Back to Journals » Psychology Research and Behavior Management » Volume 15
Women with Myocardial Infarction Present Subtle Cognitive Difficulties on a Neuropsychological Battery After Exposure to a Social Stressor
Authors Poitras M , Narvaez Linares NF , Lambert M , Browndyke JN , Plamondon H
Received 21 June 2022
Accepted for publication 8 September 2022
Published 23 September 2022 Volume 2022:15 Pages 2761—2771
DOI https://doi.org/10.2147/PRBM.S379381
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Igor Elman
Marilou Poitras,1,* Nicolás Francisco Narvaez Linares,1,* Maude Lambert,1 Jeffrey N Browndyke,2 Hélène Plamondon1
1Behavioural Neuroscience Group, School of Psychology, University of Ottawa, Ottawa, Ontario, Canada; 2Department of Psychiatry and Behavioural Medicine, Division of Behavioral Medicine & Neurosciences, Duke University Medical Centre, Durham, NC, USA
*These authors contributed equally to this work
Correspondence: Hélène Plamondon, University of Ottawa, School of Psychology, Behavioural Neuroscience Group, 136 Jean-Jacques Lussier, Vanier Hall, Room 2082, Ottawa, Ontario, Canada, Email [email protected]
Objective: Myocardial infarction (MI) is the primary cause of mortality and morbidity in women, but its sequelae remain largely understudied. Given the heart–brain relationship, our study aimed to further understand stress’s impact on regulating cognitive function post-MI. Specifically, our study evaluated the effect of stress induced using the Trier Social Stress Test (TSST), on neuropsychological function in women who have or have not experienced MI.
Methodology: To do so, women (mean age = 59.41 yrs) with (WHxMI = 13) or without () a history of MI were exposed to the TSST prior to completion of a series of standardized neuropsychological tests: the Montreal Cognitive Assessment (MoCA), Control Oral Word Association (COWA), Rey Complex Figure and Recognition (RCFT), Trail Making Test (TMT), and Auditory Consonant Triagrams (ACT).
Results: Our findings support MI to be associated with impairments in working memory affecting immediate recall of ACT, as well as visuospatial impairments in the RCFT copy trial, marked by poorer drawing accuracy and incorrect placement of figure elements. Overall, WHxMI required more time to complete the neuropsychological assessment (WHxMI 166.57 ± 12, 155.00 ± 6.57; p < 0.01).
Conclusion: Together, these findings support cognitive impairments noted following a social stressor to remain subtle in WHxMI. Our study highlights the need for the development of more sensitive tools to screen for neuropsychological impairments in women with MI and the importance of assessing performance in a variety of testing conditions.
Keywords: heart attack, cognitive function, executive functions, visual memory, verbal fluency
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide, accounting for 32.0% of mortality in 20191 and 523 million prevalent cases in 2020.2 As commonly diagnosed CVD pathologies, coronary heart disease (CHD) and myocardial infarction (MI) are associated with increased risk of cognitive decline.3–5 However, stemming from the enduring misbelief of CHD being predominantly a man’s disease,6 cardiovascular research trials have primarily focused on men’s recovery.7 Whereas CHD in women occurs on average 10 years later than men, linked to the gradual loss of estrogen’s cardioprotective effects post menopause,8 lifetime risk of developing CHD is comparable for both sexes.9 In this article, we use the terms “women” and “men” to refer to participants’ sex and the associated biological characteristics.
While the majority of CHD research has been conducted in men,10 studies including female cohorts support many clinical outcomes of CVD to be sex-specific,11,12 rendering direct application of observations in male samples to women difficult. To this effect, Haring et al13 found that women with a history of CHD were at higher risk of developing mild cognitive impairment or dementia over a mean follow-up of 8.4 years compared to women without CHD history. Research also supports an accelerated decline of verbal memory and fluency, and significantly lower verbal fluency abilities in the years following a CHD event in women.14,15 Consistent with this, a recent systematic review supported an association between CHD and cognitive impairment in women, notably through impaired word retrieval and difficulty with problem-solving.16 Indeed, this review further supported a paucity of women centric data available, further highlighting the need for studies focusing on women’s cognitive function after MI.
To our knowledge, little attention has been given to studying these populations’ cognitive function in different contexts. For example, cardiac events in rodents are known to cause a dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which modulates the stress response through the actions of glucocorticoids.17 In humans, CVD has been associated with an increased risk of mental health disorders like anxiety or depression,18,19 suggesting that HPA axis dysfunction could have an implication in the post-MI response. This is of particular interest when studying women with a history of MI, who have a higher prevalence of anxiety disorders20 and could be more impacted by altered HPA axis function.
This study assessing cognitive function was part of a larger, mixed-methods cohort study aimed at exploring controllable risk factors, physiological reactions to stress, and HPA axis activity in women with and without a history of MI. The primary aim of this study was to determine the impact of a history of MI on women’s cognitive functions, notably working memory, fluid reasoning, and verbal abilities, following a social stressor. We hypothesized that women with a history of MI (WHxMI) would obtain lower scores in all cognitive domains when compared to women without a history of MI (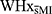 ).
).
Methodology
This study took place from August 2019 to March 2020. Women residing in the Ottawa-Gatineau region (Canada) were recruited through printed advertisements in hospital settings and ads on social media. After completing an online questionnaire on demographic variables and risk factors related to MI, we asked participants if they were interested in participating in the in-person portion of the study. All participants received a 15$ gift card and a free parking pass at the University. The study received ethical approval from the University of Ottawa Research Ethics Board (H-06-18-639). The principles of the Helsinki Declaration were followed.21
Sampling Procedures and Participant Characteristics
To be eligible for the study, women had to be aged between 45 and 80. Participants in the MI group self-reported having been diagnosed with MI by a medical professional. Their status was re-confirmed by N.F.N.L during the welcome stage. Women were excluded from the study if they: a) were on hormone replacement therapy; b) had a history (current or past) of substance abuse disorder; c) were diagnosed with a neurological disorder or dementia; d) had current psychiatric disorder; e) if menstruating, had an irregular menstrual cycle (i.e., a cycle lasting less than 21 days or more than 35 days); f) were taking contraceptive pills; or g) were pregnant or breastfeeding. We employed these exclusion criteria because they are known to influence physiological responses.22 Participants also provided a list of their medications (see Supplementary Material).
Data Collection
Once we confirmed eligibility, women were invited to the University of Ottawa’s Integrated Neurocognitive & Social Psychophysiology Interdisciplinary Research Environment (INSPIRE) Laboratory. The session lasted a maximum of 3 hours, took place between 9:00 AM and 12:00 PM, and women could complete the study in French or English. To control for confounds that could alter physiological measures, we asked participants to refrain from doing the following after 8:00 AM: a) brush their teeth or floss; b) consume alcohol; c) smoke cigarettes or consume tobacco; d) consume any food or caffeinated drinks; and e) engage in moderate or high intensity physical activity.23
Upon arrival to the laboratory, all participants provided written informed consent and completed self-reported psychological questionnaires. A 30-minute rest period followed, during which participants read a non-anxiety-inducing book of their choice or watched a nature documentary. Subsequently, participants were subjected to the Trier Social Stress Test (TSST), after which they completed the neuropsychological assessment. For more information about the procedures related to the physiological measures, please see Narvaez Linares et al.22 Only measures directly related to the objectives of the current sub-study are presented below. See Figure 1 for the experimental timeline of the larger study.
Measures
Please see Supplementary Methods for scoring and validity of all measures presented below. The following tests were selected because they are available and normed in French and English (with adequate validity and reliability), thereby providing a culture-sensitive approach to analysis.
Trier Social Stress Test
The TSST was used to induce a stress response in participants,23 and administered prior to the neuropsychological assessment. Testing procedure is described in detail in the Supplementary Methods.
Psychological Questionnaires
We used the Center of Epidemiologic Studies Depression Scale – Revised version24 (CESD-R) to measure participants’ depressive symptomatology and the General Health Questionnaire–12 item version25 (GHQ-12) to assess participants’ psychological distress.
Neuropsychological Assessment
The neurocognitive measures were administered by N.F.N.L. who has expertise in clinical neuropsychological evaluation. To ensure scoring accuracy, all tests were double-scored, and data entry was verified by M.L., who also has expertise in clinical neuropsychological evaluation.
Participants’ auditory working memory was assessed using the Auditory Consonant Triagrams26 (ACT). Participants’ phonemic and semantic verbal fluency were measured using the Controlled Oral Word Association Test27 (COWAT). The Montréal Cognitive Assessment28 (MoCA) was used as a screener of global cognitive functions. The Rey Complex Figure Test and Recognition Trial29 (RCFT) assessed participants’ perceptual organization and visual memory. The Trail Making Test30 part A (TMT-A) and B (TMT-B) served to assess participants’ processing speed and set maintenance, respectively.
Data Preparation and Statistical Analyses
We performed statistical analyses using IBM SPSS Statistics 28.0. Participants were matched for age and education based on their answers to the demographic questionnaire. Prior to the main analyses, we examined data for entry accuracy, missing data patterns, and model assumptions. Outliers, screened via boxplots, were replaced with the group’s next most extreme value ± 1. Homogeneity of variance and normality were assessed with Levene’s and Shapiro–Wilk’s tests, respectively. Chi-square tests of homogeneity were used to analyze dichotomous (2×2) and multinominal (r×2) demographic data, with Fisher’s exact test being conducted when expected count in each cell was not met (<5). We analyzed psychological questionnaires using Mann–Whitney U-tests. Raw data from neuropsychological assessments were converted into z-scores using normative data from respective neuropsychological norms (when applicable), then analyzed by independent samples t-tests or Mann–Whitney U-tests if normality was not met. The level of significance was set to p < 0.05 for all analyses. Due to the small sample size, power calculations were omitted because of possible biases in interpretation.31
Results
Self-reported socio-demographic characteristics and medical history for the analytic sample are depicted in Table 1. On average, WHxMI experienced their first MI event 2.5 years before their participation in the study and two-thirds (61.5%) experienced one MI event only. The average time to complete the assessment was 160.19 minutes (SD = 2.027).
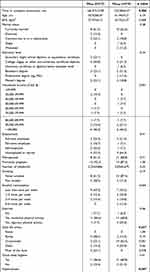 |
Table 1 Participant Characteristics |
Demographic Variables
We found that WHxMI took significantly longer to complete the neuropsychological assessment measures than 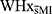 , t(17.72) = 3.12, p = 0.006, d = 1.23. Hypertension was more frequent in WHxMI (χ2[1] = 3.95, p = 0.047), and non-significant, but possible statistical trends, were found for WHxMI to have a higher BMI [t(25) = 1.91, p = 0.068, d = 0.74] and Type II diabetes (p = 0.064).
, t(17.72) = 3.12, p = 0.006, d = 1.23. Hypertension was more frequent in WHxMI (χ2[1] = 3.95, p = 0.047), and non-significant, but possible statistical trends, were found for WHxMI to have a higher BMI [t(25) = 1.91, p = 0.068, d = 0.74] and Type II diabetes (p = 0.064).
Groups also differed in the frequencies at which stress was experienced (p = 0.037). While statistical significance was lost in post-hoc analyses, possibly due to the small sample size, 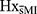 tended to report stress to be “occasionally” experienced more often (p = 0.061), whereas HxMI reported stress to be experienced “most of the time” more often than
tended to report stress to be “occasionally” experienced more often (p = 0.061), whereas HxMI reported stress to be experienced “most of the time” more often than 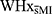 (p = 0.078). There were no group differences on any other demographic variables (see Table 1 for p values).
(p = 0.078). There were no group differences on any other demographic variables (see Table 1 for p values).
Psychological Questionnaires
There were no significant differences between WHxMI and 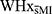 on the CEDS-R (p = 0.166) nor on the GHQ-12 (p = 0.552).
on the CEDS-R (p = 0.166) nor on the GHQ-12 (p = 0.552).
Neuropsychological Assessment
On the ACT, WHxMI had significantly poorer performance at the 0 second interval than 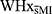 , U = 119.50, z = 2.020, p = 0.043. Groups did not differ for any other intervals (9s, 18s, 36s, or total score) or in the number of perseverance errors during the ACT (all p > 0.05; see Table 2).
, U = 119.50, z = 2.020, p = 0.043. Groups did not differ for any other intervals (9s, 18s, 36s, or total score) or in the number of perseverance errors during the ACT (all p > 0.05; see Table 2).
 |
Table 2 Neuropsychological Performance of Women with and without a History of Myocardial Infarction |
On the RCFT, WHxMI had a poorer performance during the copy trial [U = 140.00, z = 2.23, p = 0.026], and showed a trend for worse performance on the recognition trial, U = 128.50, z = 1.88, p = 0.060. There were no differences in the immediate and delayed trials (both p > 0.05). To assess for more subtle impairments, we analyzed the number of times participants inaccurately drew elements of the figure but placed them correctly, and the number of times elements were accurately drawn but incorrectly placed. During the copy trial, HxMI was associated with increased frequencies of inaccurately drawn items [U = 60.00, z = −1.99, p = 0.046] although groups did not differ in the number of incorrectly placed items (p > 0.05). In contrast, during the immediate trial, WHxMI did not differ in the number of inaccurately drawn items (p > 0.05) but had more incorrectly placed items [U = 45.50, z = −2.69, p = 0.007]. During the delayed trial, WHxMI had increased frequencies of inaccurately drawn items [U = 63.50, z = −1.88, p = 0.061]. There were no significant group differences on any of the other neuropsychological tests (all p > 0.05).
Discussion
To our knowledge, this study is the first to determine the impact of a history of MI on women’s cognitive function following exposure to the TSST, using a comprehensive neuropsychological assessment and controlling performance using an age- and education-matched group of women without a history of MI. Our findings indicate subtle impairments of cognitive function in WHxMI, as displayed by weaker performance on the ACT and the RCFT compared to 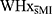 . Interestingly, recent findings from our group have supported TSST exposure to also affect stress-related cortisol secretion, physiological changes that can provide insights on HPA axis dysregulation and heart-brain continuum responses following MI.22
. Interestingly, recent findings from our group have supported TSST exposure to also affect stress-related cortisol secretion, physiological changes that can provide insights on HPA axis dysregulation and heart-brain continuum responses following MI.22
Memory and Recognition
In this study, no between-group differences were detected in visual memory performances (as indexed by scores on the immediate and delayed trials of the RCFT), suggesting visuospatial recall to be preserved. Nonetheless, WHxMI tended to obtain lower scores on the recognition trial of the RCFT, which presents insightful clues on the possible spheres of functional impairments that might be associated with a history of MI. Indeed, although WHxMI showed no difficulties recalling and drawing the complex figure from memory after delays, they did not benefit from the retrieval cues to the same extent as 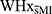 . Based on these results, WHxMI showed increased difficulty discriminating between relevant and non-relevant (distractor) stimuli, which may indicate encoding/storage deficits in survivors of MI following conditions of increased stress. This type of deficit, sometimes referred to as recognition discriminability deficits, is associated with a reduced volume of medial temporal structures, specifically the hippocampus.32 In fact, a recent functional magnetic resonance imaging study found cardiovascular risk to be associated with altered hippocampal-medial prefrontal coupling when completing a memory encoding task.33 In line with this, we also found that WHxMI made more errors in placing items during the immediate trial of the RCFT. This could indicate subtle spatial memory impairments, in which WHxMI remember pieces of the figure, but spatial location of those pieces, which are thought to be highly governed by hippocampal place cells, seemed to be an issue. This is generally consistent with the fact that MI is often a precursor to heart failure, a condition associated with decreased hippocampal volume and blood flow.34,35
. Based on these results, WHxMI showed increased difficulty discriminating between relevant and non-relevant (distractor) stimuli, which may indicate encoding/storage deficits in survivors of MI following conditions of increased stress. This type of deficit, sometimes referred to as recognition discriminability deficits, is associated with a reduced volume of medial temporal structures, specifically the hippocampus.32 In fact, a recent functional magnetic resonance imaging study found cardiovascular risk to be associated with altered hippocampal-medial prefrontal coupling when completing a memory encoding task.33 In line with this, we also found that WHxMI made more errors in placing items during the immediate trial of the RCFT. This could indicate subtle spatial memory impairments, in which WHxMI remember pieces of the figure, but spatial location of those pieces, which are thought to be highly governed by hippocampal place cells, seemed to be an issue. This is generally consistent with the fact that MI is often a precursor to heart failure, a condition associated with decreased hippocampal volume and blood flow.34,35
Information Processing and Attentional Abilities
During the copy trial of the RCFT, WHxMI obtained significantly lower scores, suggesting impaired visuo-perceptual and visuomotor integration skills. Consistent with this, Phillips & Mate-Kole36 reported CHD as a significant predictor of impaired visuospatial constructional ability during the copy trial of the RCFT. Reduced performance could be explained by slower visuospatial information processing or difficulty integrating visual information. Although information specific to women is lacking, individuals with a history of CHD have shown slower processing speeds.37 The increased number of inaccuracy errors during the copy trial of the RCFT might indicate carelessness or reduced attention to detail.38 While motor deficits in WHxMI cannot be completely ruled out, such deficits were not observed in other tasks with motor components (i.e., no significant group differences in the number of accuracy errors during the immediate trial of the RCFT or on the TMT).
Attentional difficulties are reported in individuals with a history of MI.39 In our study, a history of MI was associated with reduced recall of simple verbal information immediately after being exposed to it (no interference; ACT 0s interval). This offers an interesting parallel to difficulties observed in the RCFT copy and recognition trials, all tasks that could be argued as being the “less cognitively taxing” parts of these respective tests. This could be linked to difficulties with initial task acquisition and meeting task demands, after which WHxMI could adjust cognitive resources appropriately. Based on the current study results, both the visual and verbal attentional abilities of WHxMI appear to be impacted.
Mental Health and Time Since MI Event
The psychological questionnaires did not indicate a higher prevalence of indicators of mood problems or psychological distress in WHxMI. Interestingly however, despite similar subjective assessment of the perceived TSST stressor as 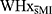 , stress-related salivary cortisol secretion appeared dysregulated in WHxMI.22 Such dissociation in the psychological and physiological responses is intriguing. Indeed, depression is elevated in MI survivors, and is associated with increased risk of impaired cardiovascular outcomes and mortality.40 Our findings highlight a need to concurrently monitor psychological and physiological measures of health and wellbeing of WHxMI to provide improved support and care. The association between cognitive complaints and poorer quality of life, increased risk of depressive symptoms, higher anxiety and higher perceived stress, and overall worse general wellbeing41,42 should also not be ignored as a factor when studying WHxMI.
, stress-related salivary cortisol secretion appeared dysregulated in WHxMI.22 Such dissociation in the psychological and physiological responses is intriguing. Indeed, depression is elevated in MI survivors, and is associated with increased risk of impaired cardiovascular outcomes and mortality.40 Our findings highlight a need to concurrently monitor psychological and physiological measures of health and wellbeing of WHxMI to provide improved support and care. The association between cognitive complaints and poorer quality of life, increased risk of depressive symptoms, higher anxiety and higher perceived stress, and overall worse general wellbeing41,42 should also not be ignored as a factor when studying WHxMI.
It has been suggested that the risk of cognitive decline augment as time since the first CHD event increases, with a most pronounced effect in individuals having experienced their event >10 years prior to neuropsychological evaluation.14 Given that most women in our sample experienced their first MI event within the past 5 years (mean 2.5 years ± 1.98), one cannot exclude that cognitive deficits might have been too subtle to be detected by the neuropsychological assessment measures. Indeed, a recent study by Gagnon et al43 showed that performance on the MoCA varied along the cardiovascular disease continuum, emphasizing the gradual cognitive decline linked to disease progression in these populations. In our study, WHxMI took significantly longer to complete the neuropsychological assessment than  . Although it is difficult to formulate a clear interpretation of this finding, speculatively, this could further highlight the subtle impairments of cognitive functioning present in our sample (e.g., needing more time to process and understand information, requiring repetition of instructions). This concords with the apparent difficulty of meeting initial task demands displayed by WHxMI, and possible slower information consolidation. Our results highlight the importance of early detection of cognitive difficulties in recent MI survivors and emphasize the need for neuropsychological measures better suited to detecting subtle cognitive impairments in this population.
. Although it is difficult to formulate a clear interpretation of this finding, speculatively, this could further highlight the subtle impairments of cognitive functioning present in our sample (e.g., needing more time to process and understand information, requiring repetition of instructions). This concords with the apparent difficulty of meeting initial task demands displayed by WHxMI, and possible slower information consolidation. Our results highlight the importance of early detection of cognitive difficulties in recent MI survivors and emphasize the need for neuropsychological measures better suited to detecting subtle cognitive impairments in this population.
Cognitive Function and Physiological Reactivity
In addition to measuring neuropsychological performance, the twin study also assessed heart rate variability –via the respiratory sinus arrythmia–, corticol secretion –via saliva samples– at 8 different points throughout the study, and anxiety level at 3 distinct study intervals (see Narvaez Linares et al22). These data supported similar levels of subjective stress and heart rate variability throughout the study in the WHxMI and 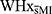 groups. However, WHxMI showed a tendency to have lower salivary cortisol secretion. This finding and its role on cognitive performance need to be further defined. Our study support lower scores on certain cognitive tests in women with a history of MI to be concurrent with reduced cortisol secretion related to a stressor. Stress and altered cortisol secretion have been associated with altered neuropsychological performance in a variety of cognitive domains (For review, see Law & Chow44). Consequently, it remains possible that dysregulated physiological responses post MI could contribute to impaired cognitive function observed in our study and also reported at delayed intervals.14,43 Further studies are needed to gain a better understanding of context-dependent impairments of cognitive function, especially in populations which have historically been neglected in CVD research.
groups. However, WHxMI showed a tendency to have lower salivary cortisol secretion. This finding and its role on cognitive performance need to be further defined. Our study support lower scores on certain cognitive tests in women with a history of MI to be concurrent with reduced cortisol secretion related to a stressor. Stress and altered cortisol secretion have been associated with altered neuropsychological performance in a variety of cognitive domains (For review, see Law & Chow44). Consequently, it remains possible that dysregulated physiological responses post MI could contribute to impaired cognitive function observed in our study and also reported at delayed intervals.14,43 Further studies are needed to gain a better understanding of context-dependent impairments of cognitive function, especially in populations which have historically been neglected in CVD research.
Limitations
The COVID-19 pandemic interrupted data collection, and terminated the study due to the vulnerability of our study population and concern for research assistants’ safety. As such, the study includes a smaller sample size than expected, possibly hindering detection of group differences. The sample size also prevented controlling for the impact of medication intake in the analyses (see Table S1 for list of medication). Larger studies should account for medication intake and its possible interaction with cognitive functions. Another consideration is the potential selection bias in recruiting participants for an in-person study, wherein relatively healthier MI survivors may be willing and able to participate in research. Considering the magnitude of the detected impairments, one cannot exclude that some selected neurocognitive measures may have lacked the overall sensitivity to detect subtle objective cognitive impairments in MI survivors. The use of self-reported data for the MI diagnosis does allow the possibility of a discrepancy between self-report and medical data. We attempted to minimize this risk by re-confirming medical history with participants during the in-person testing session. Finally, a follow-up assessment is required to explore progression of cognitive function over time.
Conclusion
Overall, our results highlight reduced recognition discriminability abilities and difficulty with task acquisition in women with a history of MI following a social stressor, along with possible dysfunctions of their attentional abilities. Furthermore, we pinpoint subtle impairments on visuospatial tasks and in the overall time needed to complete cognitive assessments. Given the substantial incidence of MI among women and the scarcity of data in this population, studies aiming to explore the impact of MI in women specifically are warranted and justified. Diverse testing conditions are also needed to better capture the wide range of situations experienced in the daily lives of MI survivors and to fully understand their impact on cognitive function.
Acknowlegments
M.P. was supported by a scholarship from the Ontario Graduate Scholarship program. N.F.N.L. was supported by a doctoral scholarship from the Fond de recherche du Québec – Nature et Technologies. This work was supported by a discovery grant to H.P. from the National Science and Engineering Research Council of Canada (RG203596-13). We would like to thank Zoey Burr, Eva Fernandes, Jeremy Oueis, Zachary Verret-Borsos, Kathel Dongnang, Yassine Hmidni, and Kristina Munelith-Souksanh for their assistance in data collection. We also want to thank our participants for taking the time to participate in this research. We acknowledge that this research was conducted in the traditional unceded territory of the Algonquin Anishnaabeg People.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
Dr. Helène Plamondon reports grants from the Natural Sciences and Engineering Research Council of Canada during the conduct of the study. This research is part of the doctoral thesis of the principal co-author.45 The authors report no conflicts of interest in this work.
References
1. World Health Organization. Cardiovascular diseases (CVDs). World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
2. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
3. Deckers K, Schievink SHJ, Rodriquez MMF, et al. Coronary heart disease and risk for cognitive impairment or dementia: systematic review and meta-analysis. PLoS One. 2017;12(9):e0184244. doi:10.1371/journal.pone.0184244
4. Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II Study. J Am Geriatr Soc. 2003;51(10):1445–1450. doi:10.1046/j.1532-5415.2003.51464.x
5. Singh-Manoux A, Britton A, Kivimaki M, Guéguen A, Halcox J, Marmot M. Socioeconomic status moderates the association between carotid intima-media thickness and cognition in midlife: evidence from the Whitehall II study. Atherosclerosis. 2008;197(2):541–548. doi:10.1016/j.atherosclerosis.2007.08.010
6. Miracle VA. Coronary artery disease in women: the myth still exists, unfortunately. Dimens Crit Care Nurs. 2010;29(5):215–221. doi:10.1097/DCC.0b013e3181ec3731
7. Gong IY, Tan NS, Ali SH, et al. Temporal trends of women enrollment in major cardiovascular randomized clinical trials. Can J Cardiol. 2019;35(5):653–660. doi:10.1016/j.cjca.2019.01.010
8. Saeed A, Kampangkaew J, Nambi V. Prevention of cardiovascular disease in women. Methodist DeBakey Cardiovasc J. 2017;13(4):185–192. doi:10.14797/mdcj-13-4-185
9. Woodward M. Cardiovascular disease and the female disadvantage. Int J Environ Res Public Health. 2019;16(7):1165. doi:10.3390/ijerph16071165
10. Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126(5):604–611. doi:10.1161/CIRCULATIONAHA.111.086892
11. Kosuge M, Kimura K, Ishikawa T, et al. Differences between men and women in terms of clinical features of ST-segment elevation acute myocardial infarction. Circ J. 2006;70(3):222–226. doi:10.1253/circj.70.222
12. Mosca L, Barrett-Connor E, Kass Wenger N. Sex/gender differences in cardiovascular disease prevention. Circulation. 2011;124(19):2145–2154. doi:10.1161/CIRCULATIONAHA.110.968792
13. Haring B, Leng X, Robinson J, et al. Cardiovascular disease and cognitive decline in postmenopausal women: results from the Women’s Health Initiative Memory Study. J Am Heart Assoc. 2013;2(6):e000369. doi:10.1161/JAHA.113.000369
14. Singh-Manoux A, Sabia S, Lajnef M, et al. History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur Heart J. 2008;29(17):2100–2107. doi:10.1093/eurheartj/ehn298
15. Xie W, Zheng F, Yan L, Zhong B. Cognitive decline before and after incident coronary events. J Am Coll Cardiol. 2019;73(24):3041–3050. doi:10.1016/j.jacc.2019.04.019
16. Narvaez Linares NF, Poitras M, Burkauskas J, et al. Neuropsychological sequelae of coronary heart disease in women: a systematic review. Neurosci Biobehav Rev. 2021;127:837–851. doi:10.1016/j.neubiorev.2021.05.026
17. de la Tremblaye PB, Raymond J, Milot MR, Merali Z, Plamondon H. Evidence of lasting dysregulation of neuroendocrine and HPA axis function following global cerebral ischemia in male rats and the effect of Antalarmin on plasma corticosterone level. Horm Behav. 2014;65(3):273–284. doi:10.1016/j.yhbeh.2014.01.003
18. Chen YY, Xu P, Wang Y, Song TJ, Luo N, Zhao LJ. Prevalence of and risk factors for anxiety after coronary heart disease. Medicine. 2019;98(38):e16973. doi:10.1097/MD.0000000000016973
19. Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28(11):1295–1302. doi:10.1093/ajh/hpv047
20. Serpytis P, Navickas P, Lukaviciute L, et al. Gender-based differences in anxiety and depression following acute myocardial infarction. Arq Bras Cardiol. 2018;111(5):676–683. doi:10.5935/abc.20180161
21. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
22. Narvaez Linares NF, Munelith-Souksanh K, Tanguay AFN, Plamondon H. The impact of myocardial infarction on basal and stress-induced heart rate variability and cortisol secretion in women: a pilot study. Compr Psychoneuroendocrinol. 2022;9:100113. doi:10.1016/j.cpnec.2022.100113
23. Narvaez Linares NF, Charron V, Ouimet AJ, Labelle PR, Plamondon H. A systematic review of the Trier Social Stress Test methodology: issues in promoting study comparison and replicable research. Neurobiol Stress. 2020;13:100235. doi:10.1016/j.ynstr.2020.100235
24. Eaton WW, Smith C, Ybarra M, Muntaner C, Tien A. Center for Epidemiologic Studies Depression Scale: review and Revision (CESD and CESD-R). In: Maruish ME, editor. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment: Instruments for Adults.
25. Goldberg DP, Williams P. A User’s Guide to the General Health Questionnaire. NFER-NELSON; 1988.
26. Stuss DT, Stethem LL, Hugenholtz H, Richard MT. Traumatic brain injury: a comparison of three clinical tests, and analysis of recovery. Clin Neuropsychol. 1989;3(2):145–156. doi:10.1080/13854048908403287
27. Benton AL. Problems of test construction in the field of Aphasia. Cortex. 1967;3(1):32–58. doi:10.1016/S0010-9452(67)80005-4
28. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
29. Osterrieth PA. Le test de copie d’une figure complexe; contribution à l’étude de la perception et de la mémoire. [Test of copying a complex figure; contribution to the study of perception and memory.]. Arch Psychol. 1944;30:206–356.
30. Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary.
31. Brysbaert M. How many participants do we have to include in properly powered experiments? A tutorial of power analysis with reference tables. J Cogn. 2019;2(1):16. doi:10.5334/joc.72
32. Bennett IJ, Stark SM, Stark CEL. Recognition memory dysfunction relates to hippocampal subfield volume: a study of cognitively normal and mildly impaired older adults. J Gerontol B Psychol Sci Soc Sci. 2019;74(7):1132–1141. doi:10.1093/geronb/gbx181
33. Meusel LAC, Greenwood CE, Maione A, Tchistiakova E, MacIntosh BJ, Anderson ND. Cardiovascular risk and encoding-related hippocampal connectivity in older adults. BMC Neurosci. 2019;20(1):37. doi:10.1186/s12868-019-0518-4
34. Suzuki H, Matsumoto Y, Ota H, et al. Hippocampal blood flow abnormality associated with depressive symptoms and cognitive impairment in patients with chronic heart failure. Circ J Off J Jpn Circ Soc. 2016;80(8):1773–1780. doi:10.1253/circj.CJ-16-0367
35. Woo MA, Ogren JA, Abouzeid CM, et al. Regional hippocampal damage in heart failure. Eur J Heart Fail. 2015;17(5):494–500. doi:10.1002/ejhf.241
36. Phillips NA, Mate-Kole CC. Cognitive deficits in peripheral vascular disease. Stroke. 1997;28(4):777–784. doi:10.1161/01.STR.28.4.777
37. Gayda M, Gremeaux V, Bherer L, et al. Cognitive function in patients with stable coronary heart disease: related cerebrovascular and cardiovascular responses. PLoS One. 2017;12(9):e0183791. doi:10.1371/journal.pone.0183791
38. Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the rey-osterrieth complex figure test. Nat Protoc. 2006;1(2):892–900. doi:10.1038/nprot.2006.115
39. Chokron S, Helft G, Perez C. Effects of age and cardiovascular disease on selective attention. Cardiovasc Psychiatry Neurol. 2013;2013:185385. doi:10.1155/2013/185385
40. van Melle JP, de Jonge P, Spijkerman TA, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med. 2004;66(6):814–822. doi:10.1097/01.psy.0000146294.82810.9c
41. Stites SD, Harkins K, Rubright JD, Karlawish J. Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, Mild Alzheimer’s Disease Dementia, and normal cognition. Alzheimer Dis Assoc Disord. 2018;32(4):276–283. doi:10.1097/WAD.0000000000000262
42. Kazukauskiene N, Fineberg NA, Bunevicius A, et al. Predictive value of baseline cognitive functioning on health-related quality of life in individuals with coronary artery disease: a 5-year longitudinal study. Eur J Cardiovasc Nurs. 2021:zvab116. doi:10.1093/eurjcn/zvab116
43. Gagnon C, Saillant K, Olmand M, et al. Performances on the montreal cognitive assessment along the cardiovascular disease continuum. Arch Clin Neuropsychol. 2021:acab029. doi:10.1093/arclin/acab029
44. Law R, Clow A. Chapter Eight - Stress, the cortisol awakening response and cognitive function. In: Clow A, Smyth N, editors. International Review of Neurobiology. Vol. 150. Academic Press; 2020: 187–217. doi: 10.1016/bs.irn.2020.01.001
45. Narvaez Linares NF. Myocardial Infarction in Women: Symptoms, Risk Factors, Neuropsychological Impairment, and Stress-Induced Physiological Changes [Doctoral dissertation]. Université d’Ottawa; 2022. doi:10.20381/ruor-27726.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

