Back to Journals » Journal of Blood Medicine » Volume 13
Waldenstrom’s Macroglobulinemia and Ascites: A Case Report
Authors Bologna C , Cozzolino A, Ferraro A, Guerra M, Guida A, Lugarà M, Coppola MG , Tirelli P, Sicignano M, Madonna P, Di Micco P
Received 18 December 2021
Accepted for publication 16 March 2022
Published 22 March 2022 Volume 2022:13 Pages 167—170
DOI https://doi.org/10.2147/JBM.S353304
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Carolina Bologna,1 Antonio Cozzolino,1 Andrea Ferraro,1 MariaVittoria Guerra,1 Anna Guida,1 Marina Lugarà,1 Maria Gabriella Coppola,1 Paolo Tirelli,1 Marilena Sicignano,1 Pasquale Madonna,1 Pierpaolo Di Micco2
1UOC Medicina Generale Ospedale del Mare ASL Na 1, Naples, Italy; 2UOC Medicina Interna Fatebenefratelli, Naples, Italy
Correspondence: Carolina Bologna, Tel +393473002271, Email [email protected]
Background: Waldenstrom’s disease is characterized by the presence of pathological changes in the B lymphocytes that are in the last stages of maturation. One characteristic of WM is the production of an abnormal high amount of IgM and hyper viscosity syndrome. The MW gets worse, symptoms such as fatigue, weight loss, night sweats, fever, recurrent infections and swollen lymph nodes develop in patients who have a known history of MGUS. In this clinical case, our patient without history of MGUS, presents for the first time for medical observation only for ascites and the presence of an interportocaval lymph node package. An atypical presentation of the disease that makes us reflect on the difficulty of making a diagnosis in the elderly patient and on pathogenetic hypotheses of ascites not yet explored.
Case Presentation: Seventy-three-year-old patient, hospitalized for the onset of ascites with sloping edema, diffuse left pulmonary opacification. At the ultrasound check, cava and portal vessels patent and of regular caliber, however with inversion of flow in correspondence with the right branch and of the door to the hilum, with a subdiaphragmatic retrocaval focus with a maximum diameter of about 3 cm, which cannot be better viewed. CT scan of the abdomen with confirmation of the presence of an interportocaval lymph node package. After evidence of the electrophoretic protein picture of a double component, probably monoclonal with positive urinary immunofixation for free K chains. IgM dosage equal to 2190 mg. Serum immunofixation practice that confirms the diagnosis of type B lymphoproliferative syndrome as per Waldenstrom’s disease, confirmed by bone marrow aspiration with morphological and flow cytometric study. Immediately begin chemotherapy with Bendamustine 120 mg. After 4 weeks of therapy with the reduction of IgM values, the patient no longer presented ascites.
Conclusion: This case has an unusual presentation of this disease and we could shed a new light on the possible pathogenesis of portal hypertension in Waldenstrom’disease.
Keywords: ascites, portal hypertension, Waldenstrom’s disease, elderly
Introduction
Background
Waldenström’s macroglobulinemia is a rare form of cancer, the estimated incidence of which, according to the Italian Association against Leukemia-Lymphomas and Myeloma (AIL), is 2.5 cases per 1,000,000 people. Waldenstrom’s disease is characterized by the presence of pathological changes in the B lymphocytes that are in the last stages of maturation.1 The presence of these alterations determines the uncontrolled growth of abnormal cells, generating numerous clones, mainly in the lymph nodes and, subsequently, in the medulla. Abnormal cells can also accumulate in other organs and tissues, such as the liver and spleen, resulting in an increase in volume (hepatosplenomegaly). Cancer cells produce only one type of immunoglobulin, called the monoclonal component or the M protein. Most people with LPS (90–95%) produce monoclonal immunoglobulin type M (IgM) and rarely produce other antibody isotypes such as IgA or IgG. Additionally please include that WM is considered a non-hodgkin’s lymphoma that it is related to hyperviscosity syndrome and describe resulting complications in more detail.
Since MW makes up the majority of cases of LPL, the two terms are sometimes used interchangeably. Usually, MW is preceded by a condition known as a monoclonal gammopathy of undetermined significance (MGUS IgM), which may or may not progress to MW. Any progression of MGUS IgM into MW takes a long time, usually years. Patients with MGUS IgM are asymptomatic; the identification of the pathology can occur following the execution of blood tests during routine investigations.2,3 A detectable (usually low) amount of abnormal blood IgM may be found in these individuals. The initial phase of MW is characterized by the absence of symptoms (asymptomatic MW) and requires close monitoring. If the MW gets worse, symptoms such as fatigue, weight loss, night sweats, fever, recurrent infections and/or swollen lymph nodes may develop. The increase in plasma viscosity (commonly refers to plasma when we speak of “hyperviscosity”) leads to the appearance of neurological symptoms due to the slow flow of blood through the vessels of the microcirculation: paraesthesia, headache may appear; the localization of vaso-occlusive phenomena in the brain can cause drowsiness, ataxia, disturbances in alertness, up to coma.
Case Presentation
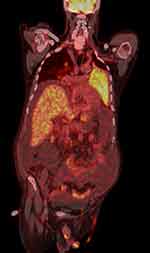
73-year-old patient with arterial hypertension, diabetes on oral hypoglycemic therapy, no history of liver disease. He is hospitalized for the onset of ascites with sloping edema, diffuse left pulmonary opacification. On a vigilant and well-oriented physical examination in time and space, with globally reduced vesicular murmur and hypophonesis in the left lung area, globose abdomen with abundant presence of ascites, non-significant pericardial circumferential detachment on the ecobedside and moderate left pleural effusion with cardiac kinetics in the norm, fibrosclerotic valve systems. On the ECG BAV I degree with atypia of repolarization. In the blood tests, moderate increase in cholestasis indices and only very slight increase in GOT, mild hypoalbuminemia, slight decline in PT, normal bilirubin, normal platelets. Major negative hepatitis markers, no alcohol intake reported. Negative tests on ascitic fluid with serum-ascites deponent gradient calculation for transudate equal to 1.3. At the ultrasound check, following the first paracentesis, liver volume increased to a finely inhomogeneous echostructure and with irregular margins, non-dilated biliary tract, suprahepatic veins, cava and portal vessels patent and of regular caliber, however with inversion of flow in correspondence with the right branch and of the door to the hilum, with a subdiaphragmatic retrocaval focus with a maximum diameter of about 3 cm, which cannot be better viewed. CT scan of the abdomen with confirmation of the presence of an interportocaval lymph node package. The PET exam shows accumulation of the metabolic tracer of a diffuse character due to the note consolidation of the upper lobe and lingula of the left lung highlighted on CT (SUV max 5.9). Concomitant further accumulation of the tracer is found in some lymph nodes in the intercavo-aortic area, interportocaval and hepatic hilum (Figure 1). After evidence of the electrophoretic protein picture of a double component, probably monoclonal with positive urinary immunofixation for free K chains. IgM dosage equal to 2190 mg. Serum immunofixation practice that confirms the diagnosis of type B lymphoproliferative syndrome as per Waldenstrom’s disease, confirmed by bone marrow aspiration with morphological and flow cytometric study. Genetic research in Waldenström’s macroglobulinemia has taken a decisive step forward in 2011, thanks to the discovery of a mutation in the MYD88 gene with a frequency of 90% or higher in moles WM patients. This same study showed that the MYD88 gene mutation, named MYD88 L265P, was not frequently found in other types of lymphoma or multiple myeloma, confirmed by subsequent follow-up studies conducted by researchers on WM around the world. The current guidelines recommend allele-specific PCR testing to detect the MYD88 L265P mutation inside the bone marrow cells in suspected cases of WM, indicating it as an essential exam for the diagnosis of the disease. Our patient had this mutation. Immediately begin chemotherapy with Bendamustine 120 mg. The patient did not receive combination therapy with rituximab because he showed intolerance to rituximab.
Discussion
This case is of importance due to severity and due to lack of information regarding this rare disease. In this clinical case, the disease started with no previous history of MGUS and with the only symptom represented by ascites. Only three cases of Waldenstrom with malignant ascites in 2004 by Stoffel,4 Marinella5 in 1994, Simos6 in 1978 have already been described in the literature.
In the first case report,4 massive peritoneal ascites developed over a short period of time in a patient without any pre-existing history of liver disease and when the liver biopsy did not show frank cirrhosis, other precipitants of ascites were considered. In patients with cirrhosis, a portal pressure gradient of 10–12 mmHg is defined as clinically significant portal hypertension and is required as a minimum for the development of ascites. The hypothesis that hyperviscosity caused the ascites is supported by the disappearance of ascites during treatment of the lymphoproliferative disorder and normalization of the serum IgM levels. Hyperviscosity that had aggravated pre-existing portal hypertension; in Marinella’s case,5 the ascites was attributed to peritoneal lymphomatosis. In Simos’s case Waldenstrom’s macroglobulinemia presents with pleural effusion and ascites.
Our patient presents ascites as the only clinical manifestation. There is no peritoneal lymphomatosis, no pleurisy or signs of hyperviscosity. Cava and portal vessels patent and of regular caliber, however with inversion of flow in correspondence with the right branch and of the door to the hilum, with a subdiaphragmatic retrocaval focus with a maximum diameter of about 3 cm, which cannot be better viewed. CT scan of the abdomen with confirmation of the presence of an interportocaval lymph node package. We tried to identify the pathogenetic mechanisms responsible for ascites, advance a hypothesis never described before in the literature: the flow inversion of the right portal branch was an expression of a slowing of the flow secondary to an intrahepatic obstacle and in particular of the small portal vessels due to probable infiltration of the wall by lymphoplasmacellular elements.
Conclusion
This case has an unusual presentation of this disease and we could shed a new light on the possible pathogenesis of this:
- Portal hypertension may be due to increased blood flow from the splenic vein secondary to splenomegaly.
- Lymph node compression may have been a contributing cause to the onset of portal hypertension (in particular on the splenomegaly aspect and sloping edema).
- The flow inversion of the right portal branch was an expression of a slowing of the flow secondary to an intrahepatic obstacle and in particular of the small portal vessels due to probable infiltration of the wall by lymphoplasmacellular elements.
Abbreviations
MGUS, Monoclonal Gammopathy of undetermined significance; MW, Macroglobulinemia Waldenstrom; ECG, electrocardiography; BAV, atrioventricular block; PT, prothrombin time.
Consent of the Patient
The authors have the consent of the patient for the publication of the case. (CARE guidelines).The authors used the patient consent form (provided by the Taylor & Francis group, of which Dove Medical Press is a part) completed, signed and securely saved. Consent to publication has been obtained: the ethics committee of the ASL Napoli 1 approved the publication of the case. The authors are able to share it with the editors of the journal if requested.
Author Contributions
CB wrote the original manuscript and edited the manuscript, AC performed ultrasound examination, MG, PM, AG, AF, MGC, ML and PT managed the patient, MS provided pathological images with descriptions, PDM performed clinical supervision. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gertz MA. Waldenström macroglobulinemia: 2021 update on diagnosis, risk stratification, and management. Am J Hematol. 2021;96(2):258–269. PMID: 33368476. doi:10.1002/ajh.26082
2. Eulitt P, Fabian D, Kelly C, Hemminger J, William BM. Waldenström’s macroglobulinemia masquerading as ovarian cancer with peritoneal carcinomatosis, ascites, and elevated CA-125. Hematol Oncol Stem Cell Ther. 2019;12(1):54–59. PMID: 28390215. doi:10.1016/j.hemonc.2017.02.004
3. Nozaki H, Tanaka K, Shimizu M. Portal hypertension in Waldestrom ‘s macroglobulinemia. J Exp Clin Med. 1988;13(4–5):245–251.
4. Stoffel EM, Spigel DR, Grace ND. Waldenstrom’s macroglobulinemia presenting as new-onset ascites. J Hepatol. 2004;41(4):689–690. PMID: 15464256. doi:10.1016/j.jhep.2004.06.020
5. Marinella MA, Kim MH, Anderson MM. Waldenstrom’s macroglobulinemia transformed into immunoblastic lymphoma presenting with malignant ascites. Am J Hematol. 1996;51(3):249–250. PMID: 8619414. doi:10.1002/(SICI)1096-8652(199603)51:3<249::AID-AJH18>3.0.CO;2-B
6. Simos A, Assco P, Hadjidimitriou G, Garzonis P, Colovos D. Waldenstrom’s macroglobulinemia presented as pleurisy of unknown origin, a case report. Acta Haematol. 1978;59:246–249. doi:10.1159/000207768
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.