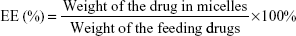Back to Journals » International Journal of Nanomedicine » Volume 11
Vitamin E succinate-conjugated F68 micelles for mitoxantrone delivery in enhancing anticancer activity
Authors Liu Y, Xu Y, Wu M, Fan L, He C, Wan J, Li P, Chen M, Li H
Received 3 January 2016
Accepted for publication 10 March 2016
Published 12 July 2016 Volume 2016:11 Pages 3167—3178
DOI https://doi.org/10.2147/IJN.S103556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lei Yang
Yuling Liu,1,* Yingqi Xu,2,* Minghui Wu,3 Lijiao Fan,1 Chengwei He,2 Jian-Bo Wan,2 Peng Li,2 Meiwan Chen,2 Hui Li1
1Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 2State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, People’s Republic of China; 3Department of Cell Biology and Anatomy, School of Medicine, University of Florida, Gainesville, FL, USA
*These authors contributed equally to this work
Abstract: Mitoxantrone (MIT) is a chemotherapeutic agent with promising anticancer efficacy. In this study, Pluronic F68-vitamine E succinate (F68-VES) amphiphilic polymer micelles were developed for delivering MIT and enhancing its anticancer activity. MIT-loaded F68–VES (F68–VES/MIT) micelles were prepared via the solvent evaporation method with self-assembly under aqueous conditions. F68–VES/MIT micelles were found to be of optimal particle size with the narrow size distribution. Transmission electron microscopy images of F68–VES/MIT micelles showed homogeneous spherical shapes and smooth surfaces. F68–VES micelles had a low critical micelle concentration value of 3.311 mg/L, as well as high encapsulation efficiency and drug loading. Moreover, F68–VES/MIT micelles were stable in the presence of fetal bovine serum for 24 hours and maintained sustained drug release in vitro. Remarkably, the half maximal inhibitory concentration (IC50) value of F68–VES/MIT micelles was lower than that of free MIT in both MDA-MB-231 and MCF-7 cells (two human breast cancer cell lines). In addition, compared with free MIT, there was an increased trend of apoptosis and cellular uptake of F68–VES/MIT micelles in MDA-MB-231 cells. Taken together, these results indicated that F68–VES polymer micelles were able to effectively deliver MIT and largely improve its potency in cancer therapy.
Keywords: F68, vitamin E succinate, mitoxantrone, polymer micelles, cancer therapy
Introduction
Mitoxantrone (MIT), an anthracene derivative, is a promising chemotherapeutic agent that has been largely used for the treatment of human malignancies, such as metastatic breast cancer, prostate cancer, acute myeloid leukemia, and non-Hodgkin’s lymphoma.1–4 MIT has a structure similar to that of anthracyclines, which induces the mitochondrial apoptosis pathway and hinders proliferation by causing DNA damage and disrupting DNA repair or synthesis.2,5 In order to enhance the anticancer activity with a low dosage of MIT, we used nanoscale drug carriers to deliver MIT to the cancer cells in this study.
Recently, nanoscale drug carriers with nontoxic, nonimmunogenic, and biodegradable benefits have attracted much attention for delivering chemotherapeutic drugs in cancer treatment since they can enhance the selective distribution via the effect of enhanced permeability and retention (EPR) and reduce inefficient cell uptake and serious side effects compared to traditional anticancer drugs.6–10 Polymeric micelles formed by amphiphilic polymers belonging to nanoscale drug carriers have been used to enhance permeation, reduce rapid renal clearance, and avoid burst drug release of chemotherapeutic drugs.11 Poloxamer 188, also known as Pluronic F68 (F68), is a block copolymer that has been accepted as a pharmaceutical excipient by the British and US Pharmacopeia and approved by the US Food and Drug Administration for intravenous injection.9,12–14 According to published reports, hydrophobic drugs could be incorporated into F68-based micelles, which were used to deliver drugs to enhance their anticancer effect.14–16 For example, cholesterol-coupled F68 micelles could enhance the anticancer activity of cabazitaxel compared to a Tween 80-based drug solution.14 However, F68 has a high critical micelle concentration (CMC) and a low drug loading (DL) capacity, resulting in poor dilution stability of the micelles.14,17 In order to overcome the drawbacks of F68 micelles, a number of methods have been proposed, including appropriate chemical modifications and mixed micelles.13,18
Vitamin E succinate (α-tocopheryl succinate, VES), a hydrophobic vitamin analog of vitamin E, displays hydrophobicity mainly due to the relatively bulky lipophilic portion in its structure, which makes it more applicable for drug solubilization.19,20 It was reported that VES-based micelles, including VES-modified Pluronic P123 micelles and VES-modified chitosan micelles, could enhance anticancer activity.20,21 In this study, the amphipathic polymer formed by the conjunction of F68 with VES was developed to enhance drug encapsulation. We synthesized the F68–VES polymer and prepared MIT-loaded F68–VES (F68–VES/MIT) micelles via self-assembly under aqueous condition (Figure 1A). The features of F68–VES/MIT micelles were further determined by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Besides, F68–VES micelles had a low CMC value of 3.311 mg/L and exhibited favorable stability even in the presence of fetal bovine serum (FBS). As compared to free MIT, F68–VES/MIT micelles had a stronger cytotoxic and pro-apoptotic effect on MDA-MB-231 cells (a human breast cancer line). In addition, we investigated the cellular uptake via autofluorescence of MIT to observe the difference between F68–VES/MIT micelles and free MIT. The results showed that F68–VES/MIT micelles had higher cellular uptake compared to free MIT in MDA-MB-231 cells. On the whole, F68-VES/MIT micelles, with efficient incorporation and controlled release of MIT, have the advantages of outstanding stability, better DL capacity, and higher cellular uptake, compared to free MIT.
Materials and methods
Materials
Pluronic F68 (molecular weight =8,350) was purchased from BASF SE (Ludwigshafen, Germany). VES was purchased from Aladdin Industrial Corporation (Shanghai, People’s Republic of China). MIT was supplied by Meilun Biological Technology Co., Ltd. (Dalian, People’s Republic of China). Dicyclohexylcarbodiimide and 4-dimethylaminopyridine were purchased from GL Biochem (Shanghai, People’s Republic of China). Millipore water was prepared by a Milli-Q Plus System (Merck Millipore Co., Billerica, MA, USA). All chemicals were of analytical grade.
Dulbecco’s Modified Eagle’s Medium (DMEM), FBS, phosphate-buffered saline (PBS), penicillin–streptomycin, and 0.25% (w/v) trypsin/1 mM EDTA were obtained from Thermo Fisher Scientific (Waltham, MA, USA). 3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl tetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St Louis, MO, USA). Hoechst 33342 and propidium iodide (PI) were purchased from Thermo Fisher Scientific.
Synthesis and characterization of F68–VES polymer
The F68–VES polymer was synthesized by conjugating VES to the terminal hydroxyl groups of F68. In brief, F68 (10 mmol), VES (25 mmol), dicyclohexylcarbodiimide (30 mmol), and 4-dimethylaminopyridine (30 mmol) were dissolved in dry dimethyl sulfoxide (DMSO, 20 mL) and stirred well at room temperature under nitrogen protection for 24 hours. The resulting solution was dialyzed (molecular weight cut-off at 3,500 Da) against DMSO to remove unreacted entities and further dialyzed against Millipore water to remove DMSO. The byproduct N, N′-dicyclohexylurea in the dialysis fluid was removed by filtration, and the final product (F68–VES) was obtained by lyophilization.
Characterization of F68–VES polymer
The structure of F68–VES polymer was confirmed by 1H nuclear magnetic resonance (1H NMR) spectroscopy and fourier transform infrared (FTIR) spectroscopy. F68–VES polymer was detected by 1H NMR spectroscopy (Bruker Optik GmbH, Ettlingen, Germany) at 400 MHz using DMSO-d6 as solvent. FTIR spectrophotometer (Nicolet, Hudson, NH, USA) was used to additionally demonstrate the structure of F68–VES in the absorbance range of 400–4,000 cm−1.
Preparation of F68-VES/MIT micelles
MIT-loaded F68–VES micelles were prepared by the solvent evaporation method as previously reported.22 Briefly, a mixture containing 15 mg F68–VES polymer and 1 mg MIT was dissolved in methanol. The solution was then added dropwise into distilled water with stirring for 6 hours at room temperature. The residual organic solvent was removed by vacuum evaporation. Finally, the F68–VES/MIT micelles were filtrated using a 0.45 μm microfiltration membrane to remove undissolved materials. Blank F68–VES micelles were also prepared by the above-mentioned method.
Characterization of F68–VES/MIT micelles
The average particle size and zeta potential of F68–VES/MIT micelles were measured by DLS with a Zetasizer Nano ZSP system (Malvern Instruments, Malvern, UK). Each sample was evenly dispersed and measured three times at 25°C. The morphology of F68–VES/MIT micelles was characterized by transmission electron microscopy (TEM; Tecnai G20; FEI Company, OR, USA) with an accelerating voltage of 200 kV. The DL and encapsulation efficiency (EE) of MIT in the drug-loaded micelles were measured by Waters e2695 HPLC system equipped with a C18 reverse-phase liquid chromatography column (250×4.6 mm). The mobile phase was methanol/0.25% acetic acid (50/50, v/v). The flow velocity was 1.0 mL/min at the maximum absorption wavelength of 670 nm. The polymeric shells were disrupted by adding methanol before HPLC analysis and the EE (%) and DL (%) of MIT in the micelles were calculated by using the following equations:
|
|
|
|
In vitro drug release of MIT from micelles
Dialysis was used to investigate the in vitro MIT release from F68-VES/MIT micelles. Free MIT was dissolved in methanol as control. The release medium was composed of PBS solution (pH 7.4) containing 0.1% (w/v) Tween 80. Two milliliters of F68–VES/MIT micelle solution or free MIT solution with equivalent drug concentration (0.1 mg/mL) was added to the dialysis tubes with a molecular weight cut-off at 3,500 Da. The dialysis tubes were immersed into 40 mL release medium and incubated in a shaking water bath at 37°C with stirring at 100 rpm. During predetermined intervals, 1 mL of release medium was taken out and the same volume of fresh medium was refilled into the identical dialysis system. Equal volume of methanol was used to dilute the collected release medium, which was filtered by a 0.45 μm microfiltration membrane. The MIT content in the collected release medium was analyzed by HPLC, as mentioned earlier. The drug release experiments were performed in triplicate.
The stability of micelles
CMC is a vital parameter reflecting the stability of micelles in the medium. In this study, the CMC of F68–VES micelles was analyzed by using pyrene as a fluorescence probe.22,23 The F68–VES micellar solutions at concentrations ranging from 3×10−5 mg/mL to 1 mg/mL were prepared with pyrene at 6×10−7 M. Pyrene fluorescence in the micellar solutions was measured by a fluorescence spectrometer (Lumina; Thermo Fisher Scientific) with an excitation wavelength of 339 nm and an emission wavelength range between 360 nm and 450 nm. The excitation spectra were scanned to analyze the relationship between fluorescence intensity ratio of pyrene I373/I384 and the micelle concentration. The fluorescence intensity ratio of pyrene I373/I384 was analyzed as a logarithm function of the F68–VES micellar concentration. The fluorescence intensity ratio of pyrene I373/I384 from the excitation spectra was analyzed as a function of the micelle concentration.23 Additionally, we applied the well-known Tyndall phenomenon to further estimate the stability of F68–VES micelles. A stable micellar solution should show a visible light beam when it is irradiated by a laser pointer.24 The initial F68–VES micellar solution was diluted with distilled water in sequence and the solutions with different concentrations were illuminated by a red laser. Micellar solutions at concentrations ranging from 0.76×10−3 mg/mL to 1.52 mg/mL were prepared at room temperature for this study.
In order to simulate the stability of micelles in blood circulation through in vitro tests, we measured the particle size of F68–VES/MIT micelles by DLS in the presence of different FBS concentrations.25 Briefly, F68–VES/MIT micellar solutions containing 20%, 30%, 40%, and 50% FBS were prepared and incubated at 37°C for different time intervals. At scheduled time points, the appearance of micellar solutions was observed and the particle size was measured.
Cell culture
The human breast cancer cell lines MDA-MB-231 and MCF-7 were obtained from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in DMEM containing 10% (v/v) heat-inactivated FBS and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C under 5% CO2. No ethical committee approval was required for this set of experiments because the experiments were performed on commercially available cell lines and the China Academy of Chinese Medical Sciences/University of Macau human subjects protection program/ ethical review board deemed it exempt.
Cytotoxicity assay
In vitro cytotoxicity of F68–VES/MIT micelles was determined using MTT assay. MCF-7 and MDA-MB-231 cells were seeded in 96-well plates at a density of 5,000 cells per well and cultured in 100 μL DMEM medium overnight. The cells were then treated with free MIT and F68–VES/MIT micelles at concentrations ranging from 0.25 μM to 4 μM for 24 hours and 48 hours, respectively. Untreated cells and cells treated with blank F68–VES micelles at equal volumes of F68-VES/MIT micelles were served as the control. After incubation, the medium was discarded and replaced by culture medium containing 1 mg/mL MTT and incubated for additional 4 hours before 100 μL DMSO was added to each well to allow the dissolution of formazan. The resulting absorbance, which represented the cell viability, was recorded by a microplate reader (SpectaMax M5; Molecular Devices LLC, Sunnyvale, CA, USA) at 570 nm.
Apoptosis assay
The Annexin V-FITC/PI assay was used to detect apoptotic cells according to the manufacturer’s instruction. To investigate the apoptotic effect of F68–VES/MIT micelles on cancer cells, MDA-MB-231 cells were plated in six-well plates at a density of 2×105 cells/well. The cells were treated with 2 μM of free MIT solution or F68–VES/MIT micelles for 24 hours. After the incubation period, both attached and unattached cells were collected and suspended in the binding buffer–dilute Annexin-FITC solution, allowing incubation in the dark for 15 minutes. Then, PI solution was added before flow cytometry analysis.
To observe morphologic change of MDA-MB-231 cells, Hoechst 33342 staining assay was carried out. MDA-MB-231 cells were plated into 96-well plates the day before being treated with free MIT and F68–VES/MIT micelles at an equivalent MIT concentration of 2 μM. After treatment for 24 hours, the medium was removed and cells were fixed with 4% paraformaldehyde (PFA) for 10 minutes. The cells were then washed twice with PBS to remove the free drugs and stained with Hoechst 33342 (10 μg/mL) at room temperature for 15 minutes later. Cells were observed and imaged by IN Cell Analyzer 2000 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA).
Cell uptake study
The cellular uptake efficiency of F68–VES/MIT micelles was monitored by flow cytometry. Briefly, 8×105 MDA-MB-231 cells were plated into 12-well plates. After overnight incubation, cells were treated with 2 μM free MIT or F68–VES/MIT and incubated at 37°C for various time periods (1 hour, 3 hours, and 6 hours). Then, cells were collected by centrifugation and washed twice to remove the free drugs. By taking advantage of the fluorescence emitted by MIT, cellular uptake of free MIT of F68–VES/MIT micelles was analyzed by flow cytometry.
The cellular uptake of free MIT and F68–VES/MIT in MDA-MB-231 cells was also imaged by using the IN Cell Analyzer 2000. After being seeded into 96-well plates with a density of 8×103 cell/well overnight, MDA-MB-231 cells were treated with 2 μM free MIT or F68–VES/MIT micelles for 6 hours in the incubator. The incubation was terminated by removing the medium and fixing the cells in 4% PFA for 10 minutes, followed by washing the cells with PBS twice and staining them with Hoechst 33342 (10 μg/mL) for 10 minutes. The cells were observed by fluorescent Cy5 and 4′,6-diamidino-2-phenylindole channels.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) based on at least three separate experiments. Statistical significance was analyzed by one-way ANOVA or Student’s t-test. P-value <0.05 was considered as statistically significant.
Results
Synthesis and characterization of F68–VES polymer
A novel amphiphilic F68–VES polymer was synthesized via the esterification reaction; Figure 1B showed the synthetic route of F68–VES polymer. The hydroxyl group of VES reacted with the carboxyl group at the end of F68 to form the F68–VES polymer. 1H NMR and FTIR techniques were used to demonstrate the successful synthesis of F68–VES polymer. The 1H NMR spectrum of F68–VES polymer was shown in Figure 2. Figure 2A–C presented the 1H NMR spectrum of F68, VES and F68–VES, respectively. The typical peaks of polypropylene oxide (PPO) in F68 were observed in the chemical shift ranging from 1.0 ppm to 3.5 ppm, including peak e (δ=1.37 ppm) for CH3 groups, peak c (δ=3.38 ppm) for CH2 groups, and peak d (δ=3.34 ppm) for CH groups. Other strong peaks (a and b) were the CH2 groups of polyethylene oxide (PEO) in F68 with a chemical shift near 3.6 ppm. Simultaneously, we discovered typical signals for VES, including peak f (δ=2.70–2.85 ppm) for the CH2 group close to carboxyl group, peak g (δ=2.08 ppm) for the CH3 group on the benzene ring and peak h (δ=2.70–2.85 ppm) for the CH2 group on the heterocyclic ring. The peaks l, m, o–p at 1.25 ppm, 1.62 ppm, and 0.91–0.96 ppm belonged to the repeating units of –CH2, –CH, and –CH3 in VES, respectively. Moreover, the structure of F68–VES polymer was verified by FTIR (Figure 3). The vibration peak associated with the ester bond formed by the reaction of the carboxyl group in F68 and the hydroxyl group in VES was at 1,713 cm−1. In short, 1H NMR and FTIR spectra indicated that the F68–VES polymer was successfully synthesized.
  | Figure 3 FTIR spectrum of F68–VES polymer. |
Characterization of F68–VES/MIT micelles
For the purpose of better understanding the characteristics of MIT-loaded F68–VES micelles, their particle size, zeta potential, morphology, DL, and EE were evaluated in this study. The particle sizes of the blank micelles and F68–VES/MIT micelles were tested by DLS, and their mean diameters were found to be 137.77±3.81 nm and 184.33±6.53 nm, respectively (Figure 4A). Both MIT-loaded micelles and blank micelles had the narrow size distribution (PDI: 0.06±0.03 for F68–VES/MIT micelles and 0.13±0.02 for blank micelles). Compared to blank micelles, F68–VES/MIT micelles showed no obvious increase in particle size. It has been reported that polymeric micelles with particle size in the range of 10–200 nm could promote drug accumulation in tumor sites on account of enhanced permeability and retention effect.26 Thus, the nanometer size and the narrow size distribution of F68–VES/MIT micelles might be suitable for accumulation at the tumor site and would not be immediately eliminated by the reticuloendothelial system.27 According to various reports, the low absolute value of zeta potential was associated with insufficient electric repulsion among particles, which would lead to aggregation.15,28 In this study, a high absolute zeta potential of −32.67±0.87 mV for the F68–VES/MIT micelles (Figure 4B) was observed, which might indicate the uniform distribution of the particles.
The TEM images showed that F68–VES/MIT micelles had homogeneous, spherical shapes and smooth edges without aggregation (Figure 4C), demonstrating that they maintained the integrity of their structure. The diameters of F68–VES/MIT micelles obtained from the TEM image matched well with those measured by DLS. Moreover, the EE and DL of F68–VES/MIT micelles measured by HPLC were 91.88%±2.20% and 5.85%±0.89%, respectively, revealing the high DL capacity of F68–VES micelles. Therefore, F68–VES micelles could effectively encapsulate MIT with high stability and integrity of the structure.
In vitro drug release of MIT from micelles
The in vitro drug release behavior of free MIT and F68–VES/MIT micelles was investigated under sink conditions via the dialysis method. In Figure 4D, we could clearly see that the cumulative release of MIT from the polymer micelles reached ~30% at 4 hours, which was much less than that of free MIT solution at 37°C. Approximately 70% of MIT was released from the free MIT solution after 8 hours, and 80% after 48 hours, while only 40% and 55% of MIT were released from the polymer micelle at 8 hours and 48 hours, respectively. The MIT release behavior reflected that the drug was incorporated into the hydrophobic core of the micelles and was slowly released even under sink conditions.29 Collectively, the results revealed that MIT-loaded F68–VES micelles had sustained release ability, which was beneficial for keeping an effective concentration during the treatment.
The stability of micelles
CMC is a common index to demonstrate the stability and physical properties of micelles.22 In this study, the CMC of F68–VES micelles was measured by using pyrene as the fluorescent probe. Pyrene, with the structure of condensed aromatics, is sensitive to polarity. The changes of fluorescence intensity of pyrene were measured by fluorescence spectrometry as a function of increasing concentrations of copolymers, and the dramatic increase in fluorescence intensity showed micelle formation. The CMC value of F68–VES micelles was ~3.311 mg/L (Figure 5A), which illustrated that F68–VES micelles were stable with a low CMC value and had a great resistance to dilution with integrated structure in body blood circulation. Besides, the classic Tyndall effect was used to estimate the stability of F68–VES micelles. A series of polymer micellar solutions with different concentrations was prepared in this study, and the distinct red light could be seen when these micellar solutions were illuminated by a red laser beam. As shown in Figure 5B, it was clear that all F68–VES micellar solutions showed a clear light beam under the red laser irradiation until the micelle concentration was <0.0076 mg/mL, which was represented by the sample S6 in Figure 5B. However, sample S7 (0.0015 mg/mL) with a distinctly darker light than S6 was almost close to the brightness of water. The low CMC value of 3.311 mg/L could ensure F68–VES micelles to maintain structural integrity under diluted conditions, which matched the results of Tyndall phenomenon.
The in vitro colloidal stability of F68–VES micelles was estimated in the presence of FBS. F68–VES micelles were diluted in water containing 20%, 30%, 40%, and 50% FBS at 37°C, and the hydrodynamic diameters were measured by DLS at different times. As shown in Figure 5C, the particle sizes of F68–VES micelles containing different FBS concentrations were stable without sharp changes as time increased up to 24 hours. When the FBS was 50%, the change in the hydrodynamic size of the polymer micelles was small compared to the original size, which was ~0.7-fold of the initial diameter after 24 hours of incubation with FBS at 37°C. In spite of the size change, the particles still exhibited good dispersion without visible particle precipitation (Figure 5D).
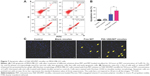
In vitro cytotoxicity of F68–VES/MIT micelles
With MTT assay, the growth inhibition of breast cancer MCF-7 and MDA-MB-231 cell lines was compared between F68–VES/MIT micelles and free MIT. Both MCF-7 and MDA-MB-231 cells showed more cytotoxicity to F68–VES/MIT micelles than free MIT. The inhibition efficiency of both F68–VES/MIT micelles and free MIT increased as the inhibition time increased. The 24-hour half maximal inhibitory concentration (IC50) values of F68–VES/MIT micelles and free MIT on MCF-7 cells were 1.71 μM and 2.79 μM, respectively. The 48-hour IC50 values of free MIT and F68–VES/MIT micelles decreased to 0.62 μM and 0.48 μM respectively. Noticeably, in MDA-MB-231 cells, the IC50 values of F68–VES/MIT micelles were lower than those of free MIT at 24-hour and 48-hour treatment time points, which were 1.64 μM versus 1.94 μM (24 hours) and 0.47 μM versus 0.59 μM (48 hours), respectively (Figure 6). Meanwhile, the cytotoxicity of blank micelles was assessed, and the result showed that the survival rates of the two cell lines reached over 90% after incubation with blank micelles of the same volume of the drug-loaded group for 24 hours and 48 hours, indicating that blank F68–VES micelles had negligible cytotoxic effect on MCF-7 and MDA-MB-231 cells. These results showed that F68–VES/MIT micelles exerted significantly enhanced cytotoxicity on MCF-7 cells and MDA-MB-231 cells.
Apoptotic effect of F68–VES/MIT micelles on MDA-MB-231 cells
MIT is one of the commonly used chemotherapeutic agents for the treatment of acute myeloid leukemia, metastatic breast cancer, and non-Hodgkin’s lymphoma, whose mechanisms of action are concerned with the inhibition of topoisomerase II, DNA intercalation, and disruption of DNA repair.2 Given that F68–VES/MIT micelles elicited cell death, we performed apoptosis assay with AnnexinV flow cytometry to evaluate the apoptotic effect of F68–VES/MIT micelles. This assay demonstrated that free MIT and F68–VES/MIT micelles showed a significant effect with apoptotic rates of 38.9% and 60.8%, respectively (Figure 7). In other words, the apoptotic rate of F68–VES/MIT micelles was 1.56-fold higher than that of free MIT. The result was further confirmed by cell nuclear morphology observation. After staining with Hoechst 33342, MDA-MB-231 cell nuclei presented as weak homogeneous blue were observed in cells untreated or treated with blank micelles, while bright chromatin condensation and nuclear fragmentation were identified in cells treated with 2 μM free MIT or F68–VES/MIT micelles. Of note, we found a higher apoptotic rate in the F68–VES/MIT micelles group than in free MIT group through nuclear morphology observation.
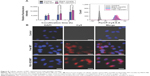
Cellular uptake of MIT released from F68–VES/MIT micelles
To confirm whether the enhanced cytotoxicity of F68–VES/MIT micelles was related to the improved intracellular uptake of MIT, the cellular uptake of F68–VES/MIT micelles in MDA-MB-231 cells was assessed by using flow cytometry, and autofluorescence of MIT was observed by IN Cell Analyzer 2000. As shown in Figure 8A, the cellular accumulation of MIT occurred in a time-dependent manner in both free MIT and F68–VES/MIT micelles treatment groups. Moreover, compared to free MIT, F68–VES/MIT micelles exhibited an enhanced uptake of MIT in cells after cellular exposure for 1 hour, 3 hours, and 6 hours. The fluorescence intensity in cells incubated with 2 μM F68–VES/MIT micelles was 22.4% higher than that in cells incubated with free MIT.
The intracellular uptake of F68–VES/MIT was further visualized with a fluorescent microscope. After a 6-hour treatment, cells treated with F68–VES/MIT micelles exhibited stronger red fluorescence than those treated with free MIT (Figure 8B), demonstrating better efficiency of entry into MDA-MB-231 cells in F68–VES/MIT micelles than in free MIT, which was consistent with the flow cytometry analysis results. The enhanced cellular uptake of F68–VES/MIT micelles caused increased accumulation of MIT in cells and eventually led to enhanced cytotoxicity and apoptosis.
Conclusion
The developed MIT-loaded F68–VES micelles had the characteristics of low CMC, optimal particle size with uniform appearance, high absolute zeta potential, EE and DL. The stability studies and in vitro drug release behavior indicated that F68–VES/MIT micelles had high stability and sustained in vitro drug release pattern. In cellular uptake assay, higher cellular uptake of F68–VES/MIT micelles than free MIT in MDA-MB-231 cells demonstrated that F68–VES polymer micelles were the effective carrier to deliver MIT through the cell membrane. In pharmacological studies, compared to free MIT, MIT-loaded F68–VES micelles showed more cytotoxicity on MDA-MB-231 and MCF-7 cells. Moreover, F68–VES/MIT micelles had a greater effect on inducing cell apoptosis in MDA-MB-231 cells than free MIT. Collectively, F68–VES micelles have the potential to become the ideal carrier for delivering chemotherapeutic agents to cancer cells, which makes great sense of performing further investigations through in vivo studies as well as clinical applications.
Acknowledgments
This study was supported by the Macao Science and Technology Development Fund (102/2012/A3), the Research Fund of the University of Macau (MYRG2014-00033-ICMS-QRCM, MYRG2014-00051-ICMS-QRCM, MYRG2015-00171-ICMS-QRCM), and the National Natural Science Foundation of China (81403120). This study was also supported by the Support Program of the Institute of Chinese Materia Medica, China Academy of Chinese Medical Sciences (Z02063, Z184).
Disclosure
The authors report no conflicts of interest in this work.
References
Pedrosa LR, ten Hagen TL, Suess R, et al. Short-chain glycoceramides promote intracellular mitoxantrone delivery from novel nanoliposomes into breast cancer cells. Pharm Res. 2015;32(4):1354–1367. | ||
Barar J, Kafil V, Majd MH, et al. Multifunctional mitoxantrone-conjugated magnetic nanosystem for targeted therapy of folate receptor-overexpressing malignant cells. J Nanobiotechnology. 2015;13:26. | ||
Guerriero E, Sorice A, Capone F, et al. Vitamin C effect on mitoxantrone-induced cytotoxicity in human breast cancer cell lines. PLoS One. 2014;9(12):e115287. | ||
Schmid P, Flath B, Akrivakis K, et al. Gemcitabine and mitoxantrone in metastatic breast cancer: a phase-I-study. Invest New Drugs. 2005;23(4):349–356. | ||
Ferrer A, Marce S, Bellosillo B, et al. Activation of mitochondrial apoptotic pathway in mantle cell lymphoma: high sensitivity to mitoxantrone in cases with functional DNA-damage response genes. Oncogene. 2004;23(55):8941–8949. | ||
Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. | ||
Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7(1):49–62. | ||
Bildstein L, Dubernet C, Couvreur P. Prodrug-based intracellular delivery of anticancer agents. Adv Drug Deliv Rev. 2011;63(1–2):3–23. | ||
Hu XL, Jing XB. Biodegradable amphiphilic polymer-drug conjugate micelles. Expert Opin Drug Deliv. 2009;6(10):1079–1090. | ||
Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2(12):751–760. | ||
Tian Y, Mao SR. Amphiphilic polymeric micelles as the nanocarrier for peroral delivery of poorly soluble anticancer drugs. Expert Opin Drug Deliv. 2012;9(6):687–700. | ||
Aoki N, Tamura M, Ohyashiki JH, Sugaya M, Hisatomi H. Poloxamer 188 enhances apoptosis in a human leukemia cell line. Mol Med Rep. 2010;3(4):669–672. | ||
Cha M-H, Choi J, Choi BG, et al. Synthesis and characterization of novel thermo-responsive F68 block copolymers with cell-adhesive RGD peptide. J Colloid Interface Sci. 2011;360(1):78–85. | ||
Song YZ, Tian QJ, Huang ZJ, et al. Self-assembled micelles of novel amphiphilic copolymer cholesterol-coupled F68 containing cabazitaxel as a drug delivery system. Int J Nanomedicine. 2014;9:2307–2317. | ||
Zhang J, Li Y, Fang X, Zhou D, Wang Y, Chen M. TPGS-g-PLGA/pluronic F68 mixed micelles for tanshinone IIA delivery in cancer therapy. Int J Pharm. 2014;476(1–2):185–198. | ||
Wang T, Wu Y, Zeng AJ. Synthesis and Characterization of amphiphilic pluronic (F68)-1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine copolymers and their micelles as a drug carrier. J Appl Polymer Science. 2010;1(117):604–613. | ||
Kulthe SS, Inamdar NN, Choudhari YM, Shirolikar SM, Borde LC, Mourya VK. Mixed micelle formation with hydrophobic and hydrophilic pluronic block copolymers: implications for controlled and targeted drug delivery. Colloids Surf B. 2011;88(2):691–696. | ||
Zhao LY, Du JC, Duan YW, et al. Curcumin loaded mixed micelles composed of pluronic P123 and F68: preparation, optimization and in vitro characterization. Colloids Surf B. 2012;97:101–108. | ||
Lian H, Sun J, Yu YP, et al. Supramolecular micellar nanoaggregates based on a novel chitosan/vitamin E succinate copolymer for paclitaxel selective delivery. Int J Nanomed. 2011;6:3323–3334. | ||
Tao YH, Han JF, Wang XW, Dou HY. Nano-formulation of paclitaxel by vitamin E succinate functionalized pluronic micelles for enhanced encapsulation, stability and cytotoxicity. Colloids and Surfaces B-Biointerfaces. 2013;102:604–610. | ||
Liang N, Sun S, Li X, et al. alpha-Tocopherol succinate-modified chitosan as a micellar delivery system for paclitaxel: preparation, characterization and in vitro/in vivo evaluations. Int J Pharm. 2012;423(2):480–488. | ||
Huang SL, Yu XH, Yang LL, et al. The efficacy of nimodipine drug delivery using mPEG-PLA micelles and mPEG-PLA/TPGS mixed micelles. Eur J Pharm Sci. 2014;63:187–198. | ||
Fang XB, Xu YQ, Zhang JM, Lu XH, Wang YT, Chen MW. Synthesis and characterization of an amphiphilic linoleic acid-g-quaternary chitosan with low toxicity. J Nanomater. 2015;2015:7. | ||
Lv SX, Tang ZH, Zhang DW, et al. Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J Control Release. 2014;194:220–227. | ||
Tang S, Yin Q, Su J, et al. Inhibition of metastasis and growth of breast cancer by pH-sensitive poly (beta-amino ester) nanoparticles co-delivering two siRNA and paclitaxel. Biomaterials. 2015; 48:1–15. | ||
Ke XY, Ng VWL, Gao SJ, Tong YW, Hedrick JL, Yang YY. Co-delivery of thioridazine and doxorubicin using polymeric micelles for targeting both cancer cells and cancer stem cells. Biomaterials. 2014;35(3):1096–1108. | ||
Yang C, Attia ABE, Tan JPK, et al. The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials. 2012;33(10):2971–2979. | ||
Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems – a review (part 1). Trop J Pharm Res. 2013;12(2):255–264. | ||
Gao Y, Li LB, Zhai G. Preparation and characterization of pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf B. 2008;64(2):194–199. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.