Back to Journals » Clinical Ophthalmology » Volume 17
Visual Impairment and Its Associated Factors Among Hypertensive Patients in Amhara Region Referral Hospitals, Ethiopia
Authors Ashenef B , Diress M , Yeshaw Y , Dagnew B , Gela YY , Akalu Y , Abdurahman A, Abebaw K
Received 8 March 2023
Accepted for publication 4 August 2023
Published 20 October 2023 Volume 2023:17 Pages 3149—3161
DOI https://doi.org/10.2147/OPTH.S408171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Baye Ashenef,1 Mengistie Diress,2 Yigizie Yeshaw,2 Baye Dagnew,2 Yibeltal Yismaw Gela,2 Yonas Akalu,2 Abdulhenan Abdurahman,3 Kassa Abebaw1
1Department of Human Physiology, School of Medicine, Debre Markos University, Debre Markos, Ethiopia; 2Department of Human Physiology, School of Medicine, University of Gondar, Gondar, Ethiopia; 3Department of Human Physiology, School of Medicine, Madda Walabu University, Bale Goba, Ethiopia
Correspondence: Baye Ashenef, Email [email protected]
Background: Visual impairment is one of the most common long-term effects of high blood pressure. It affects one’s quality of life, independence, and mobility. There is a paucity of information regarding the prevalence of visual impairment due to hypertension in Ethiopia. Therefore, this study aimed to determine the prevalence of visual impairment and its associated factors among hypertensive patients in referral hospitals of the Amhara region, Ethiopia, 2021.
Methods: An institution-based cross-sectional study was conducted among 423 study participants, which were selected by systematic random sampling technique. Data were collected by using an interviewer-administered structured questionnaire and physical measurements. The collected data were entered into Epi-data version 4.6. Then, exported into SPSS Version 26 for analysis. The crude and adjusted odds ratios and 95% Confidence intervals were calculated. Both bivariable and multivariable logistic regression analyses were done. Variables with a p-value of ≤ 0.05 were stated as significantly associated with visual impairment.
Results: The overall prevalence of visual impairment among hypertensive patients was 32.4% (95% CI: 27.9– 37.9). Duration of hypertension ≥ 5 years (AOR =3.06, 95% CI: 1.86– 4.95), history of ocular trauma (AOR =2.50, 95% CI: 1.3– 4.73), and ever alcohol drinking (AOR = 2.72, 95% CI: 1.32– 5.62) were significantly associated with visual impairment.
Conclusion: Nearly one-third of hypertensive patients were visually impaired from the study participants. Duration of hypertension ≥ 5 years, history of ocular trauma, and ever alcohol drinking was significantly associated with visual impairment among hypertensive patients. Therefore, in addition to providing medical care and follow-up services for hypertension patients, health education is needed for early and proper management of visual impairment, and to decrease their level of alcohol consumption. Furthermore, better to do this study by prospective cohort study design to identify cause-and-effect relationships.
Keywords: visual impairment, visual acuity, hypertension, Ethiopia
Introduction
Visual impairment (VI) is a condition in which the presenting distance visual acuity (VA) of the better eye is worse than 6/12.1 The leading causes of vision impairment are uncorrected refractive errors, cataracts, age-related macular degeneration, glaucoma, diabetic retinopathy, corneal opacity, hypertension, and trachoma.2–8
High blood pressure (BP) damages the heart, kidneys, and eyes, resulting in vision loss.2,9 Systemic hypertension has been linked to a variety of major eye diseases and can affect the structure and function of the eye.10,11 It is linked to a lower number of perifoveal arterioles and venules, as well as pathology and retinal vasculature.12,13 Chronically elevated BP can cause arteriosclerosis and changes in the size of the precapillary arterioles, resulting in increased resistance to blood flow and decreased perfusion to the retina, causing retinal microvascular defects, retinal vein occlusion, and diabetic retinopathy.14–16
According to the World Health Organization (WHO) in 2020, 2.2 billion people globally and 26.3 million people in African countries are visually impaired.1,17 VI is more prevalent in low- and middle-income countries (LMICs), accounting for 80–90% of the world’s visually impaired people.17,18 Ethiopia is estimated to have one of the highest rates of blindness (1.6%) and poor vision (3.7%) in the world, of which more than 80% are either treatable or preventable.19
VI is a common problem among hypertensive patients. The prevalence of visual impairment among hypertensive patients in China,20 Taiwan,21 India,22 Sri Lanka,23 and Malaysia,24 was indicated to be 8.2%,14.7%, 26.4%, 28.1%, and 50.4%, respectively. In the case of Africa, a 35.5%, 56%, and 70% prevalence of visual impairment among hypertensive patients was reported by a study in South Africa,25 Nigeria,2 and the Central Republic of Congo,26 respectively.
This implies VI has a huge impact on hypertensive patients, as well as their families and friends. The complete loss or worsening of existing eyesight can be frightening and discouraging, causing worry about independence, paying for the required medical care, retaining jobs, and providing for themselves and their families.1,27 Vision loss can also affect one’s quality of life (QOL), independence, and mobility, and has been associated with falls, injuries, and deterioration in mental health, memory, social function, jobs, and educational attainment.27
Evidence from different kinds of literature revealed that visual impairment is associated with factors like age, sex, income, educational level, duration of hypertension,2,5 obesity, duration of sleep, cigarette smoking, and alcohol consumption.19,28–32
Although there is various literature done on the prevalence and causes of visual impairment with its associated factors worldwide, there is not enough literature done on the prevalence of visual impairment among hypertensive patients. Furthermore, there is no single established study that determines the prevalence of VI among hypertensive patients in Ethiopia. Therefore, this study aimed to determine the prevalence and associated factors of visual impairment among hypertensive patients attending follow-up visits in referral hospitals of the Amhara region, Ethiopia. This study implicates hypertension patients should give attention to the visual problem due to hypertension and its prevention mechanisms.
Methods and Materials
Study Setting and Period
This study was conducted in referral hospitals of the Amhara region, Ethiopia, from April 1, 2021, to May 30, 2021. Amhara region is one of the nine regions in Ethiopia, and its capital city is Bahir Dar, which is located 565 km away, northwest of Addis Ababa, the capital city of Ethiopia. Most of the population are Christian Orthodox followers and Amharic speakers. In this region, there are eight referral hospitals. Those hospitals are providing comprehensive care and support services for more than 25 million people on follow-up and daily activities. Within these hospitals, 12,500 (unpublished data) hypertensive patients had follow-up and comprehensive services.
Study Design and Population
An institutional-based cross-sectional study was conducted among hypertensive patients in the referral hospitals of Amhara region, Ethiopia. All hypertensive patients who had a follow-up in referral hospitals of the Amhara region were considered the source population, and hypertensive patients who had a follow-up during the study period in randomly selected referral hospitals were considered the study population.
Inclusion and Exclusion Criteria
Study participants whose ages were 18 and above, and diagnosed with hypertension were included in this study, and study participants who were severely ill had mental disorders, and had hearing impairment were excluded from this study.
Sample Size and Sampling Procedures
Sample Size Determination
The sample size was determined using a single population proportion formula as follows:
Assumption: n = sample size, P = proportion of visual impairment = 50% since the study on visual impairment was not conducted in the study area, d = Margin of sampling error tolerated – 5% (0.05), α = Critical value at 95% confidence interval of certainty (1.96). After adding a non-response rate of 10%, the total sample size became 423.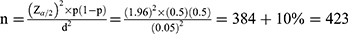 .33
.33
Sampling Procedure
Both simple random sampling and systematic random sampling methods were used in this study to select study participants. Three referral hospitals were randomly selected by lottery methods (Debre Markos referral hospital, Debre Tabor referral hospital, and Felege Hiwot referral hospitals). From study populations in the selected referral hospitals, study participants were selected using the systematic random sampling method. Every 12th participant was selected during data collection (K = N/n=12, calculated as the total hypertensive patients who had followed up during the data collection period in selected referral hospitals (5000) divided by sample size 423). A random number 3 between 1 and 12 was selected by the lottery method as a starting number, and to make the study population a good representative of the source population, a proportional number of hypertensive patients on follow-up were selected from each referral hospital. From who had followed up, those were selected as follows (Figure 1).
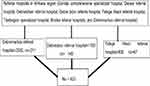 |
Figure 1 Sample size selection of study participants among hypertensive patients at three referral hospitals of Amhara region, Ethiopia 2021. |
Study Variables
The dependent variable was visual impairment (yes or no). Participants were considered to have a visual impairment if their visual acuity level in the better eye was less than 6/12 after they were examined to read letters in “Snellen’s” illiterate “E” chart.34 The independent variables for this study were age, sex, residence, income, occupation, educational level, marital status, duration of hypertension, diabetes Mellitus (DM), body mass index (BMI), history of ocular trauma, family history of visual problem, chat chewing, cigarette smoking, alcohol intake, duration of sleep, frequency of television (TV) exposure per day, regular mobile exposure, and distance of exposure.
Operational Definitions
Visual impairment: Having presenting visual acuity less than 6/12 in the better eye.1
Mild visual impairment: Having visual acuity worse than 6/12 but better than or equal to 6/18 in the better eye.1
Moderate visual impairment: Presenting distance visual acuity (VA) worse than 6/18 but better than or equal to 6/60 in the better eye.1
Severe visual impairment: Presenting distance VA worse than 6/60 but better than or equal to 3/60 in the better eye.1
Blindness: Presenting VA worse than 3/60.1
BMI: Underweight is < 18.5 kg/m2; normal weight is 18.5–24.99 kg/m2; overweight is 25–29.99 kg/m2, obese is ≥30 kg/m2.35
Current smoker: An adult who has smoked 100 cigarettes in his or her lifetime and who currently smokes cigarettes.36
Ever alcohol user: Use of alcohol, at least once in an individual’s lifetime.33
Current alcohol user: A person who consumed alcohol at least once within the last 30 days.33
Duration of hypertension: History of hypertension is classified as ≥5 years and <5 years.37
Duration of sleep: A short length of sleep of ≤5 h and long sleep of ≥9 hours.38
Exposure to mobile and television: Reading/ watching at least once a day for not less than 2 h.
Data Collection Procedure and Tools
Data were collected from March 20 to May 20 by using an interviewer-administered structured questionnaire, which was adapted from different works of the literature. The tool consists of the socio-demographic characteristics of the study participants, substance usage, medical reviews, physical measurements of blood pressure, and visual examination. Blood sugar level was extracted from the patient chart. Snellen’s illiterate “E” chart for the visual acuity test, adult-sized sphygmomanometer for blood pressure, tap meter for height, and beam balance for the measurement of weight were used. One BSc ophthalmic nurse for data collection, one optometrist for a proper eye examination, and one MSc holder in optometry as a supervisor were recruited in each selected hospital.
Examinations and Measurements
Eye Examinations
Visual acuity test was done in each eye separately, “Snellen’s” illiterate “E” chart was used by hanging on a wall at a distance of 6 meters in a well-illuminated room at a height of 2 meters.39 During the test, participants were setting or standing 6m away from the chart and cover one eye, and they read and determine the direction (for the illiterate) of the letters they see with their uncovered eyes. They repeated this process with another eye. The examiner will ask them to read smaller and smaller letters until they can no longer accurately distinguish letters.
The examination was taken place by three senior optometrists one in each selected referral hospital. Those visually impaired participants who had undetermined eye problems were consulted by the Ophthalmologist for detailed eye examination and the required data were collected after the Snellen’s chart examination was completed. All study participants who had VI were linked to the ophthalmology unit for the appropriate management and follow-up.
Anthropometric Measurements
The weight of the study participants was measured using a standard balance (MDSDS2100, Platform 10.5″ W x 22″ L x 4″ H) and the height was measured by using a height-measuring device attached to the balance. Then, BMI was calculated by dividing weight (kg) by height (m2).
Blood Pressure Measurement
Blood pressure was measured using a sphygmomanometer (XMEQSPHYRIAC14: length 54.5cm, width 14cm). A single measurement was recorded.
Data Analysis Procedure
The collected data were checked for completeness and entered into Epi data version 4.6. Then, it was exported into SPSS version 26 for analysis. Descriptive measures such as median, interquartile range, and frequencies were calculated. The crude and adjusted odds ratios were used to measure the association between study variables, and 95% confidence intervals were calculated. Model fitness was checked by Hosmer and Lemeshow test at p-value >0.05. Both bivariable and multivariable binary logistic regression models were done to identify factors associated with visual impairment. Those variables having a p-value of <0.25 in the bivariable binary logistic regression analysis were selected for multivariable binary logistic regression. Those variables with a p-value of ≤0.05 in multivariable binary logistic regression were declared as having a statistically significant association with visual impairment.
Data Quality Management
To assure the data quality, high prominence was given to designing the data collection instrument. The questionnaire was pre-tested in a setup having similar socio-cultural characteristics with the 22 study participants at Bichena primary hospitals before the actual study begins. It helps to check its wording and sort out language barriers and contextual variations on the structured questionnaire. Training for both data collectors and supervisors regarding the purpose of the study, interview, measurement techniques, and ethical issues was given for 1 day. Throughout the data collection, data collectors were supervised.
Results
Socio-Demographic Characteristics of Study Participants
A total of 423 hypertensive patients participated in the study with a 100% response rate. The age range of the participants was from 23 to 90 years with a median age of 60 ± 18 (IQR= 50–68) years. Among the study participants, 233 (55.1%) were females. More than two-thirds (67.8%) of the respondents were married, and 234 (55.08%) of the respondents were urban dwellers. Among the respondents, 269 (63.6%) were illiterate (Table 1).
 |
Table 1 Sociodemographic Characteristics of Hypertensive Patients at Amhara Region Referral Hospitals, Ethiopia, 2021 (n=423) |
Clinical, Substance Use, and Behavioral Characteristics of the Study Participants
Among the study participants, 222 (52.2%) had a history of follow-up hypertension for less than five years. Most of the study participants (86.1%) had no history of ocular trauma, and 412 (97.40%) of the respondents had no family history of eye problems. Of the study participants, 81 (19.15%) had diabetes mellitus as a comorbidity with hypertension. Two hundred and sixty-four (53.66%) of the respondents had a sleep duration of 5–8 hours. Among the respondents, one hundred and eighty-six (44%) of the respondents had exposure to TV. More than three-quarters (79.7%) of the respondents had a history of drinking alcohol in their lifetime, and only 65 (15.4%) had a history of drinking alcohol, mainly tela/tej, in the last 30 days. More than two-thirds (69.74%) of the respondents had a body mass index in the category of 18–24.99 kg/m2 (Table 2).
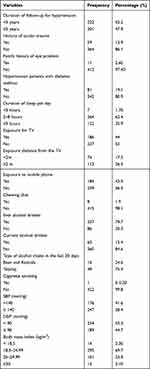 |
Table 2 Clinical and Behavioral Characteristics of Hypertensive Patients at Amhara Region Referral Hospitals, Ethiopia, 2021 (n=423) |
Prevalence of Visual Impairment
The overall prevalence of visual impairment among hypertensive patients was 32.4% (95% CI: 27.9–36.9) (Figure 2). Among the visually impaired respondents, 53 (38.7%) had mild, and 57 (41.6%) had moderate visual impairment (Figure 3).
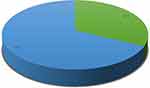 |
Figure 2 Prevalence of visual impairment among hypertensive patients at Debre Markos, Felege Hiwot, and Debre Tabor referral hospitals, Amhara regional state, Ethiopia, 2021. |
 |
Figure 3 Category of visual impairment among hypertensive patients at Debre Markos, Felege Hiwot, and Debre Tabor referral hospitals, Amhara regional state, Ethiopia, 2021. |
Factors Associated with Visual Impairment Among Hypertensive Patients
In the bivariable analysis, age, residence, educational status, occupation, income level, previous history of ocular trauma, duration of hypertension, exposure to TV, mobile exposure, ever alcohol drinking, and current alcohol drinking were associated with visual impairment. In multivariable analysis, a history of ocular trauma, duration of hypertension of five or more years, and ever alcohol drinking had a statistically significant association with visual impairment.
Those respondents who had a duration of hypertension of five or more years were 3.06 times (AOR =3.06, 95% CI: 1.86–4.95) more likely to have visual impairment as compared to respondents who had a duration of hypertension of fewer than five years. The odds of visual impairment among the respondents who had a history of ocular trauma were 2.5 times (AOR =2.5, 95% CI: 1.3–4.73). The odds of visual impairments in the respondents who had a history of drinking alcohol was 2.72 (AOR = 2.72, 95% CI: 1.32–5.62) times higher than those who had no history of drinking alcohol (Table 3).
Discussion
This study was designed to determine the prevalence of visual impairment and its associated factors among hypertensive patients in referral hospitals of the Amhara region, Ethiopia, 2021.
The prevalence of VI among hypertensive patients was 32.4%, which is higher than studies done in India at 26.4%,22 China at 8.2%,20 Eastern Taiwan at 14.7%,21 and Saudi Arabia at 13.4%.40 The possible reason for this discrepancy might be due to the differences in the definition of VI and the cut point of VA. In our study, the presenting visual acuity of the better eye was used to define VI, which can lead to a relatively higher prevalence than the above studies where they used best-corrected visual acuity to define VI. The other possible reason might be due to the visual acuity cut-point used by this study, which is less than 6/12 unlike the other studies using visual acuity cut-point of less than 6/18. These might overestimate the prevalence of visual impairment in this study. Quality of health care, health-seeking behavior, educational status, and socioeconomic differences were other variations, which might overestimate the prevalence of visual impairment in this study. Another possible reason for the discrepancy in the prevalence of VI in this study and the study done in China might be due to the age difference of the study participants. The study done in China includes all age groups, while participants included in this study were aged greater than 18, and most of the respondents were aged greater than 40 years. The other possible justification for the higher prevalence of VI in this study than in the study done in Saudi Arabia might be due to the sample size used (195), which is smaller than the sample size used by this study, and the study design used was a retrospective study design but a cross-sectional study design was used in this study.
On the contrary, the prevalence of visual impairment in this study was lower than in studies done in Malaysia 50.4%,24 Afghanistan 39.3%,29 Nigeria (56%),2 and the central republic of Congo (70.8%).26 The discrepancy might be due to the difference in age of participants, which is greater than 65 years used by studies done in Malaysia, Afghanistan, Nigeria, and the central republic of Congo while participants’ age groups greater than 18 years old were included in this study. As advancing age, the normal function of eye tissues decreases and there is an increased incidence of ocular pathology,41 which might be the reason for underestimating the prevalence of visual impairment in this study. An additional possible reason might be the population-based survey that was done in studies conducted in Afghanistan, the Central Republic of Congo, and Malaysia, but this study was a hospital-based cross-sectional study, which might have decreased the magnitude of VI. The other possible reason for the discrepancy between this study and the study done in Nigeria might be due to most of the participants in the study done in Nigeria had an ocular disease in association with hypertension like cataracts, glaucoma, diabetic retinopathy, corneal opacity, and macular degeneration. However, in this study, most of the participants had not known about visual problems diagnosed before the data collection, which might underestimate the prevalence of visual impairment among hypertensive patients.
The study conducted in Sri Lanka, 28.1%,23 and South Africa 35.1%25 was in line with this study, which might be due to the use of similar study design, and diagnostic methods, ie Better eye presenting visual acuity.
When we come to factors associated with visual impairment, a duration of hypertension ≥5 years was associated with higher odds of visual impairment which is supported by studies done in Bangladesh37 Pakistan,42 and India,15 This could be because chronic high blood pressure can harm the retina. When the blood pressure is too high, the retinal walls may thicken, causing the retinal blood vessels to restrict blood flow to the retina and limit its function, resulting in potentially permanent vision problems, including blindness.9,12,13,15,37,43
Similarly, the history of ocular trauma was associated with visual impairment which is supported by studies done in China,44 and Malawi,45 this could be because any injury to the eye, eyelid, or surrounding bone around the eye, resulting in a permanent vision loss, including blindness.46,47
The history of ever alcohol drinking was positively associated with visual impairment, which is supported by studies done in China,5,48 the United States of America,49 and Turkey.50 This might be because of the effect of alcohol on the gamma-aminobutyric acid (GABA) receptors. Alcohol does not increase GABA but mimics the effects of GABA by binding to GABA receptors in the brain (GABA agonist).51,52 GABA’s effect is to reduce neural activity by allowing chloride ions to enter the post-synaptic neuron. These ions have a negative electrical charge, which helps to make the neuron less excitable. This physiological effect is amplified when alcohol binds to the GABA receptor, probably because it enables the ion channel to stay open longer and thus lets more Cl− ions into the cell resulting in hyperpolarizing the cell and inhibiting the transmission of an action potential.39,53–55 Resulting in visual information being transmitted slowly to the visual information processing area and so visual impairment is going to happen. Moreover, by binding on GABA receptors, alcohol inhibits the superior colliculus and is involved in the control of ocular movement. This results in visual impairment due to the difficulty of rapid eye movement and failure to fixation.53
This study assessed visual acuity only, which did not include visual field testing and fundus photography examination. The majority of questions in the data-collection instrument asked about the participant’s history and would have been exposed to recall bias. This study was a cross-sectional study that might not show a cause-and-effect relationship. Since it was, a hospital-based study, a conclusion on the prevalence of visual impairment in the community cannot be made.
Conclusions
From this study, we can conclude that nearly one-third of hypertensive patients in this study were visually impaired. Duration of hypertension, history of ocular trauma, and ever alcohol drinking was positively associated with visual impairment. Even though the data were collected from all study participants with a 100% response rate, and from multi-sites, but it is better to do this study by prospective cohort study design to identify cause-and-effect relationships and use additional eye examinations like visual field assessment and fundus photography for better diagnosis.
Abbreviations
AOR, adjusted odds ratio; BMI, body mass index; BP, blood pressure; COR, crude odds ratio; DBP, diastolic blood pressure; DM, diabetes mellitus; kg, kilogram; kg/m2, kilogram per meter square; SBP, systolic blood pressure; TV, television; VA, visual acuity; VI, visual impairment.
Data Sharing Statement
All materials that were used to do this research are included in the manuscript.
Ethical Approval and Consent to Participate
All the methods were carried out in complied with the Declaration of Helsinki. Ethical clearance was obtained from the Institutional Review Committee (IRC) of the School of Medicine, University of Gondar (ref. no. 460/04/2021). A letter of cooperation was obtained from the Amhara Institution of public health (APHI) (ref. no. APHI 3/1082) and selected referral hospitals before the actual data collection was started. Written informed consent was obtained from the study participants. Privacy and confidentiality of information were kept properly, and names were not recorded. The data were collected by considering the COVID-19 protection protocol.
Acknowledgments
We would like to acknowledge the study participants, data collectors, and supervisors for their willingness, valuable support, and assistance during this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. Blindness and vision impairment; 2020. Available from: https://wwwwhoint/news-room/fact-sheets/detail/blindness-and-visual-impairment.
2. Azuamah Y, Amadi A, Esenwah E, Coa A, Ec A. Visual acuity and impairment among hypertensive adults in okagwe ohafia, abia state, Nigeria. Int J Adv Res. 2011;1:54–66.
3. Congdon N, O’Colmain B, Klaver CC, et al. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485.
4. He Y, Nie A, Pei J, et al. Prevalence and causes of visual impairment in population more than 50 years old: the Shaanxi Eye Study. Medicine. 2020;99(20):e20109. doi:10.1097/MD.0000000000020109
5. Hu JY, Yan L, Chen YD, et al. Population-based survey of prevalence, causes, and risk factors for blindness and visual impairment in an aging Chinese metropolitan population. Int J Ophthalmol. 2017;10(1):140–147.
6. Kahloun R, Jelliti B, Zaouali S, et al. Prevalence and causes of visual impairment in diabetic patients in Tunisia, North Africa. Eye. 2014;28(8):986–991. doi:10.1038/eye.2014.131
7. Maake MM, Oduntan OA. Prevalence and causes of visual impairment in patients seen at Nkhensani Hospital Eye Clinic, South Africa. World Rep Vision. 2016;18(1):232.
8. Maake MM, Oduntan OA. Prevalence and causes of visual impairment in patients seen at Nkhensani Hospital Eye Clinic, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1):728. doi:10.4102/phcfm.v7i1.728
9. Triwijoyo BK, Pradipto YD. Detection of hypertension retinopathy using deep learning and Boltzmann machines. J Phys Conf Ser. 2017;801:012039.
10. Bhargava M, Ikram MK, Wong TY. How does hypertension affect your eyes? J Hum Hypertens. 2012;26(2):71–83.
11. Schneider Rosen K. Ocular effects of hypertension. Darryl Meister Educ Forum. 2024;26(2):71–83.
12. Judy E, Kim M. Hypertensive retinopathy. Eyewiki. 2020;14(6):581–590.
13. Katsi V, Marketou M, Vlachopoulos C, et al. Impact of arterial hypertension on the eye. Curr Hypertens Rep. 2012;14(6):581–590. doi:10.1007/s11906-012-0283-6
14. Sun C, Ladores C, Hong J, et al. Systemic hypertension associated retinal microvascular changes can be detected with optical coherence tomography angiography. Sci Rep. 2020;10(1):9580.
15. Team AsMK. Hypertensive Retinopathy; 2020. Available from: https://adacom/conditions/hypertensive-retinopathy/.
16. Tien Wong PM. The eye in hypertension. Clin Exp Optom. 2007;369:425–435.
17. WHO. Eye health | WHO | Regional Office for Africa; 2020. Available from: https://wwwafrowhoint/health-topics/eye-health.
18. Gashaw M, Janakiraman B, Minyihun A, Jember G, Sany K. Self-reported fall and associated factors among adult people with visual impairment in Gondar, Ethiopia: a cross-sectional study. BMC Public Health. 2020;20(1):498. doi:10.1186/s12889-020-08628-2
19. Cherinet FM, Tekalign SY, Anbesse DH, Bizuneh ZY. Prevalence and associated factors of low vision and blindness among patients attending St. Paul’s Hospital Millennium Medical College, Addis Ababa, Ethiopia. BMC Ophthalmol. 2018;18(1):232. doi:10.1186/s12886-018-0899-7
20. Yan X, Chen L, Yan H. Socio-economic status, visual impairment and the mediating role of lifestyles in developed rural areas of China. PLoS One. 2019;14(4):e0215329.
21. Wang WL, Chen N, Sheu MM, Wang JH, Hsu WL, Hu YJ. The prevalence and risk factors of visual impairment among the elderly in Eastern Taiwan. Kaohsiung J Med Sci. 2016;32(9):475–481. doi:10.1016/j.kjms.2016.07.009
22. Marmamula S, Barrenakala NR, Challa R, et al. Prevalence and risk factors for visual impairment among elderly residents in ‘homes for the aged’ in India: the Hyderabad Ocular Morbidity in Elderly Study (HOMES). Br J Ophthalmol. 2021;105(1):32–36. doi:10.1136/bjophthalmol-2019-315678
23. Abeysena C, Champa H. Prevalence of visual impairment among adults aged forty years and above in a medical officer of health area in Sri Lanka: cross-Sectional Study. Int Arch Public Health Community Med. 2018;2:1.
24. Falahaty K. Disability among elderly people with visual impairment in two welfare homes in Malaysia. Biomed Pharmacol J. 2015;8(2):1369–1382. doi:10.13005/bpj/897
25. Mabaso RG, Oduntan OA. Risk factors for visual impairment and blindness amongst black adult diabetics receiving treatment at Government healthcare facilities in Mopani District, Limpopo province, South Africa. Afr J Prim Health Care Fam Med. 2014;6(1):E1–E8. doi:10.4102/phcfm.v6i1.623
26. Gbessemehlan A, Helmer C, Delcourt C, et al. Cardiovascular health and near visual impairment among older adults in the Republic of Congo: a Population-Based Study. J Gerontol Ser A. 2020;76(5):842–850. doi:10.1093/gerona/glaa304
27. Welp A, Woodbury RB, McCoy MA, Teutsch SM. Approaches to Reduce Vision, Impairment Making Eye Health a Population Health Imperative: Vision for Tomorrow. Washington (DC): National Academies Press (US) Copyright 2016 by the National Academy of Sciences. All rights reserved; 2016.
28. Merrie YA, Tegegne MM, Munaw MB, Alemu HW. Prevalence and associated factors of visual impairment among school-age children in Bahir Dar City, Northwest Ethiopia. Clin Optom. 2019;11:135–143.
29. Abdianwall MH, Güçiz Doğan B. Prevalence of visual impairment and related factors in Nangarhar Province of Afghanistan: a cross sectional study. Int J Ophthalmol. 2018;11(12):1968–1977.
30. Addo EK, Akuffo KO, Sewpaul R, et al. Prevalence and associated factors of vision loss in the South African National Health and Nutrition Examination Survey (SANHANES-1). BMC Ophthalmol. 2021;21(1):1. doi:10.1186/s12886-020-01714-4
31. An Y, Joo C-K. The U-shaped association between self-reported sleep duration and visual impairment in Korean adults: a population-based study. Sleep Med. 2016;26:30–36. doi:10.1016/j.sleep.2016.08.005
32. Nuertey BD, Amissah-Arthur KN, Addai J, et al. Prevalence, causes, and factors associated with visual impairment and blindness among registered pensioners in Ghana. J Ophthalmol. 2019;2019:1717464. doi:10.1155/2019/1717464
33. Diress M, Yeshaw Y, Bantihun M, et al. Refractive error and its associated factors among pregnant women attending antenatal care unit at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. PLoS One. 2021;16(2):e0246174. doi:10.1371/journal.pone.0246174
34. Ernest-Nwoke IO, Ozor MO, Akpamu U, Oyakhire MO. Relationship between body mass index, blood pressure, and visual acuity in residents of Esan West local government area of Edo State, Nigeria. Physiol J. 2014;2014:510460. doi:10.1155/2014/510460
35. CDC. Healthy weight, nutrition, and physical activity, about adult BMI; 2007. Available from: https://wwwcdcgov/healthyweight/assessing/bmi/adult_bmi/indexhtml.
36. CDC. NCHS, National health interview survey, special topics, adult tobacco use information; 2019. Available from: https://wwwcdcgov/nchs/nhis/tobacco/tobacco_glossaryhtm.
37. Mondal R, Matin M, Rani M, et al. Prevalence and risk factors of hypertensive retinopathy in hypertensive patients. J Hypertens. 2017;06:2167.
38. Peltzer K, Phaswana-Mafuya N. Association between visual impairment and low vision and sleep duration and quality among older adults in South Africa. Int J Environ Res Public Health. 2017;14(7):811. doi:10.3390/ijerph14070811
39. Karimi S, Arabi A, Shahraki T. Alcohol and the eye. J Ophthalmic Vis Res. 2021;16(2):260–270. doi:10.18502/jovr.v16i2.9089
40. Zeried FM, Alshalan FA, Simmons D, Osuagwu UL. Visual impairment among adults in Saudi Arabia. Clin Exp Optom. 2020;103(6):858–864.
41. Loh KY, Ogle J. Age related visual impairment in the elderly. Med J Malaysia. 2004;59(4):562–8, quiz 9.
42. Luqman Ali Bahoo M, Khalid MS, Haq M. Relationship between duration of hypertension and vision problems in patients hypertensive patients: a cross-sectional survey. Med Forum Mon. 2019;30:103–107.
43. Erden S, Bicakci E. Hypertensive retinopathy: incidence, risk factors, and comorbidities. Clin Exp Hypertens. 2012;34(6):397–401. doi:10.3109/10641963.2012.663028
44. Xu C, Teng C, Chow J, et al. Risk factors for visual impairment associated with corneal diseases in southern China. Clin Ophthalmol. 2016;10:777. doi:10.2147/OPTH.S103302
45. Zungu T, Mdala S, Manda C, Twabi HS, Kayange P, Ashkenazi I. Characteristics and visual outcome of ocular trauma patients at Queen Elizabeth Central Hospital in Malawi. PLoS One. 2021;16(3):e0246155. doi:10.1371/journal.pone.0246155
46. Pradhan E. Ocular trauma: prevention. Nepal J Ophthalmol. 2017;8(2):107. doi:10.3126/nepjoph.v8i2.16990
47. Aghadoost D. A brief overview of ocular trauma. Arch Trauma Res. 2014;3(2):e21639. doi:10.5812/atr.21639
48. Li Z, Xu K, Wu S, et al. Alcohol consumption and visual impairment in a rural Northern Chinese population. Ophthalmic Epidemiol. 2014;21(6):384–390. doi:10.3109/09286586.2014.967360
49. Fan AZ, Li Y, Zhang X, et al. Alcohol consumption, drinking pattern, and self-reported visual impairment. Ophthalmic Epidemiol. 2012;19(1):8–15. doi:10.3109/09286586.2011.591037
50. Ozgonul C, Sertoglu E, Mumcuoglu T. Determination of visual impairment associated with alcohol consumption. Ophthalmic Epidemiol. 2015;22(2):142–143. doi:10.3109/09286586.2015.1010690
51. Mize RR. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248.
52. Gong J-L, Lou X-T, Yuan Y-X, et al. The increased expression of GABA receptors within the arcuate nucleus is associated with high intraocular pressure. Mol Vis. 2018;24:574–586.
53. Silva JBS, Cristino ED, Almeida N, Medeiros P, Santos NAD. Effects of acute alcohol ingestion on eye movements and cognition: a double-blind, placebo-controlled study. PLoS One. 2017;12(10):e0186061. doi:10.1371/journal.pone.0186061
54. Roche DJ, King AC. Alcohol impairment of saccadic and smooth pursuit eye movements: impact of risk factors for alcohol dependence. Psychopharmacology. 2010;212(1):33–44. doi:10.1007/s00213-010-1906-8
55. Hartung B, Schwender H, Ritz-Timme S, Küppers L, Roth EH, Daldrup T. Ophthalmologic examinations under the acute influence of alcohol. Legal Med. 2020;46:101722.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

