Back to Journals » Clinical Ophthalmology » Volume 14
Visual Outcomes After Sequential Posterior Chamber Phakic IOL with Corneal Refractive Surgery (Bioptics) for the Treatment of Myopic Astigmatism
Authors Moshirfar M , Thomson RJ, West WB Jnr , McCabe SE , Sant TM, Shmunes MH , Ronquillo YC , Hoopes PC
Received 3 October 2020
Accepted for publication 6 November 2020
Published 9 December 2020 Volume 2020:14 Pages 4337—4346
DOI https://doi.org/10.2147/OPTH.S285140
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Majid Moshirfar,1– 3 Robert J Thomson,4 William B West Jnr,5 Shannon E McCabe,1,6 Thomas M Sant,5 Margaret H Shmunes,7 Yasmyne C Ronquillo,1 Phillip C Hoopes1
1Hoopes Vision Research Center, Hoopes Vision, Draper, UT, USA; 2John A. Moran Eye Center, Department of Ophthalmology and Visual Sciences, University of Utah School of Medicine, Salt Lake City, UT, USA; 3Utah Lions Eye Bank, Murray, UT, USA; 4McGovern Medical School at the University of Texas Health Science Center at Houston, Houston, TX, USA; 5University of Utah School of Medicine, Salt Lake City, UT, USA; 6Mission Hills Eye Center, Pleasant Hill, CA, USA; 7Burrell College of Osteopathic Medicine, Las Cruses, NM, USA
Correspondence: Majid Moshirfar
MD Hoopes Vision Research Center, Hoopes Vision, Draper, UT 84020, USA
Tel +1 801-568-0200
Fax +1 801-563-0200
Email [email protected]
Purpose: To report the outcomes of sequential posterior chamber phakic intraocular lens (PC-pIOL) with corneal refractive surgery in conventional (PC-pIOL prior to refractive surgery) and reverse (refractive surgery prior to PC-pIOL) bioptics for treating high myopic astigmatism.
Setting: Tertiary refractive center, Draper, Utah, USA.
Design: Retrospective case series.
Methods: Medical records of patients who underwent planned bioptics were reviewed. Surgery involved PC-pIOL placement using an implantable collamer lens (ICL) with preceding or subsequent LASIK or PRK. Pre- and postoperative manifest spherical equivalent (SEQ), visual acuity, and PC-pIOL vault were analyzed.
Results: Of the 51 eyes present at 12 months postoperatively, 49 eyes (96%) achieved target SEQ within ± 1.00 D and an identical amount achieved refractive astigmatism ≤ 1.00 D. Post-bioptics eyes achieved a postoperative UDVA equal to or better than preoperative CDVA in 45 eyes (88%). Efficacy and safety indices were 1.08 ± 0.20 (41 eyes) and 1.13 ± 0.22 (44 eyes) for conventional bioptics and 0.99 ± 0.42 (7 eyes) and 1.15 ± 0.38 (7 eyes) for reverse bioptics eyes at 12 months. The maximum PC-pIOL vault of conventional bioptics eyes (27 eyes) within 6 months before and after LASIK/PRK was 385 ± 159 μm and 377 ± 135 μm, respectively (P = 0.71).
Conclusion: Bioptics for high myopic astigmatism was safe and effective. Reverse bioptics, although not as traditional, could provide similar results. Additionally, the PC-pIOL vault does not appear to be affected by LASIK/PRK.
Keywords: bioptics, LASIK, PRK, posterior chamber phakic IOL, high myopic astigmatism, visual outcomes
Introduction
Bioptics, originally described by Zaldivar et al,1 utilizes the combination of a posterior chamber phakic intraocular lens (PC-pIOL) with excimer laser corneal ablation to split the optical correction between two planes, the corneal plane and the ciliary sulcus plane. Consequently, bioptics can treat complex and high refractive errors while maintaining a large optical zone with minimal induced spherical aberrations. As of today, the term has expanded to encompass phakic, pseudophakic, and clear lens extraction cases combined with several forms of corneal refractive procedures (LASIK, PRK, intracorneal rings, and RK).2–5 The combination of intraocular implant and corneal curvature modification expands the scope of treatment for patients with significant refractive errors that fall outside the recommended treatment range of excimer laser refractive surgery as well as patients with unfavorable corneal thicknesses that prevent full treatment of their refractive error.6–9 Only bioptics procedures involving PC-pIOL utilizing the Visian Implantable Collamer Lens V4b (ICL; STAAR Surgical, Monrovia, CA), and to a lesser extent, the Visian Toric ICL (TICL; STAAR Surgical, Monrovia, CA), will be discussed here.
Previously, PC-pIOL implantation could only address the spherical component of refractive errors, relying on a separate corneal procedure to correct the astigmatic element. With the advent of the TICL, coexisting astigmatism can now be treated using a single procedure, and several studies have already demonstrated the safety and efficacy of TICL implantation.10–14 However, TICLs are currently limited to four diopters of astigmatic correction. As a result, bioptics remains a useful treatment option in patients with large amounts of astigmatism.
The majority of reported bioptics cases involve using a PC-pIOL to correct most of the patient’s refractive error followed by a corneal refractive procedure several months later to reduce any residual refractive error. The literature on planned bioptics procedures performed in the reverse order, with corneal surgery prior to intraocular lens implantation, for the primary treatment of high refractive errors is not as extensive. This reverse order of procedures, termed by Leccisotti7 as “reverse bioptics,” could offer similar results to conventional bioptics.
This retrospective study aims to assess the safety, efficacy, and predictability of PC-pIOL in combination with LASIK and PRK bioptics procedures in both conventional and reverse orders as performed at one site.
Methods
This study followed the tenets of the Helsinki Declaration and was approved by the Hoopes Vision Ethics Board. All patients were adequately informed prior to obtaining their signed consent. Only conventional bioptics or reverse bioptics cases with planned, consecutive procedures were included in this study. Patients undergoing unplanned LASIK or PRK enhancements after or before PC-pIOL implantation and patients receiving corneal refractive surgery in addition to cataract surgery were excluded. The preoperative evaluation included uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refraction, tonometry, keratometry, pachymetry, and anterior chamber depth (ACD) measurements. Adverse events and complications were recorded at all visits.
Surgeries were performed by two surgeons (MM and PH). At least 1 week prior to PC-pIOL implantation, YAG peripheral iridotomies were performed at the 11 and 1 o’clock positions. The selection of proper Visian ICL size was based upon the Parkhurst nomogram utilizing measurements of sulcus-to-sulcus dimension, lenticular rise, and aqueous depth obtained by VUmax (Sonomed Escalon, New Hyde Park, NY) ultrasound biomicroscopy. The PC-pIOL was inserted through a temporal 3.0 mm clear corneal incision and placed into the sulcus. Postoperatively, patients received ofloxacin 0.3% or moxifloxacin 0.5% four times a day for 1 week. Prednisolone acetate 1% was also applied topically four times daily for the first week then tapered weekly.
For LASIK procedures, the AMO iFS (Abbott Medical Optics, Inc., Santa Ana, CA) femtosecond laser system was used for flap creation and the WaveLight EX500 excimer laser system (Alcon Laboratories, Inc., Fort Worth, TX) was used for stromal ablation with a 6.5 mm central optical zone and blend zone to 9.0 mm. Flap diameter was between 8.5 and 9.0 mm, and flap thickness was between 100 and 115 µm with the creation of a superior hinge. The postoperative treatment protocol included ofloxacin 0.3% or moxifloxacin 0.5% four times a day for 1 week. Patients were instructed to apply prednisolone acetate 1% every hour while awake for the first 24 hours. On postoperative day 1, the prednisolone was decreased to four times daily for 1 week and subsequently stopped.
With PRK procedures, alcohol debridement of the corneal epithelium was performed, then excimer laser was used for stromal ablation as previously discussed. Postoperative treatment included ofloxacin 0.3% or moxifloxacin 0.5% four times a day for 1 week. Ketorolac 0.5% was used twice daily for 3 days. Prednisolone acetate 1% was also applied topically four times daily for the first month, then stopped. Fluorometholone 0.1% was then used three times daily for 3 weeks, then twice daily for 3 weeks, then once daily for 3 weeks. For treatments involving an ablation depth of greater than 64 μm, a topical application of 0.02% mitomycin-C was used to decrease the risk of corneal haze. Lastly, a bandage contact lens was inserted postoperatively and removed at the 1 week post-op visit.
Follow-up after the initial procedures for both groups was performed at 1 day, 1 week, 1 month, 3 months, 6 months, and 12 months unless the second procedure occurred before the 6-month or 12-month appointment. The perioperative evaluation included UDVA, CDVA, manifest refraction, tonometry, keratometry, pachymetry, and anterior segment ocular coherence tomography (OCT). Postoperative follow-up after the final procedures occurred at 1 day, 1 month, 3 months, 6 months, and 12 months and included the above-mentioned parameters.
Statistical analysis was performed using IBM SPSS Statistics 2020 v27.0 (IBM corp., Armonk, NY). Wilcoxon rank-sum test was applied to compare outcomes in conventional and reverse bioptics. A paired t-test was used to compare changes in PC-pIOL vault prior to and after LASIK/PRK in conventional bioptics. P < 0.05 was considered statistically significant.
Results
In this retrospective study using de-identified data, the medical records of 100 eyes of 56 patients (46 male and 54 female) undergoing sequential, planned PC-pIOL implantation and corneal refractive surgery at one site between January 2012 and December 2019 were evaluated (Table 1). The mean age of patients was 33.5 ± 7.6 years (SD) (range 21 to 57).
 |
Table 1 Preoperative Baseline Characteristics for Eyes That Underwent Conventional Bioptics and Reverse Bioptics |
In 87 eyes, PC-pIOL implantation was followed by either PRK for 61 eyes (70%) or LASIK for 26 eyes (30%). Three eyes of two patients, whose procedures occurred after FDA approval of TICL in September 2018, received TICL due to high astigmatic errors (≥4.00 D). The TICL provided its maximum of four diopters of astigmatic correction and PRK corrected the remaining astigmatic (0.00 to 1.25 D) and spherical refractive error. Thirteen eyes of eight patients received PRK for two eyes (15%) or LASIK for eleven eyes (85%) prior to PC-pIOL implantation. Time between procedures was 3.6 ± 2.1 months (range 1 to 10).
Follow up was 83 eyes at 1 month, 77 eyes at 3 months, 61 eyes at 6 months, and 51 eyes at 12 months (Table 2). Although not all patients completed a 1-month postoperative visit, all patients were evaluated during at least one of the future time points analyzed. Four eyes that were purposely corrected to be myopic to achieve monovision were excluded from the postoperative UDVA and manifest spherical equivalent (SEQ) results. Refractive surgery results were reported via the standard graphs15 using data collected from the 3- and 12-month follow-ups.
 |
Table 2 Pre- and Postoperative Visual Outcomes at Postoperative Time Points |
Preoperative UDVA was worse than 20/200 in all 100 eyes. Visual outcomes at 3 and 12 months postoperatively are presented in Figure 1. Only one eye (1.3%) experienced a transient loss of 2 lines of CDVA at 3 months. However, this loss in CDVA was likely due to the initial post-PRK recovery phase and the steroid taper, as the patient’s BCVA and UCVA recovered to 20/20 at the 6-month visit. The mean preoperative SEQ was −11.30 ± 3.25 D (range −19.88 to –3.38) improving to 0.01 ± 0.35 D (−0.50 to 1.13) for the 77 eyes at 3 months. At the 12-month follow-up visit, the mean SEQ was −0.08 ± 0.31 D (−0.88 to 0.75) for 51 eyes.
Pre- and postoperative outcomes for refractive astigmatism are displayed in Figure 2. The mean preoperative refractive astigmatism was −2.67 ± 1.29 D (range −6.75 to −0.50) improving at 3 months postoperatively to −0.41 ± 0.32 D (−1.50 to 0.00). At 12 months postoperatively, the mean refractive astigmatism was −0.42 ± 0.34 D (−1.25 to 0.00)
At 3 months, the surgically induced astigmatism vector (SIA) was within ±1.00 D of target induced astigmatism vector (TIA) for 76 eyes (99%) (one eye overcorrected) and within ±0.50 D for 70 eyes (91%) (three over- and four under-corrected). At 12 months, SIA was within ±1.00 D of TIA for 49 eyes (96%) (one over- and one under-corrected) and within ±0.50 D of TIA for 43 eyes (84%) (one over- and seven under-corrected). Linear regression curves for SIA as a function of TIA demonstrated equal distribution of over- and under-corrections and mild amount of scatter (R2 of 0.92) for both 3 and 12 months postoperatively.
The mean SEQ was utilized to evaluate stability in all patients which is displayed in Figure 3. The preoperative mean SEQ of the 100 eyes was −11.30 ± 3.25 D (range −19.88 to −3.38). Mean SEQ of 83 eyes at 1 month was −0.15 ± 0.46 D (−1.50 to 1.13); 77 eyes at 3 months was 0.01 ± 0.35 D (−0.50 to 1.13); 61 eyes at 6 months was −0.03 ± 0.34 D (−0.63 to 1.13); and 51 eyes at 12 months, was −0.08 ± 0.31 D (−0.88 to 0.75).
 |
Figure 3 Stability of bioptics procedures reported as mean SEQ at 1 week and 1, 3, 6, and 12 months postoperatively. Abbreviations: SEQ, manifest spherical equivalent; D, diopters. |
Table 3 compares the change in efficacy index (mean postoperative UDVA/mean preoperative CDVA) and safety index (mean postoperative CDVA/mean preoperative CDVA) for conventional and reverse bioptics. At 1 month, the differences between the efficacy and safety indices of each group were statistically significant (P < 0.01); however, this significance was not present at any of the follow-up visits. At 12 months, 38 eyes (93%) after conventional bioptics and 4 eyes (57%) after reverse bioptics achieved a postoperative UDVA equal to or better than preoperative CDVA.
 |
Table 3 Efficacy and Safety Index for Eyes That Underwent Conventional Bioptics and Reverse Bioptics |
Table 4 compares the change in mean preoperative SEQ and refractive cylinder in both groups. Monovision eyes were excluded from the SEQ results. The difference in preoperative values and refractive cylinders at 3 months was statistically significant (P < 0.01). Differences between the two groups at all other time points were no longer significant. At 12 months postoperatively, all eyes after conventional bioptics were within ±1.00 D of target SEQ and 36 eyes (88%) were within ±0.50 D. Seven eyes (100%) after reverse bioptics achieved ±0.25 D. The astigmatic error was ≤1.00 D for all but one eye (2.3%) after conventional bioptics, and ≤0.50 D for 33 eyes (75%) and 5 eyes (71%) after conventional and reverse bioptics, respectively.
 |
Table 4 Postoperative Spherical Equivalent Refraction and Refractive Cylinder in Eyes That Underwent Conventional Bioptics and Reverse Bioptics |
PC-pIOL vault depth was recorded using the RTVue-XR Avanti OCT system (Optovue, Inc., Fremont, CA) prior to and following LASIK/PRK for 27 eyes (31%) in the conventional bioptics group to evaluate the effect LASIK/PRK had on the PC-pIOL vault (Figure 4). The vault prior to LASIK/PRK was 385 ± 159 µm (44 to 672) shifting to 377 ± 135 (130 to 610) after the ablative procedures. The difference was not significant (P = 0.71). Of the 27 available, 15 eyes (58%) had an average decrease in vault of 67 ± 88 µm (2 to 350), while the remaining 12 eyes (46%) had an average increase of 67 ± 46 µm (10 to 166).
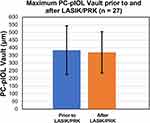 |
Figure 4 PC-pIOL vaults recorded within 6 months prior to and after LASIK/PRK in conventional bioptics. (P = 0.71). Abbreviation: PC-pIOL, posterior chamber phakic intraocular lens. |
The most common adverse event was dry eyes requiring punctal plugs, which occurred in three eyes (3%) of two patients after PC-pIOL implantation and six eyes (6%) of three patients after laser ablation surgery. Two patients had bilateral flap dislocation detected on postoperative day one requiring flap irrigation and refloat; however, neither patient experienced a loss in UDVA nor in CDVA at the final follow-up visits.
Discussion
This study examines the visual outcomes after planned bioptics procedures with a sub-analysis of eyes that underwent reverse bioptics. As reported in previous studies of planned and unplanned bioptics,1–9,16–19 our results further support bioptics as a safe, effective, and stable option for correcting myopic astigmatism.
Data on the outcomes of planned reverse bioptics in the literature is limited in comparison to that of conventional bioptics. Reverse bioptics is typically unplanned, occurring in patients who have previously undergone a corneal refractive procedure and request an enhancement but are subsequently poor corneal surgery candidates.7,20 In our study, patients receiving planned reverse bioptics showed similar visual improvements when compared to patients after conventional bioptics as well as in comparison to studies of unplanned reverse bioptics.20–23 However, due to the limited sample size (n = 13), additional studies with longer follow-up and larger sample sizes are needed to make a definitive conclusion about the outcomes of planned reverse bioptics for high myopic astigmatism. The small sample size of reverse bioptics was in part due to patient preference being the main determinant of whether or not reverse or conventional bioptics was performed. Further studies could reveal unique potential benefits of planned reverse bioptics as well.
Unlike the IOL power calculations for cataract surgery, PC-pIOL calculations are less dependent on corneal refractive power and therefore less affected by preceding corneal refractive surgery in reverse bioptics patients.23,24 Additionally, because PC-pIOL calculations are partly based on subjective refraction, reducing myopia provides a more accurate calculated PC-pIOL power.1 This in turn could lead to a larger optical zone along with diminished glare and halos in reverse bioptics patients. Reducing the power of the PC-pIOL also lessens the thickness of the lens. A thinner PC-pIOL implant would be less likely to contact and abrade the anterior crystalline lens, theoretically leading to decreased risk of anterior subcapsular cataract formation.
Due to the time required for PC-pIOL incisions to heal, LASIK is typically delayed at least 3 months to avoid potential complications from the suction ring applied in both microkeratome- and femtosecond-assisted LASIK. In reverse bioptics, prolonging the delay before the last procedure is not necessary. In our study, the average time between procedures for conventional (14 patients) and reverse bioptics (6 patients) that received LASIK was 15 and 8 weeks, respectively, demonstrating the potential decrease in inter-procedural delay.
The effects of LASIK/PRK on the PC-pIOL vault has rarely been examined in the literature. Vault narrowing has been directly linked to adverse events such as pigment dispersion syndrome and anterior subcapsular cataract formation.24,25 The multiple forces applied to the cornea during LASIK/PRK could theoretically lead to vault narrowing. However, our study found that LASIK/PRK did not cause acute vault narrowing. Future research should confirm these initial findings.
While bioptics was previously the principle method for correcting high astigmatic refractive errors, several studies have demonstrated the safety and efficacy of TICL implantation.10–14 When Choi et al27 compared 29 eyes which received TICL with 26 eyes that underwent bioptics procedures to treat moderate to high myopia with astigmatism, the two groups had similar visual outcomes at 12 months postoperatively, with quicker visual improvement and fewer postoperative higher-order aberrations in the TICL group. In a later study by Alfonso et al,28 patients who underwent TICL versus bioptics had comparable outcomes; however, the bioptics group achieved a slightly higher efficacy index (1.09 ± 0.19 compared to 0.98 ± 0.20) and refractive predictability (77 of eyes [94%] within ±0.50 D of emmetropia compared to 66 eyes [81%] in the TICL group). Regardless, TICL may overall be the preferred treatment. With a lower risk of surgical complications, decreased cost to the patient, quicker results, and the convenience of a single surgery; TICL is superior to bioptics in several regards.27
However, bioptics may yet still play a useful role, as TICL alone may be unable to address cases of high myopia (>-19.00 D) and significant astigmatism (>4.00 D). In our study, three eyes of two patients with astigmatic errors ranging from −4.00 to −5.25 D received TICL implants that provided a maximum astigmatic correction of 4 diopters followed by PRK 4.5 ± 2.6 months (3 to 9) later to reduce the remaining astigmatic and spherical error. All three eyes achieved an SEQ ± 0.50 D of emmetropia and ≤1.00 D of astigmatism with no loss in CDVA at their final follow-up visits. Our results indicate that TICL bioptics could be a viable treatment option for high astigmatic patients.
In addition to the limitations inherent in retrospective studies, this study was limited, from a statistical standpoint, by the relatively small sample size of the reverse bioptics group (n = 13). Additionally, in most of the patients, eyes were treated bilaterally rather than randomly assigning treatment to a single eye. Deciding which patients received bioptics versus reverse bioptics as well as LASIK versus PRK was left to the discretion of the surgeon and patient preference and was not randomized. Lack of stratification of the changes in PC-pIOL vault size between LASIK and PRK due to the limited sample size was an additional limitation. Specular microscopy data were not collected; however, this was not the objective of this work. The study was also limited due to a lack of follow-up beyond 12 months. Due to the higher risk of cataract formation and endothelial cell density loss after PC-pIOL implantation, the importance of long-term follow-up should be emphasized when counselling patients. Despite the limitations, this study provides useful information currently missing in the scientific literature as data regarding planned reverse bioptics is notably sparse.
In summary, bioptics procedures are safe and effective. While TICL is superior to bioptics in several regards, bioptics may still serve a purpose for treating cases of high myopia or myopia with significant astigmatism that fall outside the effective treatment ranges of PC-pIOLs and TICLs alone. The present findings suggest that PRK/LASIK does not appear to cause substantial PC-pIOL vault narrowing, though further research is needed to confirm this conclusion. Lastly, in addition to treating patients who are poor corneal enhancement surgery candidates, planned reverse bioptics could be a useful, primary treatment option for astigmatic and myopic refractive errors.
Ethics and Consent Statement
This retrospective study using de-identified data has been approved by the Hoopes Vision Ethics Board and conforms with the Helsinki Declaration of 1964, as revised in 2013, concerning human and animal rights. The patients signed informed consent.
Acknowledgments
Special thanks to Mitchell Tingey, Andrew Thomson, Derek Jezulin, and Calvin Neilsen for their assistance with data acquisition, analysis, and management.
Author Contributions
All authors take responsibility for the integrity of the work, and have given final approval to the version to be published. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This study was funded by an unrestricted grant from Research to Prevent Blindness (RPB), 360 Lexington Avenue, 22nd Floor New York, NY 10017. No support was received for the publication of this article.
Disclosure
All authors declare that they have no conflicts of interest.
References
1. Zaldivar R, Davidorf JM, Oscherow S, Ricur G, Piezzi V. Combined posterior chamber phakic intraocular lens and laser in situ keratomileusis: bioptics for extreme myopia. J Refract Surg. 1999;15(3):299–308.
2. Zaldivar R, Oscherow S, Piezzi V. Bioptics in phakic and pseudophakic intraocular lens with the Nidek EC-5000 excimer laser. J Refract Surg. 2002;18(3 Suppl):S336–S339.
3. Leccisotti A. Bioptics by angle-supported phakic lenses and photorefractive keratectomy. Eur J Ophthalmol. 2005;15(1):1–7. doi:10.1177/112067210501500101
4. Srinivasan S, Drake A, Herzig S. Early experience with implantable collamer lens in the management of hyperopia after radial keratotomy. Cornea. 2008;27(3):302–304. doi:10.1097/ICO.0b013e31815ea268
5. Abdelmassih Y, El-Khoury S, Chella E, Slim E, Cherfan CG, Jarade E. Toric ICL implantation after sequential intracorneal ring segments implantation and corneal cross-linking in keratoconus: 2-year follow-up. J Refract Surg. 2017;33(9):610–616. doi:10.3928/1081597X-20170621-02
6. Dvali ML, Tsinsadze NA, Sirbiladze BV. Bioptics with LASIK flap first for the treatment of high ametropia. J Refract Surg. 2009;25(1):S160–S162. doi:10.3928/1081597X-20090115-15
7. Leccisotti A. Bioptics: where do things stand? Curr Opin Ophthalmol. 2006;17(4):399–405. doi:10.1097/01.icu.0000233962.19004.14
8. Trivizki O, Smadja D, Mimouni M, Levinger S, Levinger E. Bioptics for high hyperopia with combined multifocal intraocular lens implantation and excimer ablation in young patients. Eur J Ophthalmol. 2019;29(4):426–430. doi:10.1177/1120672118797281
9. Pop M, Payette Y, Amyot M. Clear lens extraction with intraocular lens followed by photorefractive keratectomy or laser in situ keratomileusis. Ophthalmology. 2001;108(1):104–111. doi:10.1016/S0161-6420(00)00451-6
10. Sanders DR, Schneider D, Martin R, et al. Toric implantable collamer lens for moderate to high myopic astigmatism. Ophthalmology. 2007;114(1):54–61. doi:10.1016/j.ophtha.2006.08.049
11. Pothireddy R, Reddy KP, Senthil S, Rao HL. Posterior chamber toric phakic intraocular lenses for myopic astigmatism: first experience in India. J Cataract Refract Surg. 2012;38(9):1583–1589. doi:10.1016/j.jcrs.2012.04.032
12. Sari ES, Pinero DP, Kubaloglu A, et al. Toric implantable collamer lens for moderate to high myopic astigmatism: 3-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2013;251(5):1413–1422. doi:10.1007/s00417-012-2172-8
13. Chang J, Lau S. Toric implantable collamer lens for high myopic astigmatic Asian eyes. Ophthalmology. 2009;116(12):2340–2347. doi:10.1016/j.ophtha.2009.04.053
14. Bhikoo R, Rayner S, Gray T. Toric implantable collamer lens for patients with moderate to severe myopic astigmatism: 12-month follow-up. Clin Exp Ophthalmol. 2010;38(5):467–474. doi:10.1111/j.1442-9071.2010.02273.x
15. Reinstein DZ, Archer TJ, Randleman JB. JRS standard for reporting astigmatism outcomes of refractive surgery [published correction appears in J Refract Surg. 2015 Feb 1;31(2):129]. J Refract Surg. 2014;30(10):654–659. doi:10.3928/1081597X-20140903-01
16. Velarde JI, Anton PG, de Valentin-gamazo L. Intraocular lens implantation and laser in situ keratomileusis (bioptics) to correct high myopia and hyperopia with astigmatism. J Refract Surg. 2001;17(2 Suppl):S234–S237.
17. Sánchez-Galeana CA, Smith RJ, Rodriguez X, Montes M, Chayet AS. Laser in situ keratomileusis and photorefractive keratectomy for residual refractive error after phakic intraocular lens implantation. J Refract Surg. 2001;17(3):299–304.
18. Arne JL, Lesueur LC, Hulin HH. Photorefractive keratectomy or laser in situ keratomileusis for residual refractive error after phakic intraocular lens implantation. J Cataract Refract Surg. 2003;29(6):1167–1173. doi:10.1016/S0886-3350(03)00015-4
19. Muñoz G, Alió JL, Montés-Micó R, Belda JI. Angle-supported phakic intraocular lenses followed by laser-assisted in situ keratomileusis for the correction of high myopia. Am J Ophthalmol. 2003;136(3):490–499. doi:10.1016/S0002-9394(03)00240-X
20. Chen X, Wang XY, Zhang X, Chen Z, Zhou XT. Implantable collamer lens for residual refractive error after corneal refractive surgery. Int J Ophthalmol. 2006;9(10):1421–1426.
21. Kamiya K, Shimizu K, Komatsu M. Implantable collamer lens implantation and limbal relaxing incisions for the correction of hyperopic astigmatism after laser in situ keratomileusis. Cornea. 2010;29(1):99–101. doi:10.1097/ICO.0b013e31818a7de3
22. Pérez-Vives C, Belda-Salmerón L, García-Lázaro S, Ferrer-Blasco T, Montés-Micó R. Optical and visual simulation of standard and modified spherical aberration implantable collamer lens post myopic LASIK surgery. Eur J Ophthalmol. 2014;24(3):330–337. doi:10.5301/ejo.5000372
23. Seitz B, Langenbucher A, Nguyen NX, Kus MM, Küchle M. Underestimation of intraocular lens power for cataract surgery after myopic photorefractive keratectomy. Ophthalmology. 1999;106(4):693–702.
24. Chan TC, Liu D, Yu M, Jhanji V. Longitudinal evaluation of posterior corneal elevation after laser refractive surgery using swept-source optical coherence tomography. Ophthalmology. 2015;122(4):687–692. doi:10.1016/j.ophtha.2014.10.011
25. Kocová H, Vlková E, Michalcová L, Rybárová N, Motyka O. Incidence of cataract following implantation of a posterior-chamber phakic lens ICL (Implantable Collamer Lens) - long-term results. Výskyt katarakty po implantaci zadněkomorové fakické čočky ICL - dlouhodobé výsledky. Cesk Slov Oftalmol. 2017;73(3):87–93.
26. Gimbel HV, LeClair BM, Jabo B, Marzouk H. Incidence of implantable collamer lens-induced cataract. Can J Ophthalmol. 2018;53(5):518–522. doi:10.1016/j.jcjo.2017.11.018
27. Choi SH, Lee MO, Chung ES, Chung TY. Comparison of the toric implantable collamer lens and bioptics for myopic astigmatism. J Refract Surg. 2011;27(2):91–97. doi:10.3928/1081597X-20100414-01
28. Alfonso JF, Lisa C, Fernández-Vega Cueto L, Fernandes P, González-Méijome JM, Montés Micó R. Comparison of visual and refractive results of toric implantable collamer lens with bioptics for myopic astigmatism. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):967–975. doi:10.1007/s00417-012-2155-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


