Back to Journals » Cancer Management and Research » Volume 11
Wnt5a induces ROR1 and ROR2 to activate RhoA in esophageal squamous cell carcinoma cells
Authors Wu X , Yan T, Hao L, Zhu Y
Received 15 October 2018
Accepted for publication 27 February 2019
Published 10 April 2019 Volume 2019:11 Pages 2803—2815
DOI https://doi.org/10.2147/CMAR.S190999
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Xuping Wu,1 Ting Yan,2 Leiyu Hao,3 Yichao Zhu3,4
1The Second Hospital of Nanjing, Nanjing University of Chinese Medicine, Nanjing 210003, People’s Republic of China; 2Safety Assessment and Research Center for Drug, Pesticide and Veterinary Drug of Jiangsu Province, Nanjing Medical University, Nanjing 211166, People’s Republic of China; 3Department of Physiology, Nanjing Medical University, Nanjing 211166, People’s Republic of China; 4State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing 211166, People’s Republic of China
Background: Wnt5a is a nontransforming Wnt family member and identified as an oncogenic role on cell motility of breast cancer and glioblastoma. However, Wnt5a signaling in esophageal squamous cell carcinoma (ESCC) progression remains poorly defined.
Materials and methods: Immunohistochemistry assays were used to measure the Wnt5a expression in ESCC sections. We evaluated the role of receptor tyrosine kinase-like orphan receptor (ROR)1/2 and RhoA on the invasion of ESCC cells by using cell invasion assay, immunoprecipitation, immunofluorescence, and Rho activation assay.
Results: Wnt5a was highly expressed in invasive ESCC tissues compared with that in noninvasive and nonmalignant tissues. In vitro assay showed that sfrp2 (Wnt5a antagonist) largely blocked the invasion but not the colony formation of KYSE410 and KYSE520 ESCC cells. Anti-ROR1 mAb and ROR2-shRNA markedly inhibited the disheveled-associated activator of morphogenesis 1 (DAAM1) activity, RhoA activity, microfilament formation and the invasion of ESCC cells. Fluorescent phalloidin staining experiment showed ROR1/ROR2, receptors of Wnt5a signaling, and regulated the reassembly of actin filaments in ESCC cells. Further experiments showed that ROR1 was strongly associated with ROR2 in KYSE410 cells. The activation of RhoA, not Rac1 or Rac2, was involved in ROR1/ROR2 signaling pathway. By using DAAM1 shRNA, we found that RhoA was downstream of DAAM1, which could be rescued by the overexpression of wild-type DAAM1. This could be further proved by a RhoA inhibitor CCG-1423 which could inhibit the invasion of ESCC cells but not DAAM1 activity.
Conclusions: Wnt5a promotes ESCC cell invasion via ROR1 and ROR2 receptors and DAAM1/RhoA signaling pathway.
Keywords: ESCC, invasion, Wnt5a, DAAM1, ROR
Introduction
Esophageal squamous cell carcinoma (ESCC) is a common malignant tumor of digestive system and its mortality rate ranks sixth all around the world.1 Surgical resection is the first choice for patients with early ESCC. However, patients mainly suffer from advanced ESCC which is often accompanied by lymph node metastasis or other complications.2 The stage of ESCC invasion based on TNM staging system helps guide clinical decision and the subsequent therapeutic regimen making.3–5 Cancer cells invading the para-carcinoma tissues causes cancer cells spreading to lymph nodes or distant organs, resulting in the deterioration of this disease. However, the underlying mechanism of ESCC cell invasion is still largely unknown.
According to the comprehensive genomic analysis of 158 ESCC cases, the International Cancer Genome Consortium research project reveals that somatic variations are mainly involved in Wnt and Notch pathways.6 The Wnt signaling pathways begin with proteins passing signals into the cell through cell surface receptors, including Frizzled family receptors, lipoprotein receptor-related proteins, and receptor tyrosine kinase-like orphan receptors (RORs).7,8 Wnt signaling pathways have been characterized: the canonical Wnt pathway, the noncanonical planar cell polarity pathway, and the noncanonical Wnt/calcium pathway.9 Aberrant expression and location of beta-catenin, a member of the Wnt signaling pathway, regulates the progression of oesophageal cancer.10 Wnt2 secreted by tumor fibroblasts enhances the motility and the invasiveness of ESCC cells by inducing epithelial–mesenchymal transition.11 Treatment with galangin (an active pharmacological ingredient from propolis and Alpinia officinarum Hance) and berberine (a major component of Berberis vulgaris extract) causes the decreased expression of Wnt3a/β-catenin and induces apoptosis in ESCC cells.12
Wnt5a, as a nontransforming Wnt family member, mediates cancer initiation and metastasis.13,14 Previous studies found that Wnt5a triggers disheveled 2/disheveled-associated activator of morphogenesis 1 (DAAM1) signaling pathway and actives RhoA, resulting in the elevation of the migratory capacity of breast cancer cells and the invasion of glioblastoma cells.15,16 DAAM1, an element of cellular actin cytoskeleton, interacts with disheveled and RhoA and can polymerize actin filaments at the barbed end.13,15,17 The active DAAM1 is elevated by the treatment of Wnt5a or type IV collagen and DAAM1 participates in the breast cancer cell migration and haptotaxis.15,18 However, the role of Wnt5a in the invasion of ESCC cells is still largely unknown.
In this study, we first demonstrate that Wnt5a is upregulated in invasive ESCC tissues and promotes the invasion of ESCC cells. We also describe the mechanism underlying the Wnt5a/ROR1/2/DAAM1/RhoA signaling pathways that regulate ESCC cell invasion. Overall, these data identify ROR1/2 as the novel therapeutic targets in limiting esophageal cancer invasion.
Materials and methods
Clinical samples
Twenty-two ESCC patients were recruited by The Second Hospital of Nanjing from 2014 to 2018. The areas of higher tumor cell density for immunohistochemistry (IHC) were histopathologically confirmed by a pathologist. Formalin-fixed and paraffin-embedded tumor samples were obtained for IHC analysis of Wnt5a. Pathologic staging was performed in accordance with the Union for International Cancer Control. The study was conducted in accordance with the Declaration of Helsinki and reviewed and approved by the research ethics committee, The Second Hospital of Nanjing (No. 2018-LY-KY068). Written informed consent was taken from each participant.
Cell culture and transfections
The human ESCC cell lines KYSE410 and KYSE520 were from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). These cells were grown in RPMI-1640 medium (Cat. KGM31800-500, KeyGEN BioTECH, Nanjing, China) supplemented with 10% (v/v) FBS (Cat. SH30068.03, Hyclone, Logan, UT, USA) and 0.5 μg/mL penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2.
Short hairpin RNAs (shRNAs) specific for DAAM1 (5ʹ-GCCACTTTGTATCCTATCAGG-3ʹ), ROR2 shRNA (Cat. sc-72390-SH, Santa Cruz Biotechnology, Dallas, TX, USA) and/or wild-type (WT) ROR2 constructs were transfected into KYSE410 and KYSE520 cells using Lipofectamine 2000 reagent (Cat. 11668-019, Invitrogen, Carlsbad, CA, USA). The cells were switched to fresh medium containing 10% FBS 6 hrs after transfection and cultured for 48 hrs. All cells were cultured in a 37°C incubator with 5% CO2.
Immunohistochemistry (IHC)
ESCC pathological sections were deparaffinized at 55°C for 30 mins. Then, the sections were washed with xylene for three 5-min washes. The sections were rehydrated by 5-min successive washes in 100%, 90%, and 70% ethanol. Antigens were retrieved by microwaving the samples for 4 mins in 250 mL of 10 mmol/L sodium citrate (pH 6.0). Furthermore, endogenous peroxidase activity was blocked by incubation with 0.3% hydrogen peroxidase (ZSGB-Bio, Beijing, China) for 20 mins. The primary anti-Wnt5a (1:100 dilution, Cat. sc-365370, Santa Cruz Biotechnology) antibody was used. Diaminobenzidine (DAB) and hematoxylin counterstain (ZSGB-Bio) were used to visualize the antibody staining.
The percentage of positively stained cells was scored from 0 to 4: 0 (<10%), 1 (10–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%). The staining intensity was scored on a scale of 0 to 3: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The immunoreactivity score (IRS) for each case was multiplied by the percentages of positive cells and staining intensities. The IRS ranged from 0 to 12 (0, 1, 2, 3, 4, 6, 8, 9, and 12) and was performed without prior clinical records. All these results come from the same block in which both tumor and nonmalignant cells were counted. The invasive ESCC is judged by an experienced pathologist who showed that tumor cells penetrate the mucosa of esophagus.
Immunoblotting
KYSE410 and KYSE520 cells treated with CCG-1423 (Cat. S7719, Selleck Chemicals, Houston, TX, USA) or transfected with indicated constructs were washed with PBS and lysed with RIPA lysis buffer.19 The lysates were then clarified by centrifugation at 12,000xg for 20 mins at 4°C. The protein extracts were separated by 8% or 10% SDS-PAGE. The following antibodies were used: anti-Wnt5a (1:1,000 dilution, Cat. sc-365370, Santa Cruz Biotechnology), anti-β-actin (1:5,000 dilution, Cat. 60008-1-Ig, ProteinTech Group, Wuhan, China), anti-ROR1 (1:1,000 dilution, Cat. 20629-1-AP, ProteinTech), anti-ROR2 (1:1,000 dilution, Cat. 88639, Cell Signalling Technology, Danvers, MA, USA), anti-flag (1:1,000 dilution, Cat. 14793, Cell Signalling Technology), anti-GST (1:1,000 dilution, Cat. 2624, Cell Signalling Technology), anti-RhoA (1:1,000 dilution, Cat. 10749-1-AP, ProteinTech), and anti-DAAM1 (1:1,000 dilution, Cat. sc-100942, Santa Cruz Biotechnology) antibodies. Horseradish peroxidase-conjugated antibodies and Pierce™ ECL western blotting substrate (Cat. 32209, Thermo Fisher Scientific, Waltham, MA, USA) were used to exposure protein bands. The pictures were taken by Tanon 5200 Multi Imaging Workstation (Tanon, Shanghai, China) and the densitometry of immunoblots was analyzed by ImageCal software (Tanon).
Pulldown assays
GST-RhoA beads were used to detect active DAAM1 and first incubated with 0.1 mmol/L GTPγS (Sigma-Aldrich, St. Louis, MO, USA) at 30°C for 15 mins. Cells were washed with PBS and lysed with lysis buffer.18 The equal amount of cellular lysates was incubated with GTPγS-incubated GST-RhoA beads captured on MagneGST glutathione particles (Cat. V8611, Promega, Fitchburg, WI, USA) for 90 mins at 4°C with constant rotation. The beads were washed three times with washing buffer.18 After that, beads were gathered by a magnet in a magnetic stand. The SDS-PAGE and the measurement of the immunoblot bands density were undergone according to standard protocol.
The bacterially expressed recombinant GST-ROR1 and flag-ROR2 were purified by using GST-Sepharose 4B (Cat. 17-0756-01, GE Healthcare, Uppsala, Sweden) and Pierce Anti-DYKD4K (FLAG) Magnetic Agarose Beads (Cat. A36797, Thermo Fisher Scientific), respectively. GST-ROR1 and flag-ROR2 beads were stored for 90 mins at 4°C with constant rotation. After rinsing with washing buffer three times, the beads were enriched by centrifugation at 12,000 × g for 5 mins.
Enzyme-linked immunosorbent assay (ELISA)
KYSE410 and KYSE520 cells were grown in normal cell cultural condition. Equal volumes of cell culture media were diluted in ice-cold PBS buffer. The experiments were then performed in accordance with the protocol of the Wnt5a ELISA kit (Cat. CSB-EL026138HU, CusaBio, Wuhan, China). The concentration was calculated according to the concentration curve for Wnt5a standard samples.
Boyden chamber assays
A modified Boyden chamber system (8.0 μm pore diameter, Cat. 3422, Costar, Corning, NY, USA) were used in this study. The membranes of Boyden chambers were coated with Matrigel™ Basement Membrane Matrix (Cat. 356234, BD Biosciences, Bedford, MA, USA) for 30 mins at 37°C. KYSE410 and KYSE520 cells were treated with ROR1 rabbit mAb (1:1,000 dilution, Cat. 16540, Cell Signalling Technology) or vehicle and then suspended in serum-free culture medium supplemented with 5 μg/mL BSA. The suspensions containing 1×105 cells were added into the upper sides of Boyden chambers. The cells were allowed to invade at 37°C. After that, stationary cells were removed with a cotton-tipped applicator, and the membranes were stained with 0.5% crystal violet. The number of cells passing through the membrane was evaluated and counted in a microscope (Mshot MF53, Micro-shot Technology Co., Guangzhou, China).
Clonogenic assay
KYSE410 and KYSE520 cells were placed into 6-well plates (5,000 cells/well) and incubated with 1 μg/mL secreted Frizzled-related protein 2 (sfrp2) at 37°C for 15 days, fixed and stained with crystal violet. The pictures of tumor colonies were taken by a commercial digital camera (PowerShot S110, Canon, Japan). The number of colonies was counted using an Adobe Photoshop CS2 software from five independent replicates.
Immunoprecipitation (IP)
To test the interaction between ROR1 and ROR2, KYSE410 cells were lysed with lysis buffer.18 Cellular lysates were centrifuged at 10,000×g for 10 mins. Clarified lysates were incubated with ROR2 or lgG antibodies overnight and then treated with protein A/G conjugated agarose beads (Pierce, Rockford, IL, USA). After incubation for 1.5 hrs with agitation, beads were washed with lysis buffer for three times. After boiling in 60 μL of 2×SDS sample buffer for 5 mins, samples were subjected to SDS-PAGE and blotted with anti-ROR1 (1:1,000 dilution) or anti-ROR2 antibodies (1:1,000 dilution).
Rho GTPase activation assays (G-LISA small GTPase activation assays)
Rho GTPase activation assays (Cat. BK121 for RhoA, Cat. BK127 for Cdc42, Cat. BK125 for Rac1,2,3, Cytoskeleton Inc., Denver, CO, USA) were used to measure the Rho activity. Briefly, cells were plated onto 24-well plates (Costar) and grown to the desired confluency. Cell extracts were equalized with ice-cold lysis buffer containing protease inhibitor cocktail. Ten microliters of each lysate or lysis buffer and 290 µL of Precision Red Advanced Protein Assay Reagent were added into a 1.5 mL tube. The mixture was incubated for 1 min at room temperature. The Precision Red Advanced Protein Assay Reagent results in a red to purple/blue color change which can be characterized by an increase in absorbance at 600 nm. The absorbance of each sample was read in a blank spectrophotometer (AOE Instruments, Shanghai, China).
Immunofluorescence and actin cytoskeleton staining
Cells adhering on glass slides were fixed in 4% paraformaldehyde/PBS for 20 mins and then permeabilized in 0.2% Triton X-100. Cells were subsequently blocked in PBS containing 1% BSA for 60 mins at room temperature. Next, cells were stained with tetramethylrhodamine (TRITC)-labelled phalloidin (5 µg/mL, Sigma-Aldrich) for 40 mins in room temperature. After washing with PBS, DAPI Fluoromount G (Southern Biotech, Birmingham, AL, USA) was added to the glass slides. The images were snapshotted by a fluorescence microscope (Zeiss LSM710, Oberkochen, Germany).
Statistical analysis
All statistical analyses were calculated by SPSS 23.0 software (Chicago, IL, USA). The data of ELISA assays (Figure 1E) were analyzed by Student’s t-test, the data of correlation analysis (Table 1) were analyzed by χ2 test, and the other data were analyzed by one-way ANOVA. The number of replications was five if not noted, which were acquired in five independent experiments. For all analyses, bar charts show means ± SD and a two-sided p-value <0.05 was defined as statistically significant.
 | Table 1 Associations between Wnt5a expression and clinicopathological features of esophageal squamous cell carcinoma |
Results
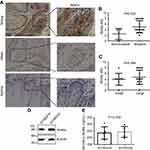
Wnt5a is highly expressed in invasive ESCC tissues
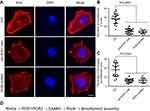
To investigate whether Wnt5a signaling regulated the pathogenesis of human ESCC, the expression patterns of Wnt5a were examined in invasive ESCC tissues. According to the analysis of 22 ESCC cases, we found that the Wnt5a was highly expressed in invasive tumor tissues compared with that in noninvasive tumor tissues (Figure 1A and B). There is no expression in nonmalignant esophageal tissues (Figure 1A). However, we did not find the differential Wnt5a expression between in small tumor tissues and in large tumor tissues (Figure 1C). Table 1 shows the clinical characteristics of 22 esophageal cancer patients. Wnt5a expression seems highly associated with lymph node metastasis (Table 1), although more patients’ samples are needed for further analysis.
We previously investigated EGFR expression and telomere in ESCC cells KYSE410 and KYSE520 and found KYSE410 and KYSE520 cells had the most difference in all nine KYSE cell lines.20–22 In this study, we found the high expression and secretion levels of Wnt5a in KYSE410 and KYSE520 ESCC cells (Figure 1D and E), which were subjected to the measure of the ability of Wnt5a-induced cell invasion. The quantity of secretory endogenous Wnt5a was 236.2±40.96 ng/mL and 243.6±35.91 ng/mL in KYSE410 and KYSE520 cells, respectively (Figure 1E).
Wnt5a induces the invasion of ESCC cells
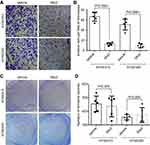
To determine whether Wnt5a induced the invasion of ESCC cells, we examined the invasion of KYSE410 and KYSE520 cells through 8.0-μm pores in membranes coated with matrigel. An approximately four-fold decrease was shown in the number of invasive cells treated with 1 μg/mL secreted Frizzled-related protein 2 (sfrp2), a Wnt5a antagonist (Figure 2A and B). Moreover, sfrp2 did not alter the ability of colony formation of KYSE410 and KYSE520 ESCC cells (Figure 2C and D). Thus, Wnt5a signaling participates in the regulation of ESCC cell invasion.
ROR1 and ROR2 participate in the invasion of ESCC cells
To investigate the effect of the receptors of Wnt5a signaling on the invasion of ESCC cells, we blocked the ROR1 and/or ROR2 signaling of ESCC cells and examined the cell invasion by Boyden chamber assays. ROR2-shRNA knocked down ROR2 expression by ~70% in KYSE410 and KYSE520 cells, which can be rescued by WT ROR2 overexpression (Figure 3A). Silence of ROR2 gave rise to a significant reduction of cell invasion, which also can be rescued by WT ROR2 overexpression (Figure 3B and C).
Next, we tested whether ROR1 physically interacted with ROR2 in human ESCC cells. Interestingly, ROR1 was strongly associated with ROR2 in KYSE410 cells (Figure 3D). An in vitro binding assay demonstrated that purified bacterially expressed recombinant ROR1 was directly binding to purified ROR2 (Figure 3E). Thus, ROR1/ROR2 receptors participate in the invasion of ESCC cells.
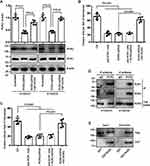
RhoA functions as a downstream target of ROR1/ROR2
The significantly decreased activity of Rac1, Rac2, and RhoA treated with anti-ROR1 mAb were found in KYSE410 ESCC cells (Figure 4A). To elucidate which specific Rho GTPases were involved in both ROR1 and ROR2 signaling pathways, we performed Rho GTPase activation assays after ROR2 knockdown. The activation of RhoA, not Rac1 or Rac2, was downregulated in ROR2 knockdown cells, which could be rescued by the WT ROR2 overexpression (Figure 4B and C). Moreover, the combination of ROR1 blockage and ROR2 knockdown showed a similar effect on the decrease of RhoA activity to the single treatment of ROR1 blockage or ROR2 knockdown (Figure 4C).
CCG-1423 (a RhoA-specific inhibitor) was used to block the RhoA activity and to study its role in cell invasion. Treatment with 1 µmol/L CCG-1423 inhibited the cell invasion of ESCC cells by approximately 59.3% and 66.3% reduction for KYSE410 and KYSE520 cells, respectively (Figure 4D). Thus, these results suggested that RhoA functions as a downstream target of ROR1/ROR2 and is participated in cell invasion of ESCC cells.
ROR1/ROR2-dependent DAAM1 activity is involved in cell invasion
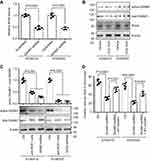
To study whether RhoA acted as the downstream of DAAM1, we silenced DAAM1 using shRNA and measured the RhoA activity. A ~0.5-fold decrease was shown in DAAM1-knockdown ESCC cells (Figure 5A). The blockage of RhoA activity by CCG-1423 treatment was unable to change DAAM1 activity (Figure 5B), suggesting the role of DAAM1 functioning as the upstream of RhoA.
Next, we examined whether DAAM1 activity was dependent on ROR1/ROR2 receptors in ESCC cells. A significant decrease in active DAAM1 levels was shown after ROR1 blockage or ROR2 knockdown (Figure 5C). The invasion of DAAM1 knockdown cells was largely inhibited, which could be rescued by the overexpression of WT DAAM1 (Figure 5D). Taken together, these experiments demonstrated that ROR1/ROR2-dependent DAAM1 activity acts as the upstream of RhoA and is involved in cell invasion of ESCC cells.
ROR1 and ROR2 regulate the reassembly of microfilaments
We next examined whether ROR1 and ROR2 signaling regulate the reassembly of actin filaments in ESCC cells. Fluorescent phalloidin staining was performed to investigate the distribution of filamentous actin (F-actin) in KYSE410 cells. We found that the formation of microfilaments was disrupted by anti-ROR1 mAb treatment, and the length of microfilaments was largely decreased (Figure 6A–C). We also found that the knockdown of ROR2 disrupted the formation of microfilaments and decreased the length of microfilaments (Figure 6A–C). Thus, the findings indicated that ROR1 and ROR2 signaling regulates the reassembly of microfilaments in ESCC cells.
Discussion
We previously found that Wnt5a stimulates the migration of breast cancer cells and promotes the invasion of glioblastoma cells via Rho signaling pathways.15,16,19 Liu et al reported that Wnt5a enhances the ability of cell mobility via RhoA signaling pathways in gastric cancer.23 In this study, we demonstrated that Wnt5a was highly expressed in invasive ESCC tissues, and the blockage of Wnt5a signaling by sfrp2 treatment largely inhibited the invasion of ESCC cells. These evidence support that Wnt5a acts as an oncogene in multiple solid tumours, including esophageal cancer, breast cancer, glioblastoma, and gastric cancer.
Evolutionarily conserved ROR1/2 are regarded as distinct receptors for Wnt5a and are involved in noncanonical Wnt signaling in organogenesis and cancer metastasis.24 Cirmtuzumab (UC-961) is a first-in-class humanized IgG1 monoclonal antibody, which is designed and developed to bind with high affinity to the extracellular domain of ROR1.25–27 Cirmtuzumab is safe and effective in a Phase I trial for patients with progressive, relapsed, or refractory chronic lymphocytic leukemia (CLL).28 Consistent with the studies for CLL, we found that anti-ROR1 mAb markedly inhibits the DAAM1/RhoA activity, microfilament formation, and the invasion of ESCC cells. This study identifies ROR1 as a novel pharmaceutical target to postpone the invasion of ESCC.
Wnt5a induces ROR1/2 heterooligomerization to strengthen leukemia chemotaxis and proliferation.24 ROR2 expressed on the plasma membrane is essential for ligand binding and Wnt signal transduction.29,30 In this study, an in vitro binding assay and immunoprecipitation assay demonstrated that purified bacterially expressed recombinant ROR1 is directly binding to purified ROR2. Knockdown of ROR2 markedly inhibits the DAAM1/RhoA activity, microfilament formation, and the invasion of ESCC cells. Moreover, anti-ROR1 mAb and ROR2 shRNA alone and combination of anti-ROR1 mAb with ROR2 shRNA play an equal effect on the invasion of ESCC cells, indicating ROR1/2 heterooligomers mediate Wnt5a-induced cell invasion.
DAAM1 is a formin protein, which links Wnt5a signal with Rho small GTPases and controls the rearrangement of microfilament.13,31 Our results demonstrate that DAAM1 functions as the downstream target of Wnt5a and ROR1/2 signaling in ESCC cells. shRNA-mediating silence of DAAM1 inhibits RhoA activity and the invasion of ESCC cells. The decrease of RhoA activity and cell invasion in DAAM1-knockdown cells indicates that DAAM1 has the ability to interact with ROR1/2 in response to Wnt5a to activate RhoA and facilitate cell invasion in ESCC (Figure 6D).
CCG-1423, a small chemical component, is a Rho inhibitor involved in potently blocking RhoA signaling through short-term treatment with CCG-1423 (<1 mmol/L).32 High dose of CCG-1423 inhibits Rho-dependent invasion of PC-3 prostate cancer cells.32 Preincubation with CCG-1423 (1 μmol/L) completely inhibits the collagen-induced haptotaxis of breast MDA-MB-231 and MDA-MB-453 cancer cells.18 CCG-1423 (0.022 μmol/L) blocks Wnt5a-induced glioblastoma U251 and T98MG cell invasion.16 Here, we found that treatment with CCG-1423 (1 µmol/L) inhibited the cell invasion of ESCC KYSE410 and KYSE520 cells. Based on these literature, CCG-1423, a pharmacological inhibitor to prevent cancer cellular events via blocking RhoA signaling, has an extremely broad range of doses.
Conclusion
This study clarified the connection between Wnt5a signaling and ESCC invasion. We demonstrated that Wnt5a mediates the invasion of ESCC cells, at least in part via ROR1/2 receptors and DAAM1/RhoA signaling pathway. Therefore, Wnt5a/ROR1/2/DAAM1/RhoA signaling pathway may be the potential therapeutic targets for human esophageal cancer.
Abbreviation list
ESCC, esophageal squamous cell carcinoma; ROR1, receptor tyrosine kinase-like orphan receptor 1; ROR2, receptor tyrosine kinase-like orphan receptor 2; DAAM1, disheveled-associated activator of morphogenesis 1; Sfrp2, secreted frizzled-related protein 2; mAb, monoclonal antibody.
Acknowledgments
This work was supported by grants from National Nature Science Foundation of China (Grant No. 81301938), the Natural Science Foundation of Jiangsu Province (Grant No. BK20181367) to Yichao Zhu, the Science and Technology Foundation of Nanjing Medical University (Grant No. 2017NJMU001) to Ting Yan and Medical Science and technology development Foundation, Nanjing Department of Health (Grant No. JQX14007) to Xuping Wu.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Reeh M, Nentwich MF, Asani S, et al. Locally advanced esophageal carcinoma: is there still a role of surgery alone without neoadjuvant treatment? J Gastrointest Surg. 2015;19(4):587–593. doi:10.1007/s11605-015-2762-y
2. Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12(7):681–692. doi:10.1016/S1470-2045(11)70142-5
3. Rice TW, Blackstone EH, Rusch VW. A cancer staging primer: esophagus and esophagogastric junction. J Thorac Cardiovasc Surg. 2010;139(3):527–529. doi:10.1016/j.jtcvs.2009.11.002
4. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: an eighth edition staging primer. J Thorac Oncol. 2017;12(1):36–42. doi:10.1016/j.jtho.2016.10.016
5. Donohoe CL, Phillips AW. Cancer of the esophagus and esophagogastric junction: an 8(th) edition staging primer. J Thorac Dis. 2017;9(3):E282–E284. doi:10.21037/jtd.2017.03.39
6. Song Y, Li L, Ou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509(7498):91–95. doi:10.1038/nature13176
7. Nusse R, van Ooyen A, Cox D, Fung YK, Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984;307(5947):131–136.
8. Nusse R. Wnt signaling in disease and in development. Cell Res. 2005;15(1):28–32. doi:10.1038/sj.cr.7290260
9. Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi:10.1016/j.cell.2006.10.018
10. Kiely B, O’Donovan RT, McKenna SL, O’Sullivan GC. Beta-catenin transcriptional activity is inhibited downstream of nuclear localisation and is not influenced by IGF signalling in oesophageal cancer cells. Int J Cancer. 2007;121(9):1903–1909. doi:10.1002/ijc.22794
11. Fu L, Zhang C, Zhang LY, et al. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/beta-catenin signalling pathway. Gut. 2011;60(12):1635–1643. doi:10.1136/gut.2011.241638
12. Ren K, Zhang W, Wu G, et al. Synergistic anti-cancer effects of galangin and berberine through apoptosis induction and proliferation inhibition in oesophageal carcinoma cells. Biomed Pharmacother. 2016;84:1748–1759. doi:10.1016/j.biopha.2016.10.111
13. Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–854.
14. Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320(5874):365–369. doi:10.1126/science.1151250
15. Zhu Y, Tian Y, Du J, et al. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS One. 2012;7(5):e37823. doi:10.1371/journal.pone.0037823
16. Liu G, Yan T, Li X, et al. Daam1 activates RhoA to regulate Wnt5ainduced glioblastoma cell invasion. Oncol Rep. 2018;39(2):465–472. doi:10.3892/or.2017.6124
17. Kerkhoff E. Actin dynamics at intracellular membranes: the Spir/formin nucleator complex. Eur J Cell Biol. 2011;90(11):922–925. doi:10.1016/j.ejcb.2010.10.011
18. Yan T, Zhang A, Shi F, et al. Integrin alphavbeta3-associated DAAM1 is essential for collagen-induced invadopodia extension and cell haptotaxis in breast cancer cells. J Biol Chem. 2018;293(26):10172–10185. doi:10.1074/jbc.RA117.000327
19. Zhu Y, Shen T, Liu J, et al. Rab35 is required for Wnt5a/Dvl2-induced Rac1 activation and cell migration in MCF-7 breast cancer cells. Cell Signal. 2013;25(5):1075–1085. doi:10.1016/j.cellsig.2013.01.015
20. Wu X, Hedman H, Bergqvist M, et al. Expression of EGFR and LRIG proteins in oesophageal carcinoma with emphasis on patient survival and cellular chemosensitivity. Acta Oncol. 2012;51(1):69–76. doi:10.3109/0284186X.2011.562239
21. Wu X, Smavadati S, Nordfjall K, et al. Telomerase antagonist imetelstat inhibits esophageal cancer cell growth and increases radiation-induced DNA breaks. Biochim Biophys Acta. 2012;1823(12):2130–2135. doi:10.1016/j.bbamcr.2012.08.003
22. Wu X, Sooman L, Lennartsson J, et al. Microtubule inhibition causes epidermal growth factor receptor inactivation in oesophageal cancer cells. Int J Oncol. 2013;42(1):297–304. doi:10.3892/ijo.2012.1710
23. Liu J, Zhang Y, Xu R, et al. PI3K/Akt-dependent phosphorylation of GSK3beta and activation of RhoA regulate Wnt5a-induced gastric cancer cell migration. Cell Signal. 2013;25(2):447–456. doi:10.1016/j.cellsig.2012.10.012
24. Hasan MK, Yu J, Widhopf GF
25. Yu J, Chen L, Cui B, et al. Cirmtuzumab inhibits Wnt5a-induced Rac1 activation in chronic lymphocytic leukemia treated with ibrutinib. Leukemia. 2017;31(6):1333–1339. doi:10.1038/leu.2016.368
26. Yu J, Chen Y, Chen L, et al. Cirmtuzumab inhibits ibrutinib-resistant, Wnt5a-induced Rac1 activation and proliferation in mantle cell lymphoma. Oncotarget. 2018;9(37):24731–24736. doi:10.18632/oncotarget.25340
27. Choi MY, Widhopf GF
28. Choi MY, Widhopf GF
29. Hasegawa D, Wada N, Yoshida S, et al. Wnt5a suppresses osteoblastic differentiation of human periodontal ligament stem cell-like cells via Ror2/JNK signaling. J Cell Physiol. 2018;233(2):1752–1762. doi:10.1002/jcp.26086
30. Dai B, Yan T, Zhang A. ROR2 receptor promotes the migration of osteosarcoma cells in response to Wnt5a. Cancer Cell Int. 2017;17:112. doi:10.1186/s12935-017-0482-y
31. Liu W, Sato A, Khadka D, et al. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105(1):210–215. doi:10.1073/pnas.0707277105
32. Evelyn CR, Wade SM, Wang Q, et al. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6(8):2249–2260. doi:10.1158/1535-7163.MCT-06-0782
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.