Back to Journals » Journal of Blood Medicine » Volume 12
What Does the Economic Burden of Acute Myeloid Leukemia Treatment Look Like for the Next Decade? An Analysis of Key Findings, Challenges and Recommendations
Authors Forsythe A, Sandman KE
Received 9 February 2021
Accepted for publication 13 April 2021
Published 5 May 2021 Volume 2021:12 Pages 245—255
DOI https://doi.org/10.2147/JBM.S279736
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Anna Forsythe, Karen Sandman
Purple Squirrel Economics, New York, NY, USA
Correspondence: Anna Forsythe
Managing Partner and CEO, Purple Squirrel Economics, 4 Lexington Ave, Suite 15K, New York, NY, 10010, USA
Tel +1-646-477-0936
Email [email protected]
Abstract: Acute myeloid leukemia (AML) is conventionally treated with chemotherapy in eligible patients. Potentially curative regimens are associated with significant toxicity, and the major cost drivers in AML historically have been hospitalization and hematopoietic stem cell transplantation. The past several years have seen a dramatic increase in the number of treatment options, including oral therapies and drugs targeted to biological pathways implicated in AML. Major current and future drivers of cost in AML include hospitalization and medical costs, stem cell transplantation for eligible patients, and medication costs. It is likely that hospitalization and medical costs will decline as more AML treatment moves to the outpatient setting. Stem cell transplantation costs may increase, if more patients are eligible for improved procedures, although the overall cost of transplantation could decrease if new procedures reduce the need for hospitalization. Medication costs are likely to increase, with various branded drugs available and in development. From a broader perspective, another driver of cost is the proportion of patients with AML who can undergo treatment. Patients who may previously have been unable to tolerate chemotherapy are more likely to be treated with the range of less intensive, more tolerable options now available. The effectiveness of newer AML treatment options also suggests that, overall, there may be more patients staying alive and on treatment longer than in the past. While certain advances, such as increased use of oral and outpatient therapies, could potentially reduce costs, the overall economic impact of AML is likely to increase as more patients are eligible for novel therapies across several phases from induction to maintenance to relapsed/refractory disease. While these novel therapies have the potential to deliver value in the form of improved efficacy, safety, and convenience, payers will need to determine how to cover a longer, more complex AML treatment pathway.
Keywords: AML, leukemia, cost of illness, economic burden
AML: A Rare and Costly Cancer
Acute myeloid leukemia (AML) arises in the bone marrow from the abnormal clonal expansion of myeloid blood cell precursors. Leukemic blast cells are found in the circulating blood as well as in the bone marrow, where they disrupt normal blood cell production, leading to myelosuppression.1 Consequences of AML include anemia, with weakness and pallor, thrombocytopenia, resulting in bleeding, and leukopenia, leading to fever and infection.
AML, which accounts for about 30% of all leukemia cases, is a relatively rare cancer, accounting for about 1.1% of all cancers in the US, with about 20,000 new cases and 11,000 deaths per year.2 Global incidence of AML in 2018 was estimated to be around 130,000.3 AML tends to affect older individuals, with a median age at diagnosis of 68 years.2
Without treatment, acute leukemias can be rapidly fatal, but with prompt initiation of intensive treatment, survival of several years or more is achievable.1 Conventional treatment for AML involves aggressive, cytotoxic induction chemotherapy, with the goal of allogeneic hematopoietic stem cell transplant (HSCT) in eligible patients. This intensive approach, while successful in some patients, has not been feasible in many elderly patients due to poor performance status and comorbidities.4 Thus, while long-term survival approaches 50% in patients under age 65, it drops to about 10% in patients over 65.5 The 5-year overall survival rate in the US from 2010 to 2016 was 28.7%.2
Based on the low overall survival rates in AML, as well as the disparities in treatment outcomes for younger and older patients, there has been considerable research in recent years to develop treatment pathways for different subgroups of patients. At the same time, an evolving understanding of AML biology has sparked the development of targeted therapies that can be tailored to patients based on genetic features of their cancer cells.4 The current standard approach is to evaluate patients’ fitness for intensive chemotherapy (IC) and HSCT. If a patient is an IC candidate, they typically receive an induction regimen, such as cytarabine plus daunorubicin, followed by HSCT if possible. Patients may also undergo consolidation therapy with high-dose cytarabine. If a patient is a candidate for non-intensive chemotherapy (NIC), they may receive a low-dose hypomethylating agent (HMA) such as azacitidine or decitabine, or they may receive low-dose cytarabine (LDAC). While these NIC options have relatively modest efficacy, in recent years there have been a series of approvals of new, targeted drugs that can be used with or without NIC, providing patients with a range of options beyond conventional chemotherapy. Patients who achieve complete remission may receive maintenance therapy with recently approved oral options (oral azacitidine approved by the US Food and Drug Administration [FDA] and midostaurin approved by the European Medicines Agency [EMA]). Relapsed or refractory (R/R) patients may receive an IC or NIC reinduction regimen, with or without a targeted agent, or single-agent targeted therapy.1,4,6
Conventional therapies for AML incur considerable costs and healthcare resource utilization (HCRU), with hospital-based chemotherapy infusions, the need for frequent monitoring, and the inevitable need to treat serious adverse effects of treatment.7 HSCT, while potentially curative, is a costly inpatient procedure. Newer treatments, with more manageable safety profiles, may help to limit medical and hospitalization costs, but in general these novel drugs cost more than conventional chemotherapy. Moreover, the higher tolerability of newer treatments increases the number of patients who can initiate and stay on treatment, potentially expanding the overall budget impact of AML on healthcare systems.
In conducting this review, we sought to first characterize the current landscape of cost and value in AML, identifying the major cost drivers and how they have evolved in recent years. We then aimed to consider how upcoming advances in treatment and care delivery may impact the economic impact of AML, so that we could identify opportunities for manufacturers, treatment centers, and others to deliver value in AML in the coming years.
AML: A Therapeutic Landscape in Transition
Historical Cost Drivers in AML
We conducted a systematic review of studies reporting economic outcomes in AML. The systematic review was performed in accordance with the methodological principles of conduct for systematic reviews as detailed in the University of York CRD’s “Guidance for Undertaking Reviews in Health Care” and in accordance with methodology established in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.8,9 SLR searches (from database inception to December 2020) were conducted in the MEDLINE, Embase, EconLit and Cochrane databases. In addition to the database searches, keyword searches of the annual proceedings of scientific meetings (American Society for Clinical Oncology [ASCO], European Haematology Association [EHA], European Society for Medical Oncology [ESMO] and American Society for Hematology [ASH]). A total of 54 records were selected from 48 original studies reporting on healthcare resource use or costs in AML. Among selected studies, 31 included US data, and 14 included EU data.
One of the large retrospective database studies on the economic burden of AML in the US before the approval of targeted agents (2008 to 2016) examined HCRU and direct costs in AML in a commercial payer database.10 The most expensive episodes of care were R/R AML ($439,104), HSCT ($329,621), induction IC ($198,657), consolidation IC ($73,428), and NIC ($53,081). Across all these groups, the main driver of cost was inpatient hospitalization, which accounted for about 70% of costs. AML symptoms and treatment toxicity were associated with higher costs, suggesting that less toxic alternatives to chemotherapy may help to control healthcare costs in AML. Several other retrospective studies confirmed these findings, noting very high costs associated with relapse/disease progression; the largest cost driver was inpatient utilization in both private and public healthcare settings.11–13
Outside of the US, the economic picture of AML is similar, as demonstrated in a claims database study (1997 to 2015) covering nearly 40,000 patients with AML in Spain.14 With mean annual direct costs of €30,775 per patient that increased by 3.7-fold from 1999 to 2011, the primary drivers were hospitalization and HSCT. A retrospective study in the Netherlands aimed at calculating the cost of initial treatment in AML also concluded that hospitalization was the major cost driver.15 A large study based on a Swedish registry (N=2954, 2007 to 2015) noted that among all AML treatment phases, the total cost from date of HSCT to death is the largest, amounting to over US$160,000, with inpatient costs accounting for 60% of the total.16
These and other database studies exploring AML costs prior to the advent of novel therapeutics depict a scenario likely to change as less toxic and more effective therapies increase in uptake. The remainder of this review explores the likely transition of key cost drivers in AML in the coming years.
Evolving Clinical and Economic Picture of AML
Following the development of the cytarabine + daunorubicin regimen and HSCT for AML in the 1970s, there were several decades without significant innovation in AML treatment.4,17 This situation changed in 2017, and the past several years have seen nine new products approved in AML. A growing understanding of the genetic features of AML cells has allowed many of these new therapies to target to specific biological pathways implicated in AML development and progression.18 Relevant to the HCRU associated with these treatments, seven of the nine newly approved drugs are orally administered.
Two are for patients with fms-like tyrosine kinase 3 positive (FLT3+) cancer: oral midostaurin may be added to IC in newly diagnosed patients, and patients with R/R AML may receive oral gilteritinib.
Patients with isocitrate dehydrogenase (IDH) mutations may receive oral enasidenib for IDH2+ RR AML, while elderly patients with IDH1+ AML may receive oral ivosidenib in the newly diagnosed or relapsed/refractory setting.
Patients with newly diagnosed AML eligible for IC who have CD33 expression may receive intravenous (IV) gemtuzumab ozogamicin in combination with IC.
A liposomal, IV administered combination of cytarabine and daunorubicin, CPX-351, is available for newly diagnosed therapy-related AML or AML with myelodysplasia-related changes (AML-MRC).
Two targeted therapy options that can be added to NIC for newly diagnosed patients are oral venetoclax and oral glasdegib.
Lastly, oral azacitidine has been approved as a maintenance therapy in patients who achieved first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) following intensive induction chemotherapy and who are not able to complete intensive curative therapy.
In addition to the rapid growth in treatment options for patients with AML, including those eligible for IC or NIC, there have been progressive improvements in supportive care for patients undergoing AML treatment. While IC remains an intensive treatment that can result in serious complications or even death, there have been substantial advances such as the introduction of broad-spectrum oral antifungals and improvements in transfusion medicine. These changes, combined with increased emphasis on patient and caregiver quality of life and management of costs, have allowed many patients to receive a greater proportion of AML treatment in the outpatient setting. Most recently, the COVID-19 pandemic has accelerated the push towards using telehealth and outpatient care when appropriate, and it is likely that these new approaches to care will influence treatment practices in the long term.7
Major drivers of cost in AML include hospitalization and medical costs, stem cell transplantation for eligible patients, and medication costs (conventional chemotherapy and novel agents). From a broader health plan or societal perspective, another driver of cost is the proportion of patients with AML who undergo treatment. Patients who may previously have been unable to tolerate chemotherapy are more likely to be treated with the range of NIC options now available. The effectiveness of newer AML treatment options also suggests that, overall, there may be more patients staying alive and on treatment longer than in the past.
Cost Drivers in Transition: Hospitalization and Medical Costs
Inpatient hospital care, and medical costs more broadly, have historically been major cost drivers in AML. Several recent factors are likely to reduce these costs, which in principle could reduce the overall economic burden of AML. These include the advent of oral AML therapies and chemotherapy regimens that can be administered in the outpatient setting, as well as improved supportive care that may reduce the need for emergency care and prolonged hospitalizations.7
AML treatments that can be administered in the outpatient setting include the seven recently approved oral options. Some of the oral therapies are administered as single agents and others as add-on therapy; choice of agent and regimen depends on biological features of the patient’s AML and the stage of treatment. In addition to the oral options, which avoid administration costs, the new liposomal formulation of daunorubicin and cytarabine, CPX-351, has a simplified dosing schedule19 that allows it to be administered to suitable patients in the outpatient setting, potentially reducing inpatient costs. In a pilot study, 14 patients were able to receive induction chemotherapy as outpatients, reducing their mean overall hospitalization by over 2 days compared with those who received the same regimen as inpatients.20 Another pilot study found that patients were able to safely receive IC for AML and high-risk myelodysplastic syndromes (MDS) as outpatients.21 Fourteen of 17 patients completed IC without needing hospital admission, although most eventually required admission for supportive care typical of patients following induction. Outpatient administration of induction regimens is not expected to prevent hospitalization altogether, but it may reduce costs by decreasing the total number of inpatient days.
Cost Drivers in Transition: HSCT and Other Cellular Therapies
Beyond hospitalization and medical costs, the other major historical cost driver in AML has been HSCT. This intensive procedure requires specialized care, with patients often hospitalized for prolonged periods and requiring extensive follow-up care. While many patients with AML have historically not been considered candidates for HSCT, improved methods for donor selection and relaxed requirements for patient fitness have expanded the eligible patient population, potentially increasing overall HSCT costs in AML.
In recent years, the ability to identify patients who are likely to benefit from allogeneic HSCT has been improved by advances in the cytogenetic and molecular risk stratification of AML as well as early assessments of measurable residual disease (MRD).22,23 Among patients who are considered suitable HSCT candidates, there are increasing options even if they do not have a matched sibling donor. Over 30 million adults are registered worldwide as potential volunteer donors, cryopreserved umbilical cord blood is growing in availability, and there is growing evidence to support the safe transplantation of haploidentical (half-matched) stem cells.24 These factors have led to a sharp increase in the number of allogeneic HSCT procedures performed in AML in the last decade.
There are several new transplantation technologies that potentially expand the donor pool. Despite the promise of umbilical cord blood stem cells in increasing the pool of potential matched donors, one challenge in using this approach in adults has been the relatively low dose of stem cells available, which can lead to graft failure and delayed bone marrow recovery.25,26 One strategy being investigated to address this challenge is UM171, a hematopoietic stem cell self-renewal agonist, which expands umbilical cord blood stem cells, thus allowing for a higher stem cell dose. Initial results in hematologic malignancies demonstrate the feasibility of this approach, with a potential for low risk of chronic graft-versus-host disease and relapse.27 An ongoing study (NCT03913026) is assessing the use of UM171-expanded cord blood cells in patients with high-risk acute leukemia/myelodysplasia. In a pilot UM171 trial (NCT02668315), among 22 patients who received a single UM171 cell bank transplant, the rate of GVHD (10%) was low, with no moderate-to-severe chronic GVHD.27 If methods such as UM171 enter clinical practice, there may be an increase in the number of patients who undergo transplants, and thus in the associated costs—both for the new technology as well as for the transplant procedure and post-transplant care.
Another possible cellular therapy on the horizon for AML is chimeric antigen receptor T-cell (CAR-T) therapy. To date, the CAR-T products that have been approved are used in B-cell malignancies, which lend themselves to this approach because they express antigens that are unique to the B-cell lineage. Myeloid cancers, by contrast, tend to express tumor antigens that are also found on various healthy cells, including hematopoietic stem or progenitor cells, making it challenging to design CAR-T therapy for AML.28 The depletion of stem cells by CAR-T cell therapy would cause prolonged myeloablation, with consequences such as infection and transfusion dependence. Despite these significant challenges, there are ongoing efforts to develop CAR-T treatments for RR AML, with ten ongoing trials identified in a search of clinicaltrials.gov on January 31, 2021 (Table 1).
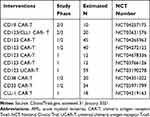 |
Table 1 Ongoing Trials of CAR-T Therapies in AML |
If investigational cellular therapies such as those outlined in Table 1 show efficacy with acceptable safety profiles in AML, they would likely carry a very high cost. The currently available CAR-T therapies cost in the range of $375,000 to $475,000 for a single infusion, in addition to the medical costs and management of complications.29 If a large number of patients with AML were considered candidates for cellular therapy, there could be a significant impact on the overall cost burden in AML. There are, however, substantial payer restrictions on coverage for the currently available CAR-T therapies. The actual cost impact therefore may be less, if only a small fraction of patients with AML are able to access the treatment.
The high cost of CAR-T therapies may be offset by their benefits if these approaches are found to be cost-effective relative to other options. Our systematic literature review did not identify any cost-effectiveness analyses for CAR-T treatments in AML. CAR-T therapy was found to be cost-effective, with an incremental cost-effectiveness ratio (ICER) of $64,600/quality-adjusted life year (QALY), in a microsimulation model of pediatric acute lymphoblastic leukemia (ALL).30 The authors of the pediatric ALL analysis note that longer-term efficacy data for CAR-T may change their findings, and it is not clear whether comparable findings would apply in adults with AML.
Cost Drivers in Transition: Drug Costs
Conventional chemotherapy for AML utilizes largely generic drugs, so that the primary costs are in administration and toxicity management rather than in direct drug costs. The large number of new AML therapies approved in the past several years largely stems from the explosion in research into abnormal genetic pathways in cancer cells and how to disrupt these pathways.18 The tailoring of therapies to specific biological pathways helps to get the right treatment to the right patient, but it also requires genetic profiling of a patient’s cancer cells to inform treatment decisions. The higher demand for genetic testing will likely contribute to the economic burden of AML.
The impact of new AML drugs on costs would best be assessed by analysing treatment patterns. While currently published treatment pattern studies are largely based on data from before new drugs became available in 2017, the novel therapies are by now fully integrated into prominent treatment guidelines, such as the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines. The NCCN guidelines include IC regimens that utilize novel branded therapies such as gemtuzumab ozogamicin, midostaurin, venetoclax, and CPX-351, alongside conventional chemotherapy options.6 For NIC induction therapy, the NCCN guidelines include a variety of novel branded therapies, such as venetoclax, glasdegib, gemtuzumab ozogamicin, ivosidenib, and enasidenib. The guidelines also include most of the novel agents as options for post-remission or maintenance therapy, as well as for R/R AML, with specific recommendations based on patient fitness, biological factors, and prior therapies.
These new targeted therapies, with more on the horizon, offer many potential benefits in terms of efficacy, safety, ease of administration, and reduced hospital time, but as branded drugs and combinations, they are likely to substantially increase spending on medications in AML, both for patients and payers. In the US, patients with private insurance may face substantial out-of-pocket copayments for the self-administered drugs, and those on Medicare may have access challenges as they may be required to justify the branded therapies over established generic options.
In the coming years, the costs of genetic testing and novel therapies are likely to grow as more targeted therapies become available. As of January 2021, there were 621 ongoing interventional Phase 1 to Phase 3 studies in AML registered on ClinicalTrials.gov, over 450 of which are Phase 2 or phase 3. Among the ongoing studies, 99 evaluate biological treatments, 25 are transplantation studies, and the remaining 496 are evaluating drug treatments and combinations. While many of these studies involve new combinations of existing drugs, the innovative pipeline is strong. Table 2 presents ongoing phase 2 or phase 3 studies of treatments targeting specific biologically or genetically defined patient subgroups or molecular pathways.
 |
Table 2 Ongoing Phase 2 and Phase 3 Trials of Therapies Targeting Biologically or Genetically Defined Patient Subgroups or Molecular Pathways in AML |
Cost Drivers in Transition: Utilization of AML Treatment
The previous three major cost drivers discussed in this review—hospitalization/medical costs, HSCT/cellular therapies, and drug costs—relate to how advances in AML treatment may impact the costs of treating a patient with AML. The final category relates to how the evolution of AML treatment may impact the overall economic burden of AML by making high-cost treatment a possibility for a larger proportion of patients with AML.
Despite the availability of NIC options, a recent literature review found that up to one-third of patients in the US and Europe receive only best supportive care for AML, with advanced age, comorbidities, and poor performance status as major factors in the decision not to administer active treatment.31 There is, however, a growing trend to use broader criteria to assess fitness for treatment: the NCCN guidelines refer to “physiologic age” rather than chronological age, to avoid declining treatment to elderly patients who are likely to tolerate and benefit from treatment.32
New treatment options may increase the likelihood of physicians to offer anticancer treatment to older patients. Glasdegib and venetoclax, for example, are approved specifically for use in elderly or unfit patients, based on pivotal trials in these populations.33,34 CPX-351 is approved without age restriction following a pivotal trial demonstrating superior efficacy and comparable safety to conventional cytarabine + daunorubicin in patients age 60 to 75.19 With increasing awareness of physiologic over chronological age, expanded options for targeted therapy with manageable safety profiles, and improved strategies for managing adverse effects of treatment, it is likely that the proportion of treated patients will increase, leading to higher overall costs in AML.
As noted above, the NCCN guidelines include a variety of options for maintenance therapy in AML. The growing role of maintenance therapy in AML is likely to increase the overall number of patients receiving treatment, as patients would continue to be treated rather than waiting for relapse before starting treatment again. Regimens of IV cytarabine and daunorubicin with or without gemtuzumab ozogamicin are recommended by the NCCN for patients under 60 years of age who are eligible for intensive chemotherapy.32 The EMA, but not the FDA, approved oral midostaurin as maintenance therapy on the basis of its phase 3 study (RATIFY; NCT00651261), which included the use of midostaurin in the induction, consolidation and maintenance settings (with progressively fewer patients completing each phase of treatment).35 Maintenance with the hypomethylating agents, IV or oral azacitidine and IV decitabine, has also shown efficacy and is recommended by the NCCN guidelines.6,36,37 With increasingly effective treatments, maintenance therapy may be of long duration: in the phase 3 trial of oral azacitidine, 71% of patients stayed on therapy for at least 6 months, while 49% were exposed for over 1 year.37
Likewise, there has been an expansion in the number of options for R/R AML, with the NCCN guidelines for R/R AML including oral therapies such as gilteritinib, enasidenib, ivosidenib, and venetoclax, and other treatments such as gemtuzumab ozogamicin.6 These novel therapies have generally manageable toxicity profiles, making treatment for R/R AML a feasible option for a broader set of patients.
There are likely to be even more options for maintenance and R/R therapy in AML in upcoming years. As of January 2021, there were 14 ongoing studies registered with ClinicalTrials.gov investigating novel targeted therapies as maintenance. Furthermore, among the 621 ongoing interventional studies in AML, 171 were specifically in the R/R population; of these, 46 were phase 2 or phase 3 studies with primary completion dates ranging from 2020 to 2029. Table 3 presents a selection of studies in R/R AML that are expected to have primary results by 2023. With the number of existing and upcoming treatment options for R/R disease and maintenance therapy, patients plausibly could stay on therapy for several years, effectively converting AML to a disease that can be managed chronically. Such a scenario would have major implications in terms of the typical patient journey and associated costs.
 |
Table 3 Notable Ongoing Phase 2 and Phase 3 Studies in RR AML with Primary Results Expected by 2023 |
Lastly, as life expectancy increases, there has been a modest uptick in AML incidence, from 3.4 per 100,000 persons in the US in 1975 to 4.3 per 100,000 in 2017.2 Thus, the overall population of patients with AML has grown, contributing to the cumulative economic impact.
Opportunities to Deliver Value in AML
The rapid evolution of treatment options for AML leaves patients and physicians with notably more decisions than in the past, while presenting payers with more costs and offsets to consider. Where previously the AML treatment pathway hinged on age and fitness, it now must begin with genetic profiling followed by consideration of a range of conventional and novel regimens based on patient factors and preferences. Patients may choose to undergo induction therapy followed by HSCT, or perhaps their initial therapy may induce a sufficient response to allow a direct transition to maintenance therapy. Those who undergo HSCT may require less time in the hospital for the procedure as methods have improved, or they may, in the near future, undergo alternative forms of stem cell transplant or cellular therapies. Patients who eventually develop R/R disease can consider a variety of targeted options, many of which can be self-administered at home. As the number of drugs with different mechanisms increases, there may be an opportunity to sequence treatments in AML as patients experience longer-term survival even in the absence of cure. These potential survival gains and clinical benefits will require investment on the part of payers, and it will be incumbent on those developing novel treatments to demonstrate the economic value with compelling evidence.
Historically, hospitalization has been a major cost driver in AML. While some evolving treatments, such as stem cell transplant and cellular therapies, include substantial hospital or medical costs, the major driver of costs in AML in the next decade is likely to be the rapid uptake of a range of novel targeted therapies. The use of these therapies in multiple phases of treatment—newly diagnosed, maintenance, and R/R—will prolong the amount of time that patients are able to stay on treatment. With survival gains will come increases in the overall cost burden of AML. The AML landscape may develop in a manner similar to what has been observed in recent years in multiple myeloma, where a treatment “desert” transformed over two decades into an opportunity to sequence patients through multiple lines of therapy while maintaining quality of life.
One opportunity to deliver value in AML treatment is the use of oral and other self-administered therapies and keeping patients in outpatient settings when feasible. While these approaches are likely to reduce costs for payers, they may shift a greater cost burden to patients, particularly in the US where novel oral drugs incur substantial copayments. The advanced age of the typical patient with AML means that they are unlikely to be employed and may be unable to cover these costs.
Patients receiving outpatient anticancer therapy still require substantial healthcare resources due to the toxicity profiles of most available treatments. Outpatient regimens such as glasdegib + LDAC or venetoclax + azacitidine are myelosuppressive, and patients may require transfusions and other supportive measures.33,34 Therefore, another way to deliver value in AML treatment is to develop treatments and regimens with improved safety profiles, with the goal of reduced spending on monitoring and treatment of adverse events. The targeting of treatments based on biological factors, while requiring investment in molecular testing, can help to focus spending on treatments most likely to be effective.
Despite the opportunities for cost offsets with innovative therapies, it would be naïve to imply that novel therapies for AML will ultimately reduce healthcare costs. The value of most cancer treatments lies in the opportunity to prolong survival while maintaining quality of life. In undertaking a holistic cost-benefit assessment of AML treatments, one must consider whether the costs translate into measurable outcomes such as increased survival, diminished symptom burden, reduced need for emergency and inpatient treatment, and decreased strain on caregivers. Such a value assessment can be used to determine the appropriate costs of innovative therapies.
Conclusion
AML is a relatively rare but costly cancer, currently characterized by high-cost intensive treatments that often require hospitalization, alongside a substantial fraction of patients who receive little or no anticancer treatment due to age or performance status. The past several years have seen a dramatic increase in the number of treatment options, and research is ongoing to further expand the therapeutic landscape in AML. While certain advances, such as increased use of oral and outpatient therapies, could potentially reduce costs, the overall economic impact of AML is likely to increase as more patients are eligible for novel therapies across several phases from induction to maintenance to R/R disease. These novel therapies have the potential to deliver value in the form of improved efficacy, safety, and convenience. In the coming years, value assessments will form the basis for price negotiations as payers determine how to cover a longer and more complex treatment pathway in AML.
Disclosure
KS and AF are both employees of Purple Squirrel Economics, a Cytel company, which acts as a consultant for various pharmaceutical clients. The authors report no other conflicts of interest in this work.
References
1. PDQ Adult Treatment Editorial Board. PDQ Cancer Information Summaries. In: Adult Acute Myeloid Leukemia Treatment (PDQ®): Health Professional Version. National Cancer Institute (US); 2002.
2. National Cancer Institute. Acute Myeloid Leukemia - Cancer Stat Facts. SEER; 2013.
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
4. Carter JL, Hege K, Yang J, et al. Targeting multiple signaling pathways: the new approach to acute myeloid leukemia therapy. Signal Transduct Target Ther. 2020;5.
5. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi:10.1016/j.blre.2019.04.005
6. Pollyea DA, Bixby D, Perl A, et al. NCCN Guidelines Insights: acute Myeloid Leukemia, Version 2.2021: featured Updates to the NCCN Guidelines. J Nat Comprehen Cancer Network. 2021;19(1):16–27. doi:10.6004/jnccn.2021.0002
7. Halpern AB, Walter RB. Practice patterns and outcomes for adults with acute myeloid leukemia receiving care in community vs academic settings. Hematology Am Soc Hematol Educ Program. 2020;2020(1):129–134. doi:10.1182/hematology.2020000097
8. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535–b2535. doi:10.1136/bmj.b2535
9. Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care; 2009.
10. Pandya BJ, Chen -C-C, Medeiros BC, et al. Economic and Clinical Burden of Acute Myeloid Leukemia Episodes of Care in the United States: a Retrospective Analysis of a Commercial Payer Database. J Manag Care Spec Pharm. 2020;26(7):849–859. doi:10.18553/jmcp.2020.19220
11. Irish W, Ryan M, Gache L, Gunnarsson C, Bell T, Shapiro M. Acute myeloid leukemia: a retrospective claims analysis of resource utilization and expenditures for newly diagnosed patients from first-line induction to remission and relapse. Curr Med Res Opin. 2017;33(3):519–527. doi:10.1080/03007995.2016.1267615
12. Meyers J, Yu Y, Kaye JA, Davis KL. Medicare fee-for-service enrollees with primary acute myeloid leukemia: an analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy. 2013;11(3):275–286. doi:10.1007/s40258-013-0032-2
13. Reyes C, Engel-Nitz NM, DaCosta Byfield S, et al. Cost of Disease Progression in Patients with Chronic Lymphocytic Leukemia, Acute Myeloid Leukemia, and Non-Hodgkin’s Lymphoma. Oncologist. 2019;24(9):1219–1228. doi:10.1634/theoncologist.2018-0019
14. Marsà A, Ascanio M, Diaz-García J, Darbà J. Epidemiology, management, and economic impact of acute myeloid leukemia and myelodysplastic syndrome in Spain at the hospital level: a claims database analysis. J Med Econ. 2020;1–8. doi:10.1080/13696998.2019.1678170
15. Leunis A, Blommestein HM, Huijgens PC, Blijlevens NMA, Jongen-Lavrencic M, Uyl-de Groot CA. The costs of initial treatment for patients with acute myeloid leukemia in the Netherlands. Leuk Res. 2013;37(3):245–250. doi:10.1016/j.leukres.2012.09.018
16. Hernlund E, Redig J, Rangert Derolf A, et al. Costs per Treatment Phase for AML Patients Receiving High-Dose Chemotherapy in Sweden. Blood. 2019;134(Supplement_1):2154. doi:10.1182/blood-2019-127957
17. Patel SA, Gerber JM, User’s A. Guide to Novel Therapies for Acute Myeloid Leukemia. Clin Lymphoma Myeloma Leuk. 2020;20(5):277–288. doi:10.1016/j.clml.2020.01.011
18. Yu J, Li Y, Zhang D, Wan D, Jiang Z. Clinical implications of recurrent gene mutations in acute myeloid leukemia. Exp Hematol Oncol. 2020;9(1):4. doi:10.1186/s40164-020-00161-7
19. Lancet JE, Uy GL, Cortes JE, et al. CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients With Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol. 2018;36(26):2684–2692. doi:10.1200/JCO.2017.77.6112
20. Kubal TE, Salamanca C, Komrokji RS, et al. Safety and feasibility of outpatient induction chemotherapy with CPX-351 in selected older adult patients with newly diagnosed AML. JCO. 2018;36(15_suppl):e19013. doi:10.1200/JCO.2018.36.15_suppl.e19013
21. Mabrey FL, Gardner KM, Shannon Dorcy K, et al. Outpatient intensive induction chemotherapy for acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood Adv. 2020;4(4):611. doi:10.1182/bloodadvances.2019000707
22. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447.
23. Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018;131(12):1275–1291. doi:10.1182/blood-2017-09-801498
24. Loke J, Malladi R, Moss P, Craddock C. The role of allogeneic stem cell transplantation in the management of acute myeloid leukaemia: a triumph of hope and experience. Br J Haematol. 2020;188(1):129–146. doi:10.1111/bjh.16355
25. Ballen KK, Gluckman E, Broxmeyer HE. Umbilical cord blood transplantation: the first 25 years and beyond. Blood. 2013;122(4):491–498. doi:10.1182/blood-2013-02-453175
26. Maung KK, Horwitz ME. Current and future perspectives on allogeneic transplantation using ex vivo expansion or manipulation of umbilical cord blood cells. Int J Hematol. 2019;110(1):50–58. doi:10.1007/s12185-019-02670-6
27. Cohen S, Roy J, Lachance S, et al. Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1–2 safety and feasibility study. Lancet Haematol. 2020;7(2):e134–e145. doi:10.1016/S2352-3026(19)30202-9
28. Mardiana S, Gill S. CAR T Cells for Acute Myeloid Leukemia: state of the Art and Future Directions. Front Oncol. 2020;10. doi:10.3389/fonc.2020.00697
29. Kansagra A, Farnia S, Majhail N, Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2020;40:e27–e34. doi:10.1200/EDBK_279151
30. Sarkar RR, Gloude NJ, Schiff D, Murphy JD. Cost-Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Pediatric Relapsed/Refractory B-Cell Acute Lymphoblastic Leukemia. J Natl Cancer Inst. 2019;111(7):719–726. doi:10.1093/jnci/djy193
31. Sikirica S. Patterns of Undertreatment Among Patients with Acute Myeloid Leukemia (AML) Not Receiving Standard Intensive Induction Chemotherapy. ASH; 2020.
32. National Comprehensive Cancer Network, Inc. NCCN® Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Acute Myeloid Leukemia; 2021.
33. DiNardo CD, Jonas BA, Pullarkat V, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med. 2020;383(7):617–629. doi:10.1056/NEJMoa2012971
34. Cortes JE, Heidel FH, Hellmann A, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33(2):379–389. doi:10.1038/s41375-018-0312-9
35. Stone RM, Mazzola E, Neuberg D, et al. Phase III open-label randomized study of cytarabine in combination with amonafide L-malate or daunorubicin as induction therapy for patients with secondary acute myeloid leukemia. J Clin Oncol. 2015;33(11):1252–1257. doi:10.1200/JCO.2014.57.0952
36. Foran JM, Sun Z, Claxton DF, et al. Maintenance Decitabine (DAC) Improves Disease-Free (DFS) and Overall Survival (OS) after Intensive Therapy for Acute Myeloid Leukemia (AML) in Older Adults, Particularly in FLT3-ITD-Negative Patients: ECOG-ACRIN (E-A) E2906 Randomized Study. Blood. 2019;134(Supplement_1):115. doi:10.1182/blood-2019-129876
37. Wei AH, Döhner H, Pocock C, et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N Engl J Med. 2020;383(26):2526–2537. doi:10.1056/NEJMoa2004444
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
