. All mice and rats were allowed to acclimatize the laboratory condition for a week before starting the experiment. Animal handling and care were carried out following international laboratory animal use and care guidelines30 throughout the experiment. At the end of the experiment, the animals were sacrificed by high dose of halothane inhalation.31 Additionally, the study protocol was approved by the Institutional Ethical Review Board of University of Gondar (SOP4/52/10).
Preparation of the Crude Extract
The leaves of J. schimperiana were washed under running tap water to remove the surface pollutants and air-dried under shade at room temperature. Thereafter, the dried leaves were coarsely powdered using mortar and pestle. Crude extract was prepared by cold maceration technique. Leaf powder (1000 g) weighted and soaked with 8000mL of 80% hydro-methanol.32 It was macerated for three days in a conical flask with occasional shaking. Then, the extract was separated from the marc by using muslin cloth and further filtered by Whatman filter paper No.1. The marc was re-macerated twice by adding fresh solvent and filtered in the same manner to maximize the yield. The collected filtrates were placed in drying oven at 40 oC to remove methanol.33 The concentrated filtrate was frozen overnight using deep freezer and then freeze dried in a lyophilizer at −50°C and 200 mBar vacuum pressure to remove water. The dried extract was stored in screw cap vials in a refrigerator at −4 oC until used for formulation of ointments and solvent fractionation.32
Solvent Fractionation of the Crude Extract
Eighty grams of the 80% hydro-methanol crude extract was subjected to a successive extraction using separator funnel with chloroform, ethyl acetate and water in the order of increasing polarity.
The dried crude extract of the plant (80 g) was suspended in 480 mL of distilled water and slightly shaken to mix it completely with the solvent. The mixture was transferred in to a separator funnel. Then, equal volume of chloroform was added to it. The new mixture was shaken gently and allowed to settle until it forms two layers and then the chloroform fraction was collected. The same volume of chloroform was added to repeat the procedure twice as described above. Then, to the remaining aqueous portion in the separating funnel, an equal volume of ethyl acetate was added followed by vigorous shaking as described for the preceding solvent fractionation. The upper layer was ethyl acetate which was separated from aqueous portion and the procedure was repeated twice. The filtrates of chloroform and ethyl acetate fractions were concentrated by rotary evaporator at 45 rpm and 40°C to obtain the chloroform and ethyl acetate fraction. The remaining aqueous residue was frozen in deep freezer overnight and then freeze dried using a lyophilizer to obtain aqueous fraction. All fractions were stored in screw cap vials in a refrigerator at −4 oC until used for formulation of ointments.32
Ointment Formulation
The crude extract and chloroform, ethyl acetate and aqueous fractions were formulated in 5% (w/w) and 10% (w/w) strength ointments. Ointments were freshly prepared on the day of the experiment for each wound model based on British Pharmacopoeia, 2009.34
Unmedicated ointment was prepared to be used as control for the physical effect it provide as protectant, emollient or lubricant.35
All ingredients of the ointment base were arranged using reduced formula to prepare 100 g ointment from the master formula which is used to prepare 1000 g ointment. The calculated amount of hard paraffin (5 g) and cecostearyl alcohol (5 g) were mixed and melted in a beaker which was placed in water bath. Then, wool fat (5 g) and white soft paraffin (85 g) were added and stirred until all the ingredients are melted. Then, it was removed from the water bath and stirred until cooled. For preparation of the medicated ointment, each of 5gm and 10gm of the 80% methanol extract and chloroform, ethyl acetate and aqueous fractions were mixed with 95 g, and 90g of the ointment bases, respectively, by levigation on the surface of an ointment slab to make ointment of uniform consistency and smooth texture. Similarly, 100gm of the entire base ingredients were taken and treated in the same way to formulate ointment exclusive of an active ingredient.33 Then, the ointments were stored in airtight amber glass containers at room temperature.35
Acute Dermal Toxicity Test
Acute dermal toxicity test of 80% methanolic extract of Justicia schimperiana was carried out according to OECD 402 guideline. Three female healthy young adult Wistar rats showing normal skin texture were used since females are slightly more sensitive than male.36 The animals were housed using group-caging and acclimatized to the laboratory condition for seven days prior to the test. The rats were anesthetized by ketamine (50mg/kg) and diazepam (5mg/kg) through intraperitoneal route of administration.36 Body surface area of the rats was calculated based on the Meeh-Rubner formula.37 On the day before application of the test chemical, around 10% of the total body surface area of the hair of rats was shaved from the dorsal and trunk area on both side. Then, single dose of 2000 mg/kg body weight of the crude extract ointment formulation and simple ointment base applied topically on respective site. Test chemical formulation and simple ointment base were held separately in contact with the shaved skin with a porous gauze dressing and non-irritating tape throughout a 24-hour exposure. During the exposure period, animals were caged individually and provided with food and water ad libitum. At the end of the exposure period, residual test substance and simple ointment were rinsed with distilled water, and the animals were returned to group housing. One hour later, the sites were examined for skin irritation. Observation of the sites was done at 24 hours after application and repeated at 48 and 72 hours thereafter.38,39
The reactions, defined as erythema and edema, were evaluated according to the scoring system for skin reactions as 0 for no erythema, 1 for very slight erythema, 2 for well-defined erythema, 3 for moderate to severe erythema, 0 for no edema, 1 for Very slight edema (barely perceptible), 2 for well-defined edema (edges of area well defined by definite raising), 3 for Moderate edema (raised approximately 1mm) and 4 for Severe edema (raised more than 1mm extending beyond the area exposure). The total possible score for primary irritation is eight.39
The score of primary irritation (SPI) was calculated for each rat. Score for erythema and edema at 24, 48 and 72 hours were summed and divided by the number of the observations for the treated sites. The SPI for the control sites were calculated in the same fashion as test.
The difference between the summation of SPI scores of three animals from the treated site and control site were calculated and used for primary irritation index (PII) determination. The PII was calculated as the arithmetical mean of the SPI values of the three rats. The irritation degree was categorized based on the PII as negligible (PII=0-0.4), slight (PII=0.5–1.9), moderate (PII=2-4.9) or severe (PII=5-8) irritation.39
Preliminary Phytochemicals Screening
The hydromethanol crude extract and chloroform, ethyl acetate and aqueous fractions of J. schimperiana were screened for the presence of secondary metabolites including alkaloids, tannins, terpenoids, flavonoids, saponins, phenols, steroids, sterols and glycosides according to standard tests.40–44
Grouping and Dosing of Experimental Animals
A total of 13 groups of mice (each with six) were used to evaluate the activity of the crude extract. Five groups of mice were used for the excision wound model. Group I (served as a negative control) was treated with simple ointment, Groups II and III were received with 5% (w/w) and 10% (w/w) crude extract ointment, respectively and Group IV (served as a positive control) was treated with nitrofurazone, 0.2% (w/w). In linear incision wound model, five groups of mice (each with six) were used. The animals of Group I–IV were treated in a similar fashion with excision wound model, but animals in Group V were left untreated (served as untreated negative control). In the burn wound model, four groups of mice (each with six) were used. The animals of Group I–III were treated in a similar fashion with excision wound model, but animals in Group IV were treated with 1% (w/w) silver sulfadiazine cream (served as a positive control).
Eight groups of mice (each with six) with circular excision wound model were used for evaluation of the activity of the solvent fractions. Excision wound model was selected because it is ideal to study physical attributes like wound contraction, epithelization and scar remodeling.45 Group I was treated with simple ointment (served as a negative control); Groups II and III were treated with 5% (w/w) and 10% (w/w) of aqueous ointment, respectively; Group IV and V were treated with 5% (w/w) and 10% (w/w) of ethyl acetate ointment, respectively; Groups VI and VII were treated with 5% (w/w) and 10% (w/w) of chloroform ointment, respectively; and Group VIII was treated with 0.2% (w/w) nitrofurazone (served as a positive control).
Evaluation of Wound Healing Activity
The hydro-methanolic extract was evaluated using excision, incision and burn wound models whereas fractions (aqueous, ethyl acetate and chloroform) were evaluated using excision wound model.
Single model is inadequate to evaluate wound healing activity and no reference exist collectively represent the various phase of the wound healing process as a whole. Hence, three different wound models (excision, incision and burn) were used to evaluate wound healing activity of 80% methanol crude extract and solvent fractions of J. schimperiana leaves.
Circular Excision Wound Model
The mice were weighed individually and anesthetized prior to creation of the experimental wounds. The surgical process was carried out using ketamine (50 mg/kg) and diazepam (5mg/kg) through intraperitoneal route of administration. Then, the back hair of the animals was shaved,36 and a full thickness circular wound (314 mm2) was excised carefully to the depth of loose subcutaneous tissues after the area was marked by using toothed forceps, surgical blades and pointed scissors. After recovery, animals were returned to their cage and the entire wounds were left opened and undressed. The wounding day was considered to be day 0. Starting from day one, the wound was treated with ointments applied topically once daily till the wounds were completely healed. The healing of wound was assessed by tracing the wound area and captured photograph at every 2 days interval post wounding. Period of epithelialization was also recoded.33,46
Measurement of Wound Contraction
The wound healing progress was assessed by measuring rate of wound contraction as the percentage reduction of the original wound size. The wound contraction was measured by a transparent polythene sheet on the wounded margin of the healing area and marked by a permanent marker. The polythene sheet was then placed on 1mm2 scale graph paper and traced out. The area was measured every other day until complete wound closure was observed, and percentage reduction in wound area was calculated. Percentage reduction in wound area (% wound contraction) of the crude extract and solvent fractionation was calculated taking initial size of the wound (314 mm2) as 100% as follows.33,47,48
Where, n= the days when measurement was taken.
Epithelialization Time
Complete epithelialization period was calculated as the number of days required for falling off of the dead tissue remnants without any residual raw wound.33,47
Linear Incision Wound Model
Animals were anesthetized in the same way described for excision wound model. The dorsal fur of each mouse was shaved. Then, three cm long and 2 mm depth of longitudinal paravertebral incision was made with a sterile blade through the marked shaved skin and subcutaneous tissue at the distance of 1.0 cm from the dorsal midline on each side.47,49 The wound was closed by means of interrupted sutures 1.0 cm apart with non-absorbable surgical thread (no. 000) with curved needle (no.11). The continuous thread on both wound edges was tightened for good closure of the wounds, and the wound was left undressed. The wounding day was considered as day 0. After 24 h of wound creation (on 1st day), animals were treated with topical formulation of vehicle, extract and standard drug daily for nine days as described in grouping and dosing section. The mice were anesthetized and all sutures were removed on day 8 post-incision, and tensile strength was measured on day 10 using continuous water flow technique.33,47 Two forceps were firmly attached 3 mm away from the edge of wound facing each other on opposite side of the incision wound. One of the forceps was fixed on stands while the other was connected to a freely suspended light weight plastic with a volume of 1000 mL through a string run over a pulley. Water was allowed to flow continuously from the reservoir slowly and steadily into the container. The water flow was arrested and the volume of water collected in the container (approximately equal to its weight) was noted at the moment the wound just opened up.50 The percent strength was also calculated as follows.33,51
Where lu= left untreated.
Burn Wound Model
Partial thickness burn wound inflicted up on animals starved overnight and anesthetized with ketamine (50mg/kg) plus diazepam (5mg/kg) through intraperitoneal route of administration. Then, hot molten bees’ wax (2gm) at 80OC poured to a metal cylinder with 314mm2 circular opening placed on the back of the animal. On solidification of wax after 8–10 minutes, the metal cylinder with wax adhered to the skin were removed leaving markedly circular burn wound. Then, animals were placed in individual cages after recovery from anaesthesia. Animals were treated with extracts, simple ointment base and standard drug applied topically once a day on the wounded area starting from the day of wounding until the day of scab falling as described under grouping and dosing section. Wound area was measured at every 2 days of post wounding and time of epithelialization was monitored.36 Photographs were captured at every 2 days of post wounding.52
Statistical Analysis
The data obtained from the experiments were expressed as mean ± SEM (standard error of the mean). Data were statistically analyzed by using one-way analysis of variance (ANOVA) followed by Tukey post-hoc test using SPSS version 23.0, and p values < 0.05 were considered statistically significant.
Results
Acute Dermal Toxicity
Maximum concentration of methanolic extract of Justicia schimperiana (10%, w/w) ointment formulation applied using a single dose of 2000mg/kg of body weight was found to be safe. At the end of 72 h, none of the animals showed either formation of edema or erythema. Moreover, no signs of toxicity as well as no mortality were noted during the 14 days of cage side observation. Their Primary irritation index values were zero implying the non-irritant nature of the test samples as per the dermal irritation scoring system. Hence, ointment formulation of Justicia schimperiana leaves extract can be used safely as topical preparation to treat skin disease such as wound.
Phytochemical Screening
A total of 1000 g of Justicia schimperiana coarsely powdered leaves provided 192 g of crude hydromethanol extract making the yield, 19.2% (w/w). During solvent fractionation, 7.2 g chloroform, 13 g ethyl acetate and 59.8 g aqueous fractions were produced from 80 g of crude extract with the percentage yield of 9%, 16.25% and 74.75%, respectively. The Preliminary phytochemical screening of the 80% methanol extract of Justicia schimperiana leaves and its solvent fractions is depicted in Table 1. The phytochemical screening revealed the presence of alkaloids, tannins, saponins, flavonoids, terpenoids, phenols, steroids, and glycosides in the crude extract. All the phytochemicals found in crude extract were also present in the aqueous and ethyl acetate fractions except steroid. However, only Phenols and steroids were detected in the chloroform fraction.
 |
Table 1 Phytochemical Screening of the Crude Extract and Its Solvent Fractions |
Effect of the Crude Extract on Excision Wound
Wound Contraction
The progress of wound contraction induced by topical application of 80% methanol extract of J. schimperiana leaves ointment, simple ointment base, and nitrofurazone 0.2% (w/w) ointment is shown in Table 2. Topical application of 5% (w/w) and 10% (w/w) methanolic extract and standard drug showed better wound contraction compared to the negative control group. The 10% (w/w) methanolic extract ointment treated group showed significant (P<0.05) wound contraction starting from the second day of post wounding compared to the negative control group. As shown in Table 2, there was no significant difference in activity between the 10% (w/w) and 5% (w/w) extract but higher rate of wound closure was observed with 10% (w/w) ointment. The 10% (w/w) ointment also showed better wound contraction than the standard drug on the eighth and tenth day although the difference failed to reach statistical significance. The animals treated with 5% (w/w) methanolic extract ointment formulation showed significant (p<0.001) wound contraction on sixth day of post wounding as compared to negative control group and the maximum rate of wound contraction was seen on the sixth day which was 44.84%. The maximum rate of wound contraction was observed with the standard drug (nitrofurazone ointment, 0.2% (w/w)) on the sixth and eighth day of post wounding (p<0.001) compared to the negative control which was 48.25% and 53.4%, respectively.
 |
Table 2 Effect of Crude Extract on Wound Contraction of an Excision Wound in Mice |
Epithelialization Period
Animals treated with 5% (w/w) and 10% (w/w) ointments of the extract as well as the standard drug showed shorter epithelization period than the negative control group (Table 3 and Figure 1). The period of epithelialization was 20.00±0.516, 17.40±0.678, 16.83±0.601 and 17.00±0.447 days for negative control group, 5% (w/w) extract, 10% (w/w) extract and standard drug, respectively.
 |
Table 3 Effect of Crude Extract on Epithelization Period of an Excision Wound in Mice |
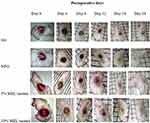 |
Figure 1 Wound repair at different post wounding days in excision wound of mice. Abbreviations: SO, simple ointment; NFO, nitrofurazone ointment; MLE, methanol leaf extract. |
The 10% (w/w) extract treated group showed faster rate of epithelialization (p<0.01) compared to negative control group. However, there was no significant difference in epithelialization period among the extract doses as well as compared to the standard drug (Table 3).
Effect of Solvent Fractions on Excision Wound
Wound Contraction
Wound contraction was significantly higher (p<0.001) with the standard drug starting from the 2nd post wounding day onwards as compared to the negative control. Similarly, aqueous, ethyl acetate and chloroform fractions with 5% (w/w) and 10% (w/w) ointment formulation showed enhanced wound contraction starting from the 6th post wounding day onwards (p<0.001) compared to the negative control group. When aqueous, ethyl acetate and chloroform fraction with 5% (w/w) and 10% (w/w) ointment formulations were compared to each other, the higher dose tended to show higher rate of wound contraction than the smaller dose though the difference was not statistically significant. There is no significant difference in activity among the fractions and the standard drug (Table 4).
 |
Table 4 Effect of Solvent Fractions on Contraction of Excision Wound in Mice |
Epithelization Period
As depicted in Table 5, epithelization period was significantly shorter (p<0.001) for animals treated with 5% (w/w) and 10% (w/w) of aqueous, ethyl acetate and chloroform fractions compared to negative control group. There was no significant difference in days of epithelization among the fractions and the standard drug (Table 5). Although the difference failed to reach statistical significance, animals treated with ointment containing the aqueous and ethyl acetate fractions showed shorter epithelization period than chloroform fraction.
 |
Table 5 Effect of Fractions on Epithelialization Period of Excision Wound in Mice |
Effect of the Crude Extract on Incision Wound
Both 5% (w/w) and 10% (w/w) ointments of 80% methanol extract of J. schimperiana showed significant increase (p<0.001) in wound tensile strength compared to negative control group (Table 6). The mean tensile strength in the group treated with simple ointment base tended to increase by about 13.19% compared to untreated control group which failed to reach statistical significance. Both 5% and 10% (w/w) ointment formulations showed significantly increased tensile strength (P<0.001) by 46.75% and 55.25%, respectively compared to simple ointment (Table 6).
 |
Table 6 Effect of Crude Extract on Breaking Strength of an Incision Wound in Mice |
Effect of the Crude Extract on Burn Wound
Wound Contraction
The progress of wound contraction induced by topical application of 80% methanol leaf extract of J. schimperiana ointments, simple ointment base, and silver sulfadiazine, 1% (w/w) cream is shown in Table 7. The 10% (w/w) crude extract ointment treated group showed significant (p<0.001) wound contraction starting from the fourth day whereas the 5% (w/w) extract treated group showed significant (p<0.001) wound contraction starting from the 6th day of post wounding compared to the negative control group. There was no significant difference in activity between the extracts and the standard drug (Table 7). Although the difference failed to reach statistical significance, higher rate of wound closure was observed with 10% (w/w) extract ointment compared to the 5% (w/w). The maximum rate of wound contraction for silver sulfadiazine, 1% (w/w) cream was seen from day 4 onwards (p < 0.001) as compared to the negative control group.
 |
Table 7 Effect of Crude Extract on Wound Contraction in Burn Wound of Mice |
Epithelization Period
As presented in Table 8, significantly decreased epithelialization period (p<0.001) was observed in groups treated with 5% (w/w) and 10% (w/w) extract ointments with 18.33±0.558 days and 17.83±0.477 days, respectively compared to the control group (21.83±0.167 days). The difference in epithelialization period between the plant extract and standard drug was found to be statistically insignificant (Table 8 and Figure 2).
 |
Table 8 Effect of Crude Extract on Period of Epithelialization of Burn Wound in Mice |
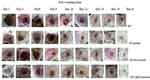 |
Figure 2 Contraction of burn wound on the back of mice at different days. Abbreviations: SO, simple ointment; SS, silver sulfadiazine; MLE, methanol leaf extract. |
Discussion
Wound healing is a stepwise process which consists of different phases such as hemostasis, inflammation, proliferative and remodeling or maturation. Treatment with methanolic extract of J. schimperiana leaves and its solvent fractions showed faster healing as compared to the untreated group. The better wound healing progression with J. schimperiana leaf extract may be due to intervening one or more phases of healing thereby facilitating the changes in wound contraction, period of epithelization and wound breaking strength.
Previous studies carried out on the related species, Adhatoda vasica of genus Adhatoda, reported significant wound healing activity53 suggesting the genus Adhatoda is endowed with wound healing activity.
Wound contraction indicates the rate of reduction of unhealed area during the healing process. Thus, fast rate of wound closure indicates better efficacy of medication.54 Wound contraction is attributed to increased proliferation of fibroblasts and their subsequent transformation to myofibroblasts. Enhanced contractile property of myofibroblast results in shorter epithelialization period helping the wound to heal faster.55 The proliferative phase starts on the third day after wounding and lasts for about two weeks thereafter. It is characterized by fibroblast migration and deposition of newly synthesized extracellular matrix acting as a replacement for the provisional network composed of fibrin and fibronectin.56
The shorter epithelialization periods produced by the ointments of the crude extract and solvent fractions might be due to facilitated proliferation and migration of epithelial cells and/or increased viability of epithelial cells. Wound contraction shortens the healing time because it decreases the size of the wound and reduces the amount of extracellular matrix needed to repair the defect. Contraction also facilitates re-epithelization by shortening the distance migrating keratinocytes must travel.57
Polar solvents like water are used to extract more polar compounds like flavonoids, glycosides, tannins and some alkaloids.58 Moreover, slightly higher healing effect of the aqueous fraction may be associated with the easy release of polar compounds from the simple ointment. The most polar compounds in the aqueous fraction are better released from non-polar base as compared to relatively less polar compounds.47
Better tensile strength produced by the crude extract in incision wound might be due to increased collagen synthesis and its maturation, angiogenesis and stabilization of fibers. Cumulative effect of all these phenomena improves circulation, thus providing oxygen and nutrients essential for the healing process of the wound site.55 Tensile strength of a wound mainly depends on an increase in collagen concentration and stabilization of the fibers.47
Stimulation of fibroblasts is believed to be one of the mechanisms of plant extracts to facilitate wound healing, and fibroblasts in granulation tissue regulate the production, deposition, and their subsequent maturation of collagen fibers that impart physical strength to the tissue.59 Incisional wounds treated with the crude extract of J. schimperiana in both strengths (5% and 10%, w/w) showed greater tensile strength as compared to the negative control, and this might be due to increased collagen synthesis per cell and facilitated cross-linking of the proteins.
In this study, 10% (w/w) of the crude extract and the solvent fraction ointment formulations showed better wound healing effect in terms of increased rate of wound contraction, reduction in period of epithelialization and improved wound tensile strength as compared to 5% ointment formulation treated mice. Thus, wound healing effect of the experimental plant seems dose dependent. Wound healing activity of J. schimperiana might be due to the presence of phytochemicals like alkaloids, tannins, saponins, flavonoids, terpenoids, phenols and glycosides. The wound healing action of the plant could be a result of either the individual or additive effects of these phytoconstituents.
Previous in vitro and in vivo studies showed that J. schimperiana have anti-inflammatory,28 antibacterial13,28 and antioxidant activities.27
Plants which are rich in a wide variety of secondary metabolites belonging to chemical classes such as tannin, terpenoid, alkaloid and polyphenol are generally superior in their anti-microbial activities.25 Several studies have reported that flavonoids, tannins, alkaloids and polyphenols possess antibacterial properties.60
Flavonoids and triterpenoids are known to promote the wound-healing process mainly due to their anti-microbial and free radical scavenging activities which seems to be responsible for wound contraction and increased rate of epithelialization.61 Flavonoids increases collagen synthesis, promote the cross-linking of collagen and decrease the degradation of soluble collagen.62 Alkaloids, triterpenoids, tannins and flavonoids are known to promote the wound healing process due to their astringent and antimicrobial properties.63
Tannins promote wound healing through chelating with free radicals and reactive oxygen species, shrinking proteins due to their astringent effect, promoting contraction of the wound and increasing the formation of capillary vessels and fibroblasts.64 Saponins possess anti-inflammatory, ant-microbial and antioxidant activities.65 Terpenoids have antibacterial properties and play an active role in wound healing, strengthen the skin, increase the concentration of antioxidants in wounds and restore inflamed tissues by increasing blood supply.66 Phenols possess strong antioxidant, antibacterial and numerous biological activities. Several studies have indicated that phenols are very effective in scavenging free radicals due to their redox properties.60 Steroids have been reported to have antibacterial66 as well anti-inflammatory activities.47 These findings collectively indicate that phytoconstituents present in J. schimperiana may initiate or facilitate a host defense mechanisms responsible for wound healing either individually or synergistically.
Conclusion
This study revealed that ointment formulations prepared from the crude 80% methanol extract of Justicia schimperiana leaves and its solvent fractions (aqueous, ethyl acetate and chloroform) were capable of promoting wound healing as compared to the negative control group, and this justifies the use of the leaves of Justicia schimperiana for wound treatment in the Ethiopian traditional medicine.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Statement
The experiment was conducted in accordance with the Guide for the Care and Use of Laboratory Animals (30) and the study has been approved by the ethical review committee of school of Pharmacy, University of Gondar (Reference number, SOP4/52/10).
Acknowledgments
We are thankful to Amhara Regional State Health Bureau for funding this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. SGG conducted the actual experiment.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work.
References
1. Wong SY, Manikam R, Muniandy S. Prevalence and antibiotic susceptibility of bacteria from acute and chronic wounds in Malaysian subjects. J Infect Dev Countries. 2015;9(09):936–944.
2. Järbrink K, Ni G, Sönnergren H, et al. Prevalence and incidence of chronic wounds and related complications: a protocol for a systematic review. Syst Rev. 2016;5(1):152.
3. Panda V, Sonkamble M, Patil S. Wound healing activity of Ipomoea batatas tubers (sweet potato). Functional Foods Health Dis. 2011;1(10):403–415.
4. Fikru A, Makonnen E, Eguale T, Debella A, Mekonnen GA. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. J Ethnopharmacol. 2012;143(2):469–474.
5. Teka A, Rondevaldova J, Kokoska L, et al. In vitro antimicrobial activity of plants used in traditional medicine in Gurage and Silti Zones, south central Ethiopia. BMC Complement Altern Med. 2015;15(1):286.
6. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem District, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4.
7. Ragunathan M, Abay SM. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria District, Northwestern Ethiopia. Int J Med. 2009;2:436.
8. Giday M, Teklehaymanot T, Animut A, Mekonnen Y. Medicinal plants of the Shinasha, Agew-awi and Amhara peoples in northwest Ethiopia. J Ethnopharmacol. 2007;110(3):516–525.
9. Agisho H, Osie M, Lambore T. Traditional medicinal plants utilization, management and threats in Hadiya Zone, Ethiopia.. J Med Plants. 2014;2(2):435.
10. Birhanu T, Abera D, Ejeta E. Ethnobotanical study of medicinal plants in selected Horro Gudurru Woredas, western Ethiopia. J Biol Agri Healthcare. 2015;5(1):83–93.
11. Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):40.
12. Suleman S, Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia). Ethiopia J herbs. 2012;18(1):34–57.
13. Kloos H, Menberu T, Tadele A, et al. Traditional medicines sold by vendors in Merkato, Addis Ababa: aspects of their utilization, trade, and changes between 1973 and 2014. Ethiopian J Health Dev. 2016;28(2):656.
14. Araya S, Abera B, Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre District, Southern Tigray, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):22.
15. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo District, Tigray Region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65.
16. Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):25.
17. Teklehaymanot T. An ethnobotanical survey of medicinal and edible plants of Yalo Woreda in Afar regional state, Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):40.
18. Yineger H, Yewhalaw D, Teketay D. Ethnomedicinal plant knowledge and practice of the Oromo ethnic group in southwestern Ethiopia. J Ethnobiol Ethnomed. 2008;4(1):11.
19. Chekole G. Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District, Northern Ethiopia. J Ethnobiol Ethnomed. 2017;13(1):55.
20. Wubetu M, Abula T, Dejenu G. Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern Ethiopia. BMC Res Notes. 2017;10(1):157.
21. Yirga G. Assessment of indigenous knowledge of medicinal plants in Central Zone of Tigray, Northern Ethiopia. African J Plant Sci. 2010;4(1):006–11.
22. Teklehaymanot T, Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, Northwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):12.
23. Yineger H, Kelbessa E, Bekele T, Lulekal E. Plants used in traditional management of human ailments at Bale Mountains National Park, Southeastern Ethiopia. J Med Plants Res. 2013;2(6):132–153.
24. Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124(1):69–78.
25. Geyid A, Abebe D, Debella A, et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. J Ethnopharmacol. 2005;97(3):421–427.
26. Murthy P, Moges G, Hymete A, Gebremariam T. Antimicrobial and phytochemical screening of Justicia Schimperiana. Eth Pharm. 1993;11:47–53.
27. Umer S, Asres K, Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharm Biol. 2010;48(4):461–468.
28. Assefa A, Urga K, Melaku D, Guta M, Mekonnen W. Bronchodilator and anti-inflammatory activities of Adhatoda schimperiana. Ethiop J Health Sci. 2008;18:2.
29. Shah A, Amini-Nik S. The Role of Phytochemicals in the Inflammatory Phase of Wound Healing. Int J Mol Sci. 2017;18(5):1068.
30. National Research Council. Guide for the Care and Use of Laboratory Animals. National Academies Press; 2010.
31. Rigalli A, Di Loreto V. Experimental Surgical Models in the Laboratory Rat. CRC Press; 2016.
32. Fentahun S, Makonnen E, Awas T, Giday M. In vivo antimalarial activity of crude extracts and solvent fractions of leaves of Strychnos mitis in Plasmodium berghei infected mice. BMC Complement Altern Med. 2017;17(1):13.
33. Mulisa E, Asres K, Engidawork E. Evaluation of wound healing and anti-inflammatory activity of the rhizomes of Rumex abyssinicus J. (Polygonaceae) in mice. BMC Complement Altern Med. 2015;15(1):341.
34. British Pharmacopoeia Commission. British Pharmacopoeia 2009. British Pharmacopoeia Commission; 2011.
35. Allen L, Ansel HC. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems. Lippincott Williams & Wilkins; 2013.
36. Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complem Alternative Med. 2011;1:43.
37. Gouma E, Simos Y, Verginadis I, Lykoudis E, Evangelou A, Karkabounas S. A simple procedure for estimation of total body surface area and determination of a new value of Meeh’s constant in rats. Lab Anim. 2012;46(1):40–45.
38. Koeter HB. OECD Guidelines for the Testing of Chemicals: 402 Acute Dermal Toxicity: Fixed Dose Procedure. Human Exp Toxicol. 2017;1:43.
39. More B, Sakharwade S, Tembhurne S, Sakarkar D. Evaluation for skin irritancy testing of developed formulations containing extract of Butea monosperma for its topical application. Int J Toxicol Applied Pharmacol. 2013;3(1):10–13.
40. Yadav R, Agarwala M. Phytochemical analysis of some medicinal plants. J Phytol. 2011;3:12.
41. Hossain MA. Study of total phenol, flavonoids contents and phytochemical screening of various leaves crude extracts of locally grown Thymus vulgaris. Asian Pac J Trop Biomed. 2013;3(9):705–710.
42. Ismail AM, Mohamed EA, Marghany MR, Abdel-Motaal FF, Abdel-Farid IB, El-Sayed MA. Preliminary phytochemical screening, plant growth inhibition and antimicrobial activity studies of Faidherbia albida legume extracts. J Saudi Soc Agr Sci. 2016;15(2):112–117.
43. Khanam Z, Wen CS, Bhat IUH. Phytochemical screening and antimicrobial activity of root and stem extracts of wild Eurycoma longifolia Jack (Tongkat Ali). J King Saud Univ Sci. 2015;27(1):23–30.
44. Pandey S. Preliminary phytochemical screening and in vitro antibacterial activity of Bauhinia variegata Linn. against human pathogens. Asian Pacific J Tropical Dis. 2015;5(2):123–129.
45. Rawat S, Singh R, Thakur P, Kaur S, Semwal A. Wound healing agents from medicinal plants: a review. Asian Pac J Trop Biomed. 2012;2(3):S1910–S7.
46. Kokane DD, More RY, Kale MB, Nehete MN, Mehendale PC, Gadgoli CH. Evaluation of wound healing activity of root of Mimosa pudica. J Ethnopharmacol. 2009;124(2):311–315.
47. Mekonnen A, Sidamo T, Asres K, Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J Ethnopharmacol. 2013;145(2):638–646.
48. Umeh VN, Ilodigwe EE, Ajaghaku DL, Erhirhie EO, Moke GE, Akah PA. Wound-healing Activity of the Aqueous Leaf Extract and Fractions of Ficus exasperata (Moraceae) and its Safety Evaluation on Albino Rats. J Traditional Complem Med. 2014;4(4):246–252.
49. Süntar IP, Akkol EK, Yılmazer D, et al. Investigations on the in vivo wound healing potential of Hypericum perforatum L. J Ethnopharmacol. 2010;127(2):468–477.
50. Ilango K, Chitra V. Wound healing and anti-oxidant activities of the fruit pulp of Limonia acidissima Linn (Rutaceae) in rats. Tropical J Pharmaceutical Res. 2010;9(3):e54.
51. Gebrehiwot M, Asres K, Bisrat D, Mazumder A, Lindemann P, Bucar F. Evaluation of the wound healing property of Commiphora guidottii Chiov. ex. Guid. BMC Complement Altern Med. 2015;15(1):282.
52. Fahimi S, Abdollahi M, Mortazavi SA, Hajimehdipoor H, Abdolghaffari AH, Rezvanfar MA. Wound healing activity of a traditionally used poly herbal product in a burn wound model in rats. Iran Red Crescent Med J. 2015;17(9):34.
53. Subhashini S, Arunachalam KD. Investigations on the phytochemical activities and wound healing properties of Adhatoda vasica leave in Swiss albino mice. African J Plant Sci. 2011;5(2):133–145.
54. Ghosh S, Samanta A, Mandal NB, Bannerjee S, Chattopadhyay D. Evaluation of the wound healing activity of methanol extract of Pedilanthus tithymaloides (L.) Poit leaf and its isolated active constituents in topical formulation. J Ethnopharmacol. 2012;142(3):714–722.
55. Samanta R, Pattnaik AK, Pradhan KK, Mehta BK, Pattanayak SP, Banerjee S. Wound healing activity of silibinin in mice. Pharmacognosy Res. 2016;8(4):298.
56. Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528–1542.
57. Strodtbeck F. Physiology of wound healing. Newborn Infant Nursing Rev. 2001;1(1):43–52.
58. Sarker SD, Latif Z, Gray AI. Natural Products Isolation. Springer Science & Business Media; 2005.
59. Upadhyay A, Chattopadhyay P, Goyary D, Mitra Mazumder P, Veer V. Ixora coccinea enhances cutaneous wound healing by upregulating the expression of collagen and basic fibroblast growth factor. ISRN Pharmacol. 2014;2014:w356.
60. Sagbo IJ, Afolayan AJ, Bradley G. Antioxidant, antibacterial and phytochemical properties of two medicinal plants against the wound infecting bacteria. Asian Pac J Trop Biomed. 2017;7(9):817–825.
61. Arun M, Satish S, Anima P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. leaves. Avicenna j Phytomed. 2016;6(3):295.
62. Lodhi S, Jain AP, Rai G, Yadav AK. Preliminary investigation for wound healing and anti- inflammatory effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med. 2016;7(1):14–22.
63. Muhammad AA, Arulselvan P, Cheah PS, Abas F, Fakurazi S. Evaluation of wound healing properties of bioactive aqueous fraction from Moringa oleifera Lam on experimentally induced diabetic animal model. Drug Des Devel Ther. 2016;10:1715.
64. Ashok PK, Upadhyaya K. Tannins are astringent. Journal of Pharmacognosy and. Phytochemistry. 2012;1(3):896.
65. Guo N, Tong T, Ren N, Tu Y, Li B. Saponins from seeds of Genus Camellia: phytochemistry and bioactivity. Phytochemistry. 2018;149:42–55.
66. Samejo MQ, Sumbul A, Shah S, Memon SB, Chundrigar S. Phytochemical screening of Tamarix dioica Roxb. ex Roch. J Pharmacy Res. 2013;7(2):181–183.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.