Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Visual Performances of a New Extended Depth-of-Focus Intraocular Lens with a Refractive Design: A Prospective Study After Bilateral Implantation
Authors Spadea L , Giannico MI, Formisano M , Alisi L
Received 23 May 2021
Accepted for publication 5 July 2021
Published 16 July 2021 Volume 2021:17 Pages 727—738
DOI https://doi.org/10.2147/TCRM.S320422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Leopoldo Spadea, Maria Ilaria Giannico, Martina Formisano, Ludovico Alisi
Eye Clinic, Policlinico Umberto 1, “Sapienza” University of Rome, Rome, Italy
Correspondence: Leopoldo Spadea
Head Eye Clinic, Policlinico Umberto 1, “Sapienza” University of Rome, Via Benozzo Gozzoli 34, Rome, 00142, Italy
Tel +39 06 519 3220
Fax +39 06 8865 7818
Email [email protected]
Purpose: The aim of the present study was to evaluate the visual outcome of a new extended depth-of-focus (EDOF) intraocular lens (IOL) after bilateral implantation. A qualitative and quantitative analysis was performed and data were compared with those given by other studies regarding multifocal IOLs, which have the same purpose of giving spectacle independence to the patients.
Methods: The study enrolled 40 eyes of 20 patients who underwent cataract surgery with bilateral implantation of an EDOF IOL (Evolve Soleko, Rome, Italy). The mean age was 74.5± 9 years (range 59– 83ys). Refractive outcomes and contrast sensitivity were evaluated preoperatively and at 6-month follow-up. We also examined reading speed, glare, halos, difficulties in the night driving, the requirement for spectacles, and overall satisfaction with vision. Two questionnaires were administered for this purpose.
Results: At 6 months, the percentage of eyes within ± 0.50 diopters (D) from emmetropia was 82.5%. Of all patients, 90% were satisfied with their vision. The percentage of spectacle-free for near and distance vision patients was 70% and 95%, respectively. A postoperative binocular uncorrected 60cm intermediate visual acuity (UI60VA) of 0.2 logMAR or better was achieved in 92% of patients. Contrast sensitivity significantly improved postoperatively (p< 0.001) and mean reading speed was good.
Conclusion: This new EDOF IOL seems to provide an effective alternative to patients who desire a spectacle-free lifestyle postoperatively. These lenses can supply a satisfactory distance, intermediate and near vision, and retain good contrast sensitivity, with most patients reporting excellent satisfaction.
Keywords: cataract surgery, EDOF, IOL, presbyopia
Introduction
Intraocular lens (IOL) technology has advanced significantly over the past several decades, and cataract surgery has become a form of refractive surgery. Many patients request and expect spectacle independence for both distance and near vision. Furthermore, the intermediate working distance is becoming more important with the increased use of computers, smartphones, and tablets. Compared to traditional multifocal IOLs that allow patients to focus on images in multiple focal planes, achieving a fuller visual range and a greater spectacle independence than monofocal IOLs,1,2 in the “extended depth-of-focus” (EDOF) technology, the focus is extended longitudinally with the main aim of providing a single elongated focus without gaps between the foci; thus, improving the range of vision from far to intermediate, and creating a more natural and smoother defocus curve. This technology leads to fewer or less severe visual disturbances and better contrast sensitivity.2–4
In this study, we report our initial experience with bilateral implantation of a new EDOF IOL, analyzing the early postoperative clinical outcomes and patient-reported outcomes.
Methods
The present study has been performed in accordance with the principles stated in the Declaration of Helsinki and obtained ethical approval from the local Institutional Review Board (IRB) of the “Sapienza” University of Rome, Umberto I Hospital (#0569/2019). Informed consent was obtained from all patients before enrollment.
The study was designed as a prospective, noncomparative interventional study and it was conducted between July 2019 and December 2019.
Twenty patients (10 males and 10 females, mean age: 74.5ys ±9; range 59–83ys) with age-related cataracts in both eyes were selected. Inclusion criteria for the study were primary procedure with no previous refractive surgery and absence of any ocular pathology other than refractive error or cataract. Eyes with previous ocular trauma, coexistent ocular pathologies, such as diabetic retinopathy, age-related macular degeneration, glaucoma, pseudoexfoliation, corneal guttae, eyes with photopic (luminance level 10 cd/m2) pupillary diameter <2mm and mesopic (luminance level 1 cd/m2) pupillary diameter >5mm (pMetrics 1.30 pupillometry, iVis Technology, Taranto, Italy) were excluded from the study. Indeed, pupil size is known to be related to the visual outcome, photopic phenomena, and contrast sensitivity when implanting a non-monofocal lens.2–4 Mean preoperative scotopic pupil size was 5mm ±0.9, mean mesopic pupil size was 3.9mm ±0.4, mean photopic pupil diameter was 3mm ±0.5. Eyes with corneal astigmatism >1D were selected for toric customized IOL implantation (7 eyes). The second eye surgery was performed 1 month after the first IOL implantation.
Consenting patients underwent preoperative ophthalmic evaluation including uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) (Early Treatment of Diabetic retinopathy Study - ETDRS Chart - at 6m), uncorrected near visual acuity (UNVA), and corrected near visual acuity (CNVA) at 40cm (RADNER Reading Charts). Uncorrected and corrected 60cm intermediate visual acuity (UI60VA and CI60VA) were tested with the MNREAD App designed to run on an iPad. Slit-lamp evaluation, Goldmann applanation tonometry, dilated fundus examination were also carried out. Biometry was performed using the IOLMaster optical biometry Carl Zeiss IOLMaster® Advanced Technology V. 7.7 (Carl Zeiss Meditec AG, Jena, Germany). Minimal hyperopic spherical equivalent was aimed for target refraction in IOL power calculation (mean +0.3D ±0.2). This indication was suggested by the IOL manufacturer to enhance visual performance at near distance.
MNREAD App was also applied to test speed reading. It is a digital version of the printed MNREAD Acuity Charts, a continuous-text reading-acuity test suitable for measuring the reading acuity and the reading speed of normal and low-vision patients. This test was developed at the Minnesota Laboratory for Low-Vision Research, University of Minnesota, Minnesota, USA. During the test, the patients read a series of 14 sentences, as quickly and accurately as possible. The physical print size ranges from 6.3M to 0.32M (in Sloan M notation). From the recommended viewing distance of 40cm, the corresponding angular print size ranges from 1.2 to 0.1logMAR (Snellen equivalents 20/320 to 20/16) with print decreasing in size by 0.1 log unit (each sentence is 0.1logMAR units smaller than the previous sentence). The App displays the speed reading in words per minute (wpm) automatically at the end of the test.
Contrast sensitivity examination was performed monocularly with the Monpak3 Metrovision Contrast Sensitivity test (Pérenchies, France) with the best spectacle correction. It consists of sinusoidal gratings whose parameters (luminance, contrast, and spatial frequency) are controlled by the computer. Each grating is first presented with very low contrast, then the contrast is progressively increased. The patient presses a button when he/she detects the grating bars. The test is usually performed under photopic conditions (average luminance of the grating: 80cd/m2). The results of the exam are represented by a curve obtained by plotting the contrast sensitivity versus spatial frequency. The response curve from a normal subject shows a maximum for medium spatial frequencies (around 3 cycles per degree). The highest spatial frequency perceived at maximum contrast is around 30 to 45 cycles per degree.
At the preoperative and 6-month postoperative follow-up visit, patients completed a purpose-developed satisfaction questionnaire and an overall qualitative analysis was tested with the 14-item Visual Function Questionnaire (VFQ-14). It consists of 14 questions covering 14 aspects of visual function. Each item was scored between 0 and 4 points. The degree of difficulty experienced while performing activities related to vision was assessed as no difficulty (4 points), a little difficulty (3 points), a moderate amount of difficulty (2 points), a great deal of difficulty (1 point), and unable to do the activity (0 points). The average score was calculated; higher scores indicate less difficulty in performing activities.
Patients were evaluated 1 hour postoperatively, after 1 day, 1 week, 1 month, 3 months, and 6 months. At each visit, refraction, CDVA, UDVA, UI60VA, and UNVA were measured and any adverse events were noted.
Surgical Technique
Lens calculations were performed using the IOLMaster optical biometry using the Hoffer Q formula for axial length <22mm, the Holladay formula for axial length ≥22mm and <26mm, the SRK T formula for axial length ≥26mm.5
The refractive aim in all the eyes was a slight postoperative hyperopic spherical equivalent (mean +0.3D±0.2). All cataract surgeries were performed by a single surgeon (LS) via a 2.2mm clear corneal incision, using the Infiniti phacoemulsificator (Infiniti® Vision System, Alcon, Fort Worth, USA). After the incision, an approximately 5mm capsulorhexis was fashioned, nucleus fragmented using chopping technique and emulsified, followed by cortical clearance and IOL implantation in the capsular bag using the Viscojet-Bio 2.2mm injector (Medicel AG, Altenrhein, Switzerland). In the case of toric IOL implantation, the demarcation points at the 0°–180° axis were signed at the limbus before starting cataract surgery, using a dermographic pen. Residual viscoelastic was washed out of the paracentesis and antibiotic solution was injected into the anterior chamber before the hydro-sutures of the corneal incisions (Figure 1).
 |
Figure 1 Digital slit lamp examination 6 months after surgery. The extended depth-of-focus intraocular lens Evolve is well centered in the capsular bag. |
Postoperatively, patients were instructed to instill one drop of dexamethasone 1mg/mL and tobramycin 3mg/mL (Tobradex, Novartis Pharmaceuticals, London, UK) three times a day for 15 days and one drop of indomethacin (5mg/mL) (Indom, Alfa Intes S.r.l., Casoria-NA, Italy) three times a day for 1 month.
IOL
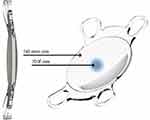
The EDOF IOL Evolve (Soleko, Rome, Italy) received the Conformité Européene (CE) marking on April 2020 and is based on a refractive design principle to continuously extend the depth-of-focus. The lens has an aberration-neutral aspheric design and optimized chromatic aberration to improve the quality of vision (Figure 2). It is a new refractive one-piece foldable IOL made of acrylic hydrophilic material (25% water content), specially designed for pupil size ranging from 3.50mm to 2.50mm, with 40% of the light redirected to intermediate focus through aspheric optics (Figure 3). The IOL add power is +2.50 Diopters (D), resulting in about +1.75D in the spectacle plane. The aspheric add power of +2.50D in the optic zone generates a positive spherical aberration that induces an extended depth-of-focus of about ±0.5D, in case of 3mm pupil size, for an intermediate vision at about 55cm. The planned comfort zone of the lens ranges from 45 to 50cm for distance, even under night driving conditions. On the other hand, near vision is conditioned by the pupil diameter and the light conditions of the surroundings. The lens performance is better under photopic conditions.
The aspheric optic shape is biconvex, with 360 square edge morphology and UV-light white filter. The lens has a small central refractive EDOF zone, circular in shape, 1.7mm large, for near vision (+2.50D addition), surrounded by a far vision zone. The body size is 6.0mm with four-point closed haptic fixation with optic angulation of 5 degrees, and an overall size ranging from 11.2mm to 11.8mm (depending on selected diopter). The range of diopters is −5.0D/+30.0D (step 0.5D) with a toric variant available; the refractive index is 1.46 (546nm, 20°C in water). Recommended A constants for optical biometry are SRKT 119.1, Holladay I SF 1.90, Hoffer Q pACD 5.68; by using ultrasound biometry the A constant is 118.7 and with immersion technique 119.0. The toric version of the lens is also available offering cylinder correction up to 15.00D in 0.25D steps; a special characteristic is that the rotation is not required as the lens is customized by the manufacturer and it is always positioned along the 0°–180° axis.
Statistical Analysis
Preoperative and postoperative clinical parameters were described by means and standard deviation or percentages. All calculations were performed using “Primer of biostatistics” software, Stanton A. Glantz, 2007, 6th Edition and Numbers version 10.0 MacOS. Normality for data samples was evaluated with the Kolmogorov–Smirnov test. If the parametric analysis was possible, Student’s t-test for paired data was used to calculate the change in the manifest spherical equivalent and visual acuity, whereas for non-parametric analysis the Wilcoxon rank test was applied to assess the significance of such differences. The chi-square test was used to compare percentages. A P value of less than 0.05 was considered statistically significant.
Results
The present study included 40 eyes of 20 patients with a mean age of 74.5ys ±9 (range 59–83ys), who underwent bilateral EDOF IOL implantation for age-related cataract. Baseline characteristics of the study group and refractive outcomes are shown in Table 1.
 |
Table 1 Demographics and Preoperative and Postoperative Clinical Data |
Refractive Predictability and Stability
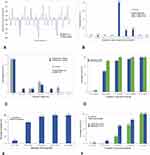
Mean preoperative and postoperative sphere, cylinder, and manifest refractive spherical equivalent (MRSE) are presented in Table 1. Figure 4A shows the achieved postoperative MRSE with respect to the attended values for each patient in the study group. The mean prediction error (the difference between attempted predicted MRSE as estimated by the formulas used for biometric calculation and achieved MRSE) was −0.17±0.43D at 6 months. Figure 4B depicts the distribution of postoperative MRSE. At 6 months, 33 eyes (82.5%) and 39 eyes (97.5%) were within ±0.50 and ±1.00D of emmetropia, respectively. Figure 4C shows the residual postoperative refractive cylinder with respect to the preoperative values. At 6 months, 28 eyes (70%) had the magnitude of the refractive cylinder of 0.50D or less.
UDVA and CDVA
Figure 4D shows the comparison between postoperative UDVA and CDVA. Of all eyes, 90% (36 eyes) achieved monocular UDVA of 0.1logMAR or better at 6 months.
Binocularly (Figure 4E), the percentage of patients who achieved UDVA of at least 0.1logMAR or better was 95% (38 eyes). Cumulative UNVA is shown in Figure 4F. The UNVA of 0.5logMAR or better was measured in 25 eyes (62.5%) monocularly and in 16 of patients (80%) binocularly. The mean logMAR values for UDVA, UI60VA, and UNVA are shown in Table 1. Regarding monocular and binocular UI60VA, they both improved significantly at 6 months follow-up; a postoperative binocular UI60VA of 0.2 logMAR or better was achieved in 92% of patients.
Patients maintained good CDVA at the 6-month check-up, with a mean logMAR value of 0.01±0.04 (between −0.1 and 0.1logMAR).
Speed Reading
The mean speed reading was 152.1±48.2 wpm (range 92–243wpm) at the final follow-up. The test was performed monocularly with the best spectacle correction.
Contrast Sensitivity
The results of contrast sensitivity at different spatial frequencies are depicted in Figure 5. There was a significant improvement in contrast sensitivity for spatial frequency >3cpd (range 0.5–18cpd) at 6 months postoperatively. The mean contrast sensitivity changed from 11±0.8dB preoperatively, to 16.8±1.1dB at the 6-month follow-up visit (p<0.001).
 |
Figure 5 Mean contrast sensitivity outcomes under photic conditions preoperatively and 6 months after surgery with different spatial frequencies. Abbreviation: cpd, cycles per degree. |
Patient-Reported Outcomes
The results of the VFQ14 questionnaire are summarized in Table 2. Patients reported high levels of satisfaction with visual recovery for distance and near vision. Most of the patients showed no difficulty in performing common daily activities (range 50–95%), a small percentage of them referred a little or a moderate amount of difficulty, respectively (range 0% to 25% for both). Table 3 summarizes the main outcomes of the “patient experience questionnaire”. The percentage of patients who were very satisfied or satisfied with their vision at 6 months was 90%. Additionally, all patients stated that their vision met or exceeded their expectations. When questioned about postoperative spectacle wear, 95% of patients at 6 months reported not wearing any correction for distance vision. For near vision, the percentage of patients not requiring any correction at all was 70%. None of the patients complained of photic phenomena (halos, glare) and this was correlated with postoperative satisfaction.
 |
Table 2 Results of VFQ-14 Questionnaire |
 |
Table 3 Patient Experience Questionnaire |
Intraoperative and Early Postoperative Adverse Events
The IOL Evolve represents a reliable alternative for patients who need optimal intermediate and distance vision even in unfavorable lighting conditions and with reduced pupil size.
No serious complications occurred during surgery (posterior capsule perforation, iridodialysis, etc.) or during the follow-up. Postoperative events included cystoid macular edema, diagnosed by optical coherence tomography in one eye, 1 month postoperatively, which was resolved in 2 weeks with topical and systemic anti-inflammatory therapy. No glistening of the IOL was detected, nor posterior capsular bag opacification. There have been no explants of the IOL Evolve due to unwanted optical side effects or other reasons in the early postoperative period.
Discussion
Providing high-quality VA and the best levels of spectacle independence for near, intermediate, and distance vision has been the primary aim of multifocal IOL implantation. Bifocal IOL implantations allow to obtain successful outcomes in distance and near vision but did not improve enough intermediate VA, mainly because of the characteristics of the lens and patient selection. Furthermore, postoperative photic functional disorders, such as haloes (ie a circle of white or colored light around the sun, moon, or other luminous body), rings around lights, glare (ie to shine with a harsh uncomfortably brilliant light), and photopsia significantly limited visual quality.6–11 The use of trifocal IOLs has been reported to also improve intermediate VA.12–14 The EDOF IOL constitutes the most recent form of multifocal technology and has been reported to provide a significantly increased range of vision with minimal optical side effects. They present an extended far focus area that reaches the intermediate distance. Tecnis Symfony IOL (Johnson & Johnson Vision Care, Inc.) was the first EDOF IOL approved in 2016 by the US Food and Drug Administration (FDA),15 based on a diffractive principle, but other technologies have been used for EDOF IOLs such as a progressive multifocal IOL, a bioanalogic IOL, or IOLs based on the pinhole effect.2,16
In previous studies, the EDOF IOLs were able to restore excellent far and intermediate VA with functional near vision compared to other multifocal IOL designs. This new generation of lenses demonstrated a superior range of vision and spectacle independence compared to monofocal lenses.17–20 However, in a recent study, Cochener et al reported that near vision was statistically better in the trifocal lenses compared to the EDOF ones.21
Few data are available on the safety and efficacy of different EDOF IOLs’ designs. Schallhorn et al recently evaluated clinical and patient-reported outcomes of a new EDOF IOL, the AT LARA 829MP IOL (Carl Zeiss Meditec AG, Jena, Germany). The IOL is based on a diffractive design with chromatic correction and manufacturing technology designed to minimize light scatter. They reported good refractive predictability with 86.7% of eyes within ±0.5 D of emmetropia and observed a small hyperopic shift during the 3-month follow-up, probably due to early postoperative keratometric changes and capsular contractions moving the IOL posteriorly. Improvement in distance vision was excellent with 87.5% of patients having 20/20 or better binocular UDVA at 3 months and 95.4% reporting no use of optical correction for distance vision. Furthermore, more than 90% of patients attained binocular UNVA of 20/50 or better.16
Pedrotti et al recently reported better improvement in both uncorrected and corrected monocular and binocular intermediate and near visual acuity after bilateral implantation of the Mini Well intraocular lens, when compared to the bilateral implantation of the aspheric monofocal Mini-4-Ready IOL (SIFI S.p.A., Catania, Italy). They suggested that the Mini Well EDOF IOL offer good vision at all distances for activities of daily living, without a decrease in contrast sensitivity, even in the presence of halo phenomena not subjectively perceived.22
Schojai et al presented a prospective randomized comparative clinical trial comparing a group of patients implanted with a monofocal 1-piece Tecnis Z B00 IOL (Johnson & Johnson Vision Care, Inc.) in the dominant eye and an IC-8 IOL (AcuFocus) in the nondominant eye with a control group that was implanted with the Tecnis Symfony IOL bilaterally. The target refraction of the dominant eye was emmetropia while the target in the nondominant eye was myopia (mini-monovision, −0.75D). The UDVA was excellent in both groups with statistically significantly better results in the IC-8 Group; no significant differences were reported for the uncorrected near and intermediate visual acuity, but subjective patient satisfaction was higher in the IC-8 Group.23 Interestingly, Tarib et al showed that refractive results for near vision might be better with a mixed approach. In their study, the implantation of an EDOF IOL in the dominant eye and of a trifocal IOL in the other eye archived better UNVA results when compared to bilateral implantation of EDOF IOL.24
In our study, distance visual outcomes were excellent in all patients with a range of UDVA from 0.0 to +0.22logMAR, with a value of 20/20 achieved in 36 eyes (90%). This confirmed the ability of this EDOF IOL to successfully restore distance visual function. These results are similar to the outcomes observed in previously published data on other types of multifocal lenses.21,25 Unlike multifocal IOLs which require emmetropia as target refraction to achieve the best visual outcomes, being small amounts of refractive error the cause of degrading in visual performance, the IOL Evolve power calculation was targeted for slight hyperopic refraction (mean +0.3±0.2D) to enhance visual performance at near distance. In the current study, we achieved good refractive predictability with 33 eyes (82.5%) within ±0.50D. Regarding the postoperative MRSE, the IOL Evolve showed a stable tolerance to postoperative refractive errors. This characteristic adds an additional value to the optical property of this lens and renders the lens versatile for different clinical situations, which is a key factor for a high satisfaction rate.
Patients had excellent distance vision, with 19 (95%) having 0.1 logMAR or better binocular UDVA at 6 months. In our study, the excellent visual outcome at a far distance was consistent with the high level of spectacle independence, with 95% of the implanted patients reporting no use of optical correction for distance vision. Such low levels of spectacle dependence are comparable to those reported for multifocal lenses. However, the performance of the EDOF IOL for distance vision is better than for near vision, as previously suggested.14,18,21,26 Synthetically, visual outcome and spectacle independence were excellent for far and intermediate vision, good for near vision with minimal optical phenomena. These differences may be ascribable to factors such as variances in visual acuity measurement tests, residual refractive errors, and study population.
In our study 16 patients (80%) obtained binocular UNVA of 0.5logMAR or better. A better indication of a patient’s near vision achievements might be the outcomes of a postoperative questionnaire, where 14 patients (70%) at 6 months claimed to be spectacle-free for near vision. When questioned about difficulties performing near vision tasks, most of the patients claimed not to have any difficulty in performing common daily activities (range 50–95%), and a limited percentage of them referred a little or a moderate amount of difficulty, respectively (range 0–25% for both). The question involved common tasks related to the use of close-up vision and intermediate distances (ie reading, playing cards, cooking, sewing).
Optical side effects in this study were evaluated 6 months postoperatively. None of the patients complained of any visual disturbance. In multifocal IOLs, one image is in focus, while the out-of-focus image is neuronally suppressed (simultaneous vision) yet still produces such unwanted dysphotopsia.27 In other studies reporting bilateral implantation of multifocal IOLs, 25% to 60% of patients reported difficulties due to perception of photic phenomena postoperatively. Furthermore, optical side effects at early postoperative visits are difficult to interpret because they can be associated with factors other than the IOL design, such as early postoperative inflammation, corneal edema, dry eye, etc.26,28,29 We followed selective inclusion criteria to enroll patients based on the pupil diameter under different light conditions and this could explain our successful results. There was no statistical difference in the mean mesopic pupil sizes measured pre- and postoperatively at 6 months follow-up (p=0.647).
In the present study, the best levels of contrast sensitivity were achieved at spatial frequencies >3cpd and, probably, the absence of a negative change at medium or higher spatial frequencies (6–18cpd) may indicate the absence of posterior capsular opacification 6 months after surgery.
We believe that the achieved outcomes support the idea that EDOF IOLs can be a valuable option to restore the visual acuity of the pseudophakic eyes. The use of this new design concept of IOLs promises an expanded depth of field without the drawbacks associated with a multifocal visual system. However, although the findings of this study are encouraging, further comparative studies with other EDOF IOLs are warranted to examine whether these characteristics translate into better clinical outcomes.
In our experience, the Evolve EDOF IOL produced a spectacle independence for far and intermediate vision with a good rate of satisfaction and spectacle independence for near vision. The advantages are given by the reduction of the optical phenomena that are typically seen with multifocal IOLs. Indeed, this IOL is based on a refractive design with chromatic correction and manufacturing technology designed to minimize light scatter. These features seem to offer good vision at all distances for activities of daily living, without a decrease in contrast sensitivity, even in the presence of halo phenomena not subjectively perceived.
This study has several notable limitations. First, the refractive outcomes and visual acuity tolerance to the postoperative MRSE with bilateral implantation of other EDOF IOLs should be assessed. Ideally, three or more groups of IOLs would have to be included to minimize confounding factors and analyze the optical performance of different IOL designs. Furthermore, another limitation of the study is the lack of objective measurement of the higher-order aberrations induced by the IOL. Previous studies have shown that most IOLs including the EDOF IOLs induced some amount of higher-order aberrations such as coma and trefoil and these are pupil-dependent.30 However, we have recorded the subjective feelings of patients in terms of glare, halo, etc., which may be used as surrogates to indicate higher-order aberrations. Numerous further studies will be necessary to evaluate the efficacy of the different EDOF technologies, as stated by several reviews.2,31 Additionally, further comparative studies with other multifocal IOLs with minimal added power could be useful to evaluate the efficacy in restoring clear vision from far to near distances.32,33
Conclusions
The present work widens the partial results of our previously published article.34 The EDOF IOL Evolve is a promising tool for producing excellent visual rehabilitation as a treatment for presbyopia. We found that the lens was effective in improving distance and near vision in the majority of patients who reported great satisfaction levels with postop vision. Future research will defectively potentiate the development of new EDOF IOLs designs that will provide spectacle independence and excellent visual outcomes after cataract surgery at all distances.
Acknowledgment
Presented in part at the 1st SOR (Società Oftalmologica Romana) Congress, Rome, Italy on January 2020.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sieburth R, Chen M. Intraocular lens correction of presbyopia. Taiwan J Ophthalmol. 2019;9(1):4–17. doi:10.4103/tjo.tjo_136_18
2. Kohnen T, Suryakumar R. Extended depth-of-focus technology in intraocular lenses. J Cataract Refract Surg. 2020;46(2):298–304. doi:10.1097/j.jcrs.0000000000000109.
3. Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10:1965–1970. doi:10.2147/OPTH.S114890.
4. Savini G, Schiano-Lamoriello D, Balducci N, Barboni P. Visual performance of a new extended depth-of-focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018;34(4):228–235. doi:10.3928/1081597X-20180125-01.
5. Aristodemou P, Cartwright NEK, Sparrow JM, Johnston RL. Formula choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. doi:10.1016/j.jcrs.2010.07.032.
6. Ang R, Martinez G, Cruz E, Tiongson A, Dela CA. Prospective evaluation of visual outcomes with three presbyopia-correcting intraocular lenses following cataract surgery. Clin Ophthalmol. 2013;7:1811–1823. doi:10.2147/OPTH.S49848.
7. Gundersen KG, Potvin R. Comparative visual performance with monofocal and multifocal intraocular lenses. Clin Ophthalmol. 2013;7:1979–1985. doi:10.2147/OPTH.S52922.
8. Braga-Mele R, Chang D, Dewey S, et al. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40(2):313–322. doi:10.1016/j.jcrs.2013.12.011.
9. Shah S, Peris-Martinez C, Reinhard T, Vinciguerra P. Visual outcomes after cataract surgery: multifocal versus monofocal intraocular lenses. J Refract Surg. 2015;31:658–666. doi:10.3928/1081597X-20150611-01.
10. Calladine D, Evans JR, Shah S, Leyland M. Multifocal versus monofocal intraocular lenses after cataract extraction. Sao Paulo Med J. 2015;133(1):68. doi:10.1590/1516-3180.20151331T2.
11. Maurino V, Allan BD, Rubin GS, Bunce C, Xing W, Findl O; Moorfields IOLSG. Quality of vision after bilateral multifocal intraocular lens implantation: a randomized trial-AT LISA 809 M versus AcrySof ReSTOR SN6AD1. Ophthalmology. 2015;122(4):700–710. doi:10.1016/j.ophtha.2014.10.002.
12. Mojzis P, Pena-Garcia P, Liehneova I, Ziak P, Alio JL. Outcomes of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2014;40(1):60–69. doi:10.1016/j.jcrs.2013.06.025.
13. Voskresenskaya A, Pozdeyeva N, Pashtaev N, Batkov Y, Treushnicov V, Cherednik V. Initial results of trifocal diffractive IOL implantation. Graefes Arch Clin Exp Ophthalmol. 2010;40:60–69. doi:10.1007/s00417-010-1424-8.
14. Sheppard AL, Shah S, Bhatt U, Bhogal G, Wolffsohn JS. Visual outcomes and subjective experience after bilateral implantation of a new diffractive trifocal intraocular lens. J Cataract Refract Surg. 2013;39(3):343–349. doi:10.1016/j.jcrs.2012.09.017.
15. Zvorničanin J, Zvorničanin E. Premium intraocular lenses: the past, present and future. J Curr Ophthalmol. 2018;30(4):287–296. doi:10.1016/j.joco.2018.04.003
16. Schallhorn SC, Teenan D, Venter JA, Hannan SJ, Schallhorn JM. Initial clinical outcomes of a new extended depth of focus intraocular lens. J Refract Surg. 2019;35(7):426–433. doi:10.3928/1081597X-20190530-01
17. deMedeiros AL, de Araujo Rolim AG, Motta AFP, et al. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol. 2017;11:1911–1916. doi:10.2147/OPTH.S145945.
18. Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi:10.1016/j.jcrs.2017.03.037
19. Ruiz-Mesa R, Abengozar-Vela A, Aramburu A, Ruiz-Santos M. Comparison of visual outcomes after bilateral implantation of extended range of vision and trifocal intraocular lenses. Eur J Ophthalmol. 2017;27(4):460–465. doi:10.5301/ejo.5000935.
20. Hogarty DT, Russell DJ, Ward BM, Dewhurst N, Burt P. Comparing visual acuity, range of vision and spectacle independence in the extended range of vision and monofocal intraocular lens. Clin Exp Ophthalmol. 2018;46(8):854–860. doi:10.1111/ceo.13310.
21. Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507–514. doi:10.3928/1081597X-20180530-02.
22. Pedrotti E, Chierego C, Talli PM. Extended depth of focus versus monofocal IOLs: objective and subjective visual outcomes. J Refract Surg. 2020;36(4):214–222. doi:10.3928/1081597X-20200212-01.
23. Schojai M, Schultz T, Jerke C, Böcker J, Dick HB. Visual performance comparison of 2 extended depth-of-focus intraocular lenses. J Cataract Refract Surg. 2020;46(3):388–393. doi:10.1097/j.jcrs.0000000000000068.
24. Tarib I, Kasier I, Herbers C, et al. Comparison of visual outcomes and patient satisfaction after bilateral implantation of an EDOF IOL and a mix-and-match approach. J Refract Surg. 2019;35(7):408–416. doi:10.3928/1081597X-20190417-02.
25. Gundersen KG. Rotational stability and visual performance 3 months after bilateral implantation of a new topic extended range of vision intraocular lens. Clin Ophthalmol. 2018;12:1269–1278. doi:10.2147/OPTH.S173120
26. Law EM, Aggarwal RK, Kasaby H. Clinical outcomes with a new trifocal Intraocular lens. Eur J Ophthalmol. 2014;24(4):501–508. doi:10.5301/ejo.5000407.
27. Alba-Bueno F, Vega F, Millan MS. Halos and multifocal intraocular lenses: origin and interpretation. Arch Soc Esp Oftalmol. 2014;89(10):397–404. doi:10.1016/j.oftal.2014.01.002.
28. Konen T, Title C, Bohm M. Trifocal intraocular lens implantation to treat visual demands in various distances following lens removal. Am J Ophthalmol. 2016;161:71–7e1. doi:10.1016/j.ajo.2015.09.030.
29. Lubinski W, Gronkowska-Serafin J, Podboraczynska-Jodko K. Clinical outcomes after cataract surgery with implantation of the tennis ZMB00 multifocal intraocular lens. Med Sci Monit. 2014;20:1220–1226. doi:10.12659/MSM.890585.
30. Breyer DRH, Kaymak H, Ax T, et al. Multifocal intraocular lenses and extended depth of focus intraocular lenses. Asia Pac J Ophthalmol (Phila). 2017;6(4):339–349. doi:10.22608/APO.2017186.
31. Kanclerz P, Toto F, Grzybowski A, Alio JL. Extended depth-of-field intraocular lenses: an update. Asia Pac J Ophthalmol (Phila). 2020;9(3):194–202. doi:10.1097/APO.0000000000000296.
32. Hayashi K, Yoshida M, Hayashi H. All-distance visual acuity and contrast visual acuity in eyes with a refractive multifocal intraocular lens with minimal added power. Ophthalmology. 2009;116(3):401–408. doi:10.1007/s10384-008-0626-7.
33. Gil MA, Varon C, Cardona G, Buil JA. Visual acuity and defocus curves with six multifocal intraocular lenses. Int Ophthalmol. 2020;40(2):393–401. doi:10.1007/s10792-019-01196-4.
34. Giannico MI, Formisano M, Alisi L, Spadea L. Preliminary outcomes of a new extended depth of focus intraocular lens: a prospective study after bilateral implantation. J Cataract Refract Surg. 2021. Epub ahead of print. doi:10.1097/j.jcrs.0000000000000616.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.