Back to Journals » Clinical Ophthalmology » Volume 18
Visual and Refractive Outcomes of a New Hydrophobic Trifocal Toric Intraocular Lens
Authors Daya S , Espinosa Lagana M
Received 12 January 2024
Accepted for publication 28 March 2024
Published 3 April 2024 Volume 2024:18 Pages 997—1007
DOI https://doi.org/10.2147/OPTH.S453565
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sheraz Daya, Marcela Espinosa Lagana
Centre for Sight, East Grinstead, West Sussex, UK
Correspondence: Sheraz Daya, Centre for Sight, East Grinstead, West Sussex, RH19 4RH, UK, Email [email protected]
Purpose: To evaluate the visual outcomes and efficacy of astigmatism correction using a new hydrophobic trifocal toric intraocular lens (IOL).
Methods: This study involved 62 eyes implanted with the FineVision HP Toric IOL. The visual and refractive outcomes were assessed preoperatively and 6 weeks after the surgery. Specifically, monocular uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), uncorrected intermediate visual acuity (UIVA) at 80 and 60 cm and uncorrected near visual acuity at (UNVA) at 40 cm were evaluated. The rotational stability of the lens was also assessed.
Results: Sixty-one eyes (98.39%) were within ± 1.00D and 55 eyes (88.71%) were within ± 0.50 D of spherical equivalent, with a mean value of 0.09± 0.39 D. 51 (82.26%) and 61 (98.39%) eyes had a UDVA of ≥ 20/20 and ≥ 20/25, respectively, and for CDVA these values were as follows: 59 (95.16%) and 62 eyes (100%), respectively. The mean UDVA and CDVA were 0.01± 0.06 and − 0.01± 0.04logMAR, respectively. Greater than or equal to unaided 20/20 vision was achieved at 40 cm in 42 (67.74%), UIVA at 60 cm in 42 (67.74%) and 50 eyes (80.65%) at 80 cm. Those achieving ≥ 20/25 were 56 (90.32%, 40 cm), 59 (95.16%, 60 cm), and 62 eyes (100%, 80 cm). Postoperative mean values were 0.04± 0.07, 0.03± 0.07, and 0.00± 0.07logMAR for UCNVA, UIVA at 60 cm, and UIVA at 80 cm, respectively. The mean rotation of the IOL was 5.8 degrees.
Conclusion: This hydrophobic trifocal toric IOL provides good refractive outcomes with excellent visual acuity across multiple distances, providing a full range of focus.
Keywords: astigmatism, trifocal, toric, multifocal, cataract
Introduction
Optical design and materials are two parameters that have accounted for considerable advances in the development of intraocular lenses (IOLs). Complex IOL optical surface designs involving diffractive technology provide patients with good vision at not only at distance but also intermediate and near. Patients implanted with trifocal IOLs achieve better intermediate visual acuity than patients with bifocal IOLs without any adverse effects on far distance or near visual acuity.1,2 A recent systematic review and Bayesian network meta-analysis concluded that bilateral implantation of trifocal IOLs might be an optimal option without compromising far distance visual acuity.3 For astigmatic eyes, it has been suggested that useful visual acuity may be achieved with a trifocal IOL when the astigmatism is ≤0.75 D, inferring astigmatic correction is necessary when the astigmatism is greater than 0.75 D.4 Options to correct astigmatism include limbal relaxing incisions or astigmatic keratotomies; however, the amount of correction is limited and can vary based on age, incision length and distance from the visual axis.5 The inclusion of astigmatic optical correction with trifocal IOLs is thus necessary for higher magnitudes of astigmatism. In relation to the IOL material, use of hydrophobic acrylic IOLs has previously been associated with a high risk of glistening formation, although they usually produce little or no posterior capsule opacification (PCO)6,7 and little or no risk of calcification and in turn opacification.8 In contrast, hydrophilic acrylic materials result in less or no glistenings;9 however, PCO is more common.10 Glistening-free hydrophobic IOL materials have more recently been developed and are now commercially available. One of these, GF, was analyzed to determine its suitability for use in IOLs9 and was found to be glistening-free along with properties that may also reduce PCO.
A hydrophilic toric trifocal IOL currently in use, is the FineVision POD FT (Beaver-Visitec International, Inc. [BVI], Waltham, USA), which has been reported to have good refractive and visual acuity outcomes in eyes with varying amounts of corneal astigmatism.11–23 Recent studies have analyzed the outcomes achieved with the FineVision POD F GF IOL model, which is made of GF hydrophobic material,21–31 and comparative studies between hydrophilic and hydrophobic version of the lens have concluded that there are no significant differences between them in terms of visual and refractive outcomes.25,27,29 Although earlier studies of the non-toric version of the FineVision POD F GF IOL have been published, to our knowledge, there are no studies reporting the clinical outcomes achieved with the toric lens.
To provide clinical evidence of the benefit of using the hydrophobic glistening-free material in a toric design, this study assessed the refractive and visual outcomes at different distances of eyes implanted with the trifocal toric FineVision HP IOL after cataract and refractive lens replacement surgery.
Methods
Study Design and Patients
This was a single-center retrospective study conducted at the Centre for Sight, West Sussex (United Kingdom). The retrospective study was carried out in accordance with the tenets of the Declaration of Helsinki and approved as a retrospective audit for the institution (Number 2022-12-1). Eyes of patients who underwent either cataract surgery or refractive lens replacement implanted with the FineVision GF Toric trifocal lenses were included. All patients had more than 0.75D of keratometric astigmatism, as noted by corneal topography and keratometric measurement. No patients had any conditions that would normally exclude them from implantation of trifocal lenses such as age-related macular degeneration, irregular astigmatism or poor ocular surface. Patients who had a previous refractive procedure (corneal laser surgery and conductive keratoplasty) were excluded from the review.
Intraocular Lens
The FineVision HP toric IOL lens is a diffractive biconvex apodized aspheric toric trifocal lens made of hydrophobic acrylic glistening-free material with a refractive index of 1.52 (Abbe number of 42). It has a 6.0 mm optic diameter with an overall diameter of 11.4 mm. The lens includes a filter for ultraviolet and blue light. The haptic design is a double C-loop with 5 degrees of haptic angulation (Figure 1A). In addition to the spherical component for distance, the lens diffractive properties create two further foci, one for intermediate vision (+1.75 D) and one for near vision (+3.50 D). The spherical power ranges from +10.0 D to +35.0 D in 0.50 steps available in the following cylinder powers (at IOL plane): 1.00 D, 1.50D, 2.25 D, 3.00 D, 3.75 D, 4.50 D, 5.25 D, and 6.00 D.
Surgical Procedure
All surgical procedures were conducted by one of two surgeons using the Victus femtosecond laser (Bausch & Lomb Inc., USA) and the Stellaris phacoemulsification system (Bausch & Lomb Inc., USA) through a 1.8 mm scleral incision. The incisions were placed at the 110 to 120 degree position in all cases. The orientation of the toric lens implant was based on topography-derived aberrometry, specifically isolating Zernike polynomial of astigmatism (Z2). The patient’s eye was marked with a Gentian violet pen at a slit lamp installed with a graduated protractor in 5-degree increments rotated to the desired positive axis. Following crystalline lens removal and cortical aspiration, the IOL was implanted into the capsular bag and rotated to the axis marked on the cornea (Figure 1B).
Preoperative and Postoperative Assessment
All patients included in this study had the following preoperative parameters documented: monocular uncorrected distance visual acuity (UDVA), corrected-distance visual acuity (CDVA), refraction, corneal topography and corneal, internal and overall wavefront aberrometry (OPD-Scan III, Nidek CO. LTD, Aichi, Japan), and optical biometry (IOLMaster 500, Carl Zeiss Meditec AG, Jena, Germany). The A constant used for optical biometry was 119.4, and the IOL power was calculated using the Holladay 2 formula. The IOL cylinder power and target IOL axis were calculated using the online FineVision Toric Calculator available at http://www.physioltoric.eu. The targeted refraction was towards emmetropia with hyperopes targeted to be on the plus side and myopes on the myopic side of emmetropia.
Postoperatively, the following measurements were taken at 6 weeks: monocular and binocular UDVA, CDVA, uncorrected intermediate visual acuity (UIVA) at 60 and 80 cm, and uncorrected near visual acuity (UNVA) at 40 cm. The refractive error, sphere and cylinder, was measured and, specifically, an astigmatism vector analysis was performed by applying the double-angle tool32 using the refractive cylinder and keratometry results from before and after the surgery. Any adverse events or complications were also recorded. Postoperatively, patients were not dilated postoperatively and had wide-field fundus photography (Clarus, Carl Zeiss Meditec AG, Jena, Germany). As they were not dilated, the rotational orientation of the IOL was determined using OPD3 internal aberrometry. The orientation of internal astigmatism is derived from the Z2 Zernike polynomial by subtracting the corneal aberrometry from the overall aberrometry. This method has been previously described.33 This is not as accurate as direct observation of the lens following pupil dilation and errors of rotational angle and magnitude can occur from lens tilt. Lens rotation was measured by comparing the intended axis of implantation, as noted on the operative form, to the orientation of the lens on internal aberrometry at the 6 weeks follow-up visit.
Statistical Analysis and Sample Size
The outcomes were analyzed using Excel (2019, version 16.43, Microsoft Corporation, Redmond, WA, USA). Mean values and ± the standard deviation (SD) with ranges were calculated. Assuming a sample size of 50 eyes, a 95% confidence interval and a standard deviation of 0.12 logMAR for distance visual acuity, the precision for the primary estimate would be about 0.0335 logMAR. This was considered adequate for the main purpose of this study. A review of 60+ consecutive eyes was deemed sufficient.
Results
This study included 62 consecutively treated eyes of 34 patients implanted with the FineVision HP Toric IOL of which 22 patients were female (67.64%) (Table 1). The mean patient age was 62.39±6.96 years (ranging from 51 to 80 years). Complete postoperative data were available for all treated eyes, and none were lost to follow-up. There were no surgical complications or adverse events, and no post-surgery refractive enhancements were required. One eye of one patient developed capsular phimosis with a mild hyperopic shift from posterior lens displacement. This was resolved by a YAG laser radial anterior capsulotomy, which resulted in a reduction in hyperopia.
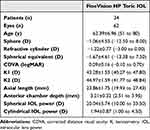 |
Table 1 Demographic Characteristics of Participants Shown as Means, Standard Deviations (SD) and Ranges |
Standard graphs for reporting refractive and visual acuity outcomes were constructed. Evaluation of spherical equivalent (Figure 2A) revealed 50% within ±0.13 D and 24.19% in the range +0.50 to +0.14 D. 61 eyes (98.39%) were within ±1.00 D, and 55 eyes (88.71%) were within ±0.50 D. The mean postoperative SE was 0.09±0.39 D (ranging from 1.25 to −0.88 D). Analysis of postoperative refractive cylinder (Figure 2B) indicated 53 eyes (85.48%) were ≤0.25 D. The mean postoperative refractive cylinder was −0.15±0.24 D, ranging from 0 to −1.00 D. Double angle plots of corneal and refractive cylinder pre- and post-operatively (Figure 3) revealed little change in corneal astigmatism with the preoperative centroid of 1.29 D @ 92 degrees and postoperatively 1.18 D @ 88. The centroid of the refractive astigmatism decreased from 0.51 D @ 98 degrees before the surgery to 0.07 D @ 51 degrees after the surgery.
 |
Figure 2 (A) Distribution of postoperative spherical equivalent (D), (B) Distribution of postoperative refractive cylinder (D). |
 |
Figure 3 Double-angle plots for preoperative and postoperative corneal and refractive astigmatism. Centroids and mean absolute values with standard deviations are also shown. |
Monocular change of CDVA preoperatively to UDVA postoperatively revealed no change in the majority (55 eyes, 88.7%) and an improvement in the remainder (Figure 4). Postoperatively, UDVA of 20/20 or better was achieved in 51 eyes (82.26%) and 20/25 or better in 61 eyes (98.39%, Figure 5A). For CDVA, these percentages improved to 95.16% (59 eyes) and 100% (62 eyes), respectively. The postoperative mean values of monocular UDVA and CDVA were 0.01±0.06 logMAR (range from −0.10 to 0.20) and −0.01±0.04 logMAR (range from −0.10 to 0.10), respectively. Cumulative monocular postoperative UCNVA of 20/20 or better was found in 42 eyes (67.74%) and UIVA at 60 cm in 42 eyes (67.74%) and UIVA at 80 cm in 50 eyes (80.65%) (Figure 5B). These percentages increased to 90.32% (56 eyes), 95.16% (59 eyes), and 100% (62 eyes), respectively, for values of 20/25 or better. The postoperative mean values for UCNVA were 0.04±0.07 logMAR (range from 0.00 to 0.20), UIVA at 60cm 0.03±0.07 logMAR (range from −0.10 to 0.20), and UIVA at 80cm o 0.00±0.07 logMAR (range from −0.10 to 0.10).
 |
Figure 4 Change in visual acuity lines between the postoperative uncorrected distance visual acuity (UDVA) and best-corrected distance visual acuity (CDVA). |
Comparing the intended angle of implantation to the axis noted on internal astigmatic aberrometry, the mean difference (implied rotation) was 5.84 degrees (SD 6.41). Thirty-eight eyes (61.3%) were within 5 degrees of the intended orientation, 18 (29%) between 6 and 10 degrees and 6 (9.7%) were more than 10 degrees away from the intended axis.
Discussion
There is considerable and growing interest in the use of hydrophobic acrylic material as opposed to hydrophilic acrylic in intraocular lens implants. The rationale is the reduced incidence of posterior capsule opacification, which can adversely affect lens performance, especially that of diffractive lenses where energy distribution for intermediate and near is low. Additionally, with hydrophilic lenses there is an established increased risk of lens calcification, especially if there is ever secondary intervention, in particular where an air bubble is involved. On the other hand, glistenings from small loculations of liquid have been reported in hydrophobic acrylic materials, which in turn can affect lens performance. Newer hydrophobic materials have come a long way, largely eliminating glistenings. Changes from hydrophilic to hydrophobic are accompanied by changes in refractive index as well as Abbe number. The hydrophobic version of this lens has a refractive index of 1.52 and an Abbe number of 42 compared with the hydrophilic of 1.46 and 58, respectively. This in turn potentially affects lens performance and translation of lens implant design along with its impact needs to be considered and warrants evaluation.
Several published studies have shown the refractive and visual outcomes of the toric hydrophilic FineVision POD FT IOL11–23 and the spherical model of the hydrophobic FineVision POD F GF IOL.24–31 To date, there is no published work detailing the results for the toric hydrophobic FineVision HP IOL. This retrospective review evaluates the visual and refractive outcomes in patients who have had cataract or refractive lens exchange with this hydrophobic toric trifocal lens implant.
Spherical outcomes were highly predictable, with the majority within ±0.13 D (48.15%), 88.89% within ±0.50 D and 100% within ±1.00 D (Figure 2A). Refractive cylinder was ≤0.25 D in 81.48% of eyes (Figure 2B). Mean postoperative SE and refractive cylinder values were 0.08±0.37 D and −0.18±0.25 D, respectively. The double-angle plot analysis (Figure 3) revealed a reduction in the centroid of the refractive astigmatism from 0.51 D @ 98 degrees preoperatively to 0.07 D @ 51 degrees postoperatively at 6 weeks. These outcomes reveal predictable astigmatic outcomes. Similar outcomes have been found with the hydrophilic version of the toric trifocal lens. In a study of 145 eyes, Nistad et al12 found a mean SE of −0.09±0.39 D 3 months post-surgery. Poyales and Garzón15 reported a mean SE of −0.08±0.21 D and a cylinder of −0.14±0.31 D in 29 eyes with a similar follow-up timeframe, and Ribeiro and Ferreira17 in a study of 60 eyes with 3 months follow-up showed a mean SE of −0.20±0.39 D and a cylinder of −0.27±0.39 D. The same authors also analyzed 60 eyes implanted with the AcrySof IQ PanOptix toric IOL and found a mean SE value of −0.07±0.35 D and a mean cylinder of −0.17±0.45 D for that lens with no statistically significant differences between the two groups of eyes in relation to SE, sphere, and cylinder (p=0.091, p=0.105 and p=0.821, respectively). In two more recent studies, Orts-Vila et al found a mean SE of 0.07±0.28 D and a cylinder of −0.23±0.32 D18 (99 eyes) and −0.02±0.23 D and −0.16±0.22 D19 (26 eyes in low cylinder IOL power). Our results with the hydrophobic lens were similar to those published using the hydrophilic model, despite a different refractive index and Abbe number for the two materials. The different curvature and thickness of the new model (related to the different optical properties of the hydrophobic material) did not affect refractive accuracy nor final outcome. An in vivo longitudinal chromatic aberration study reported that the chromatic difference of focus (from 480 to 700 nm) for far distance vision was significantly higher than for intermediate and near vision in the hydrophobic trifocal model,26 a similar finding was reported for the hydrophilic trifocal model.24 However, the authors found a consistently higher longitudinal chromatic aberration value in patients implanted with the hydrophobic model compared with the hydrophilic model.
To ascertain the influence of different materials on refraction values, Garzón et al29 compared refractive outcomes in 100 patients (50/50) implanted with one of the two IOL models (non-toric version) at 1-month post-surgery, using subjective and objective methods. They concluded that, based on the outcomes of their study on hydrophobic and hydrophilic trifocal IOLs, no current objective measuring technique is as reliable as the subjective method with which the patient gets their best visual acuity possible. They also reported better results with the hydrophobic material for all methods evaluated, with the difference between subjective refraction and objective refraction being very close to zero.
The high level of refractive accuracy was consistent with good visual acuity outcomes. All eyes showed similar or improved differences between UDVA and CDVA (Figure 4). In this study, the postoperative mean values of monocular distance UDVA and CDVA were 0.01±0.06 logMAR and −0.01±0.04 logMAR, respectively. Poyales and Garzón15 found a UDVA and CDVA of 0.05±0.08 and 0.02±0.03 logMAR, respectively, and Ribeiro and Ferreira17 obtained values of 0.04±0.12 logMAR and 0.03±0.11 logMAR, respectively. As indicated previously, these authors analyzed eyes implanted with the AcrySof IQ PanOptix toric IOL and found similar outcomes with no significant differences between the two groups of eyes: 0.06±0.11 logMAR (p=0.701) and 0.03±0.09 logMAR (p=0.643), respectively. The outcomes reported by Orts-Vila et al18 were also similar: 0.03±0.07 logMAR and 0.01±0.05 logMAR, respectively. This study revealed 82.26% were 20/20 or better and 98.39% 20/25 or better, respectively (Figure 5A). Corrected distance visual acuities improved to 95.16% (≥20/20) and 100% (≥20/25). Poyales and Garzón15 reported UDVA ≥20/25 in 93% and ≥20/32 in 97%; CDVA was≥20/20 in 81% and ≥20/32 in 98%. By comparison, Orts-Vila et al18 found that 81% and 96% of eyes had UDVA and CDVA of 20/20 (100% of eyes were 20/25). At near (40 cm) and intermediate (60 and 80 cm) distances, outcomes of this lens demonstrated good visual performance with high cumulative percentages of eyes obtaining 20/20 and 20/25 visual acuities (Figure 5B). At 60 and 80 cm, 67.74% and 80.65% of eyes were 20/20 or better, and 95.16% and 100% were 20/25 or better, respectively. Postoperative mean values for UIVA were 0.03±0.07 logMAR at 60cm and 0.00±0.07 at 80 cm. These outcomes are slightly better than those previously published by other authors. Ribeiro and Ferreira17 found a mean monocular UIVA of 0.11±0.09 logMAR at 60 cm and 0.09±0.10 logMAR at 80 cm with the hydrophilic toric lens. With the AcrySof IQ PanOptix toric IOL, mean acuities were 0.05±0.08 logMAR at 60cm and 0.07±0.09 logMAR at 80 cm. In terms of UCNVA, 67.4% were ≥20/20 and 90.32% 20/25 or better for this hydrophobic toric IOL. The mean UCNVA in this series was 0.04±0.07 logMAR. Ribeiro and Ferreira17 obtained a mean UNVA of 0.07±0.11 logMAR for the FineVision POD FT and 0.05±0.10 logMAR for the AcrySof IQ PanOptix toric IOL groups. Similar to previous findings, using the hydrophilic FineVision POD FT IOL, this hydrophobic trifocal toric IOL improves intermediate vision without adversely affecting far distance or near vision.
In terms of rotational stability, the mean value of rotation using the methodology of internal aberrometry in comparison to the intended axis was 5.84±6.41 degrees. This method is not as accurate as direct observation of lens positioning with a dilated pupil. This method is highly dependent on the quality of the test performed and prone to error from lens tilt. However, the rotational stability observed using this method is in line with those reported by other authors using direct observation of lens orientation postoperatively with the hydrophilic version of the lens and other trifocal toric IOLs. Specifically, for the hydrophilic Finevision model, 1-month post-surgery, Sheen-Ophir et al20 found a mean value of 3.52±3.38 degrees; Poyales et al reported a value of 1.18±1.18 degrees at 3 months postoperatively.14 Ribeiro et al reported 1.33±0.90 degrees,16 and in another series Ribeiro and Ferreira reported a mean value of 1.89±0.31 degrees.17 Ribeiro and Ferreira17 also reported a mean value of 1.59±2.15 degrees for the AcrySof IQ PanOptix toric IOL group. Studies with longer follow-up also support the good rotational stability of the hydrophilic lens. Vandekerckhove13 reported mean values of 2.56±2.22 and 2.55±2.62 degrees at 6- and 12-months post-surgery, respectively. An error of 5 degrees in the alignment of a toric IOL will reduce the cylindrical correction efficacy by about 15%.34 Higher levels of residual astigmatism would thus be expected; however, in this series, post-surgery refractive astigmatism reduction was excellent (double angle plot graph in Figure 3-bottom right) with the concentration of plots at 0.0 and a mean value of the centroid close to zero (0.07 D). Considering the refractive cylindrical outcomes, it seems plausible that in reality there was minimal if any cylindrical correction efficacy reduction in this series in spite of the slightly higher magnitude of rotation found. This increase in the magnitude of rotation may be from error inherent in the methodology used.
While retrospective studies are considered less robust than prospective, in this study, in keeping with the institution’s patient pathway protocols, all follow-up data were complete. Patients, as per pathway, are typically discharged at 6 weeks unless there is an issue that needs to be addressed, such as delayed adaptation or residual refractive error. While rotational change is unlikely to take place, refractive change can conceivably change with alteration of lens positioning and ongoing capsular fibrosis and would be reflected at a later date. Only one patient required intervention at 6 weeks for hyperopic shift secondary to capsular phimosis. Although visual acuities were measured at different distances (40, 60 and 80cm), defocus curve measurements would have provided more detailed information on the range of focus of this lens. Similarly, more information about lens performance would have been useful with measurements of contrast in varied light conditions. Quality of Vision questionnaires would also have been beneficial in understanding patient reported outcomes. Patients were treated along the lines of the organization’s agreed care pathway, which did not include defocus, contrast or quality of vision questionnaires as standard processes.
Conclusion
The study reflects real-world audits with no biased pre-selection or treatment demonstrating good refractive outcomes, rotational stability and in turn excellent visual results at 40, 60, 80 cm and distance with the Finevision HP hydrophobic Toric lens.
Data Sharing Statement
Data not available for sharing.
Disclosure
Sheraz Daya reports grant support from Allotex and Johnson & Johnson Vision; consultant/advisor of, lecture fees, and grant support from Bausch + Lomb; consultant/advisor of and lecture fees from BVI – Physiol; consultant/advisor of Cristalens, Carl Zeiss Meditec, Nidek, Inc., Oyster Point, Tarsus, and Vialase; consultant/advisor of, stock options, grant support from Excellens; owner of company of Infinite Medical Ventures (EO); consultant/advisor for and stock options from PRN. The authors report no other conflicts of interest in this work.
References
1. Shen Z, Lin Y, Zhu Y, Liu X, Yan J, Yao K. Clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2017;7:45337. doi:10.1038/srep45337
2. Zhong Y, Wang K, Yu X, Liu X, Yao K. Comparison of trifocal or hybrid multifocal-extended depth of focus intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2021;11(1):6699. doi:10.1038/s41598-021-86222-1
3. Cho JY, Won YK, Park J, et al. Visual outcomes and optical quality of accommodative, multifocal, extended depth-of-focus, and monofocal intraocular lenses in presbyopia-correcting cataract surgery: a systematic review and bayesian network meta-analysis. JAMA Ophthalmol. 2022;e223667. doi:10.1001/jamaophthalmol.2022.3667
4. Hayashi K, Yoshida M, Igarashi C, Hirata A. Effect of refractive astigmatism on all-distance visual acuity in eyes with a trifocal intraocular lens. Am J Ophthalmol. 2021;221:279–286. doi:10.1016/j.ajo.2020.07.051
5. Abu-Ain MS, Al-Latayfeh MM, Khan MI. Do limbal relaxing incisions during cataract surgery still have a role? BMC Ophthalmol. 2022;22(1):102. doi:10.1186/s12886-022-02327-9
6. Gregori NZ, Spencer TS, Mamalis N, Olson RJ. In vitro comparison of glistening formation among hydrophobic acrylic intraocular lenses. J Cataract Refract Surg. 2002;28(7):1262–1268. doi:10.1016/S0886-3350(02)01224-5
7. Li Y, Wang J, Chen Z, Tang X. Effect of hydrophobic acrylic versus hydrophilic acrylic intraocular lens on posterior capsule opacification: meta-analysis. PLoS One. 2013;8(11):1.
8. Zhao Y, Yang K, Li J, Huang Y, Zhu S. Comparison of hydrophobic and hydrophilic intraocular lens in preventing posterior capsule opacification after cataract surgery: an updated meta-analysis. Medicine. 2017;96(44):e8301. doi:10.1097/MD.0000000000008301
9. Pagnoulle C, Bozukova D, Gobin L, Bertrand V, Gillet-de Pauw MC. Assessment of new-generation glistening-free hydrophobic acrylic intraocular lens material. J Cataract Refract Surg. 2012;38(7):1271–1277. doi:10.1016/j.jcrs.2012.02.041
10. Chang A, Kugelberg M. Posterior capsule opacification 9 years after phacoemulsification with a hydrophobic and a hydrophilic intraocular lens. Eur J Ophthalmol. 2017;27(2):164–168. doi:10.5301/ejo.5000831
11. Gundersen KG, Potvin R. Comparison of visual outcomes after implantation of diffractive trifocal toric intraocular lens and an diffractive apodized bifocal toric intraocular lens. Clin Ophthalmol. 2016;10:455–461. doi:10.2147/OPTH.S103375
12. Nistad K, Göransson F, Støle E, Shams H, Gjerdrum B. The use of capsular tension rings to reduce refractive shift in patients with implantation of trifocal intraocular lenses. J Refract Surg. 2017;33(12):802–806. doi:10.3928/1081597X-20170829-02
13. Vandekerckhove K. Rotational stability of monofocal and trifocal intraocular toric lenses with identical design and material but different surface treatment. J Refract Surg. 2018;34(2):84–91. doi:10.3928/1081597X-20171211-01
14. Poyales F, Garzon N, Pizarro D, Cobreces S, Hernandez A. Stability and visual outcomes yielded by three intraocular trifocal lenses with same optical zone design but differing material or toricity. Eur J Ophthalmol. 2019;29(4):417–425. doi:10.1177/1120672118795065
15. Poyales F, Garzon N. Comparison of 3-month visual outcomes of a spherical and a toric trifocal intraocular lens. J Cataract Refract Surg. 2019;45(2):135–145. doi:10.1016/j.jcrs.2018.09.025
16. Ribeiro FJ, Ferreira TB, Relha C, Esteves C, Gaspar S. Predictability of different calculators in the minimization of postoperative astigmatism after implantation of a toric intraocular lens. Clin Ophthalmol. 2019;13:1649–1656. doi:10.2147/OPTH.S213132
17. Ribeiro FJ, Ferreira TB. Comparison of visual and refractive outcomes of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2020;46(5):694–699. doi:10.1097/j.jcrs.0000000000000118
18. Orts P, Piñero DP, Aguilar S, Tañá P. Efficacy of astigmatic correction after femtosecond laser-guided cataract surgery using intraoperative aberrometry in eyes with low-to-moderate levels of corneal astigmatism. Int Ophthalmol. 2020;40(5):1181–1189. doi:10.1007/s10792-020-01283-x
19. Orts-Vila P, Aguilar-Córcoles S, Tello-Elordi C, Ramos-Alzamora M, Montés-Micó R, Tañá-Rivero P. Trifocal toric intraocular lenses in eyes with low amount of corneal astigmatism. Int J Ophthalmol. 2020;13(10):1567–1573. doi:10.18240/ijo.2020.10.09
20. Sheen-Ophir S, Reitblat O, Levy A, Assia EI, Kleinmann G. Deviation from the planned axis of three toric intraocular lenses. Sci Rep. 2022;12(1):13760. doi:10.1038/s41598-022-17811-x
21. Yoo YS, Paik DW, Lim DH, Chung TY. One-year long-term clinical outcomes following diffractive trifocal toric intraocular lens implantation: retrospective observational case series study. Ann Transl Med. 2022;10(21):1159. doi:10.21037/atm-22-1007
22. Ruiz-Mesa R, Tañá-Sanz P, Tañá-Sanz S, Orts-Vila P, Tañá-Rivero P. Visual and refractive outcomes of a trifocal toric intraocular lens implanted in eyes with high corneal astigmatism. J Refract Surg. 2023;39(4):229–234. doi:10.3928/1081597X-20230127-01
23. Ang RET. Long-term trifocal toric intraocular lens outcomes in Asian eyes after cataract surgery. J Cataract Refract Surg. 2023;49(8):832–839. doi:10.1097/j.jcrs.0000000000001195
24. Vinas M, Gonzalez-Ramos A, Dorronsoro C, et al. In vivo measurement of longitudinal chromatic aberration in patients implanted with trifocal diffractive intraocular lenses. J Refract Surg. 2017;33(11):736–742. doi:10.3928/1081597X-20170814-01
25. Nagy ZZ, Popper-Sachetti A, Kiss HJ. Comparison of visual and refractive outcomes between hydrophilic and hydrophobic trifocal intraocular lenses sharing the same optical design. J Cataract Refract Surg. 2019;45(5):553–561. doi:10.1016/j.jcrs.2018.11.034
26. Vinas M, Gonzalez-Ramos AM, Aissati S, et al. Longitudinal chromatic aberration in patients implanted with trifocal diffractive hydrophobic IOLs. J Refract Surg. 2020;36(12):804–810. doi:10.3928/1081597X-20200930-01
27. Poyales F, Pérez R, López-Brea I, Zhou Y, Rico L, Garzón N. Comparison of visual performance and patient satisfaction outcomes with two trifocal IOLs with similar optical design but different materials. Clin Ophthalmol. 2020;14:3237–3247. doi:10.2147/OPTH.S273641
28. Mayer CS, Son HS, Łabuz G, et al. Laboratory and clinical experience with a diffractive trifocal intraocular lens sutured to an artificial iris. J Refract Surg. 2022;38(1):61–68. doi:10.3928/1081597X-20211209-02
29. Garzón N, Poyales F, García-Montero M, Vega F, Millán MS, Albarrán-Diego C. Impact of lens material on objective refraction in eyes with trifocal diffractive intraocular lenses. Curr Eye Res. 2022;47(1):51–61. doi:10.1080/02713683.2021.1946563
30. Benyoussef AA, Reboux N, Cochener B. Comparison of bilateral reading performance among two presbyopia-correcting intraocular lenses. J Refract Surg. 2022;38(7):428–434. doi:10.3928/1081597X-20220516-02
31. Kim JW, Eom Y, Park W, et al. Comparison of visual outcomes after two types of mix-and-match implanted trifocal extended-depth-of-focus and trifocal intraocular lenses. Graefes Arch Clin Exp Ophthalmol. 2022;260(10):3275–3283. doi:10.1007/s00417-022-05710-w
32. Abulafia A, Koch DD, Holladay JT, Wang L, Hill WE. Editorial. Pursuing perfection in IOL calculations IV: astigmatism analysis, SIA and double angle plots. J Cataract Refract Surg. 2018;44(10):1169–1174. doi:10.1016/j.jcrs.2018.07.027
33. Gualdi L, Cappello V, Giordano C. The use of NIDEK OPD Scan II wavefront aberrometry in toric intraocular lens implantation. J Refract Surg. 2009;25(1 Suppl):S110–S115. doi:10.3928/1081597X-20090115-06
34. Felipe A, Artigas JM, Díez-Ajenjo A, et al. Residual astigmatism produced by toric intraocular lens rotation. J Cataract Refract Surg. 2011;37(10):1895–1901. doi:10.1016/j.jcrs.2011.04.036
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


