Back to Journals » Clinical Ophthalmology » Volume 16
Visual and Refractive Outcomes Following Exchange of an Opacified Multifocal Intraocular Lens
Authors Stewart SA , McNeely RN , Chan WC, Moore JE
Received 26 February 2022
Accepted for publication 13 May 2022
Published 9 June 2022 Volume 2022:16 Pages 1883—1891
DOI https://doi.org/10.2147/OPTH.S362930
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Stephen A Stewart,1,2 Richard N McNeely,1 Wing C Chan,1 Jonathan E Moore1,3,4
1Cathedral Eye Clinic, Belfast, Northern Ireland, UK; 2School of Medicine, Dentistry and Biomedical Sciences, Queen’s University Belfast, Belfast, Northern Ireland, UK; 3Biomedical Sciences Research Institute, University of Ulster, Coleraine, Northern Ireland, UK; 4Department of Ophthalmology, Tianjin Medical University, Tianjin, People’s Republic of China
Correspondence: Stephen A Stewart, Cathedral Eye Clinic, Belfast, Northern Ireland, UK, Tel +44 28 9032 2020, Email [email protected]
Purpose: To assess the visual and refractive outcomes following exchange of an opacified multifocal intraocular lens (IOL).
Patients and Methods: A consecutive series of 37 eyes (31 patients) that underwent IOL exchange between November 2015 and May 2021 were included in this study. The indication for surgery in all cases was opacification of a multifocal IOL. Outcome measures included design and anatomical location of the secondary IOL, intraoperative and postoperative complications, visual acuity and refractive accuracy.
Results: An opacified Lentis Mplus multifocal IOL was explanted from all eyes and replaced with a monofocal IOL in 21 eyes (57%) and multifocal IOL in 16 eyes (43%). Secondary IOLs were implanted in the capsular bag or sulcus or were iris-fixated. IOL exchange was performed at a mean interval of 7 years after the primary surgery. Anterior vitrectomy was required for vitreous prolapse in 9 eyes (24%). Mean corrected distance visual acuity (CDVA) postoperatively was − 0.02 ± 0.08 logMAR for eyes with a monofocal secondary IOL and 0.02 ± 0.08 logMAR for eyes with a multifocal secondary IOL. Mean refractive prediction error was − 0.57 ± 0.67 D in the multifocal-monofocal group and − 0.33 ± 0.59 D in the multifocal–multifocal group.
Conclusion: An opacified multifocal IOL can be exchanged for a monofocal or multifocal IOL, depending on available capsular support and the patient’s desired refractive outcome. Vitreous prolapse requiring anterior vitrectomy is the most common intraoperative complication. An improvement in visual acuity and a low postoperative complication rate were achieved in this cohort of patients.
Keywords: cataract surgery, multifocal IOL, opacified IOL, IOL exchange
Introduction
Intraocular lens (IOL) opacification is a recognized late complication of cataract surgery or refractive lens exchange. If the opacification becomes visually significant, IOL exchange is required to restore visual function. This phenomenon is the indication for up to three-quarters of all IOL exchanges.1–5
IOL opacification occurs due to calcium and phosphate deposition on the surface of the IOL or within the IOL substance.6 It is most common in hydrophilic acrylic lenses but has also been reported in hydrophobic acrylic and silicone IOLs.7
A proprietary IOL design (“Hydrosmart”, Oculentis GmbH, Berlin, Germany) that incorporates a hydrophobic surface coating on a primarily hydrophilic IOL was intended to retain the advantages of a hydrophilic IOL but lower the risk of opacification.8 However, a cluster of cases of opacification of these lenses was reported, prompting a recall of Lentis foldable intraocular lenses with specific model numbers and expiry dates.8–10 The recalled lenses included some models of multifocal IOLs.
Previously described cases of IOL for exchange opacification of a multifocal IOL report secondary implant of a monofocal IOL.11–13 For patients who were happy with their range of unaided vision prior to IOL opacification, it may be appropriate to consider implanting a multifocal IOL at the time of IOL exchange. This study presents a case series of patients undergoing exchange of an opacified multifocal intraocular lens for a monofocal or multifocal IOL.
Patients and Methods
Consecutive patients who underwent IOL exchange of an opacified Lentis Mplus IOL (Oculentis GmbH) from November 2015 to May 2021 were included in this single-center retrospective cohort study. This study used only unidentifiable patient data and additionally all patients gave their written informed consent for their anonymised data to be submitted for audit and publication. The research adhered to the tenets of the Declaration of Helsinki. The study was approved as an audit study by the Cathedral Eye Clinic Ethics Committee (study reference number CECREC18-02).
Patient Assessment
Preoperatively, all patients had a full ophthalmic examination that included keratometry, topography, autorefraction (OPD-Scan aberrometer, Nidek Co, Ltd.), subjective refraction, uncorrected (UDVA) and corrected (CDVA) distance visual acuities, uncorrected near visual acuity (UNVA), slit-lamp examination, Goldmann tonometry, dilated fundoscopy, and biometry using swept-source optical coherence tomography (IOLMaster 700, Carl Zeiss Meditec AG, Jena, Germany). The history and clinical evidence of previous Nd:YAG posterior capsulotomy were documented. Vision examination included UDVA and CDVA (logMAR, original Early Treatment Diabetic Retinopathy Study chart 1 at 4m) and UNVA at 40cm with Radner reading charts under a standard mesopic lighting condition.
The optical biometer was used to measure corneal curvature, anterior chamber depth and axial length (AL). IOL power calculation for the lens to be implanted was calculated based on IOLMaster biometry using the Haigis formula for eyes with an AL of 22 mm or more, and the Hoffer Q formula for eyes with an AL of less than 22mm.
Intraocular Lens
All explanted lenses were the Lentis Mplus IOL (Figure 1), a single-piece refractive multifocal hydrophilic acrylic IOL with a hydrophobic surface modification. It has a sector-shaped embedded near zone that can vary in strength from +1.5 to +3.0D. Following explant of the opacified IOL, the decision regarding which model of IOL to implant and its location was influenced by the patient’s desired visual outcome and capsular status.
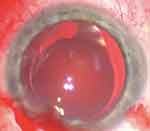 |
Figure 1 Diffusely opacified Lentis Mplus IOL prior to explantation. |
Surgical Technique
All surgeries were performed by a single experienced anterior segment surgeon (JE Moore), with assistance from a vitreoretinal surgeon (WC Chan) in one case. A 2.75 mm incision was made adjacent to the long axis of the IOL at a position to facilitate the transectional cutting of the IOL and was enlarged to 3.0 mm to facilitate removal of the IOL remnants. A dispersive ophthalmic viscoelastic device (Viscoat) was first injected into the anterior chamber followed by a cohesive OVD (Z-Hyalin) with the aim of providing adequate protection of the endothelium during surgical manoeuvres within the anterior chamber.
If there was significant anterior capsular phimosis, the capsulorhexis was enlarged using intraocular microscissors and forceps. Capsular hooks (Capsule Retractors, MicroSurgical Technologies, Washington, USA) were used in some cases to support the capsulorhexis at either end of the long axis of the IOL, either prior to or after enlargement of the capsulorhexis. Viscoelastic was injected at the midpoint behind the IOL to push the posterior capsule back and the IOL forwards. A bimanual technique was used to prolapse the IOL optic through the capsulorhexis and dial the proximal haptic out of the capsular bag.
The opacified IOL was grasped with intraocular forceps and then cut across its short axis using intraocular scissors (Packer/Chang IOL Cutter, MicroSurgical Technologies, Washington, USA). The trailing haptic was then mobilised from the capsular bag. Both portions of the IOL were removed through the corneal section. Anterior vitrectomy was carried out if there was a posterior capsular defect with vitreous prolapse. A capsular tension ring was implanted depending on the flexibility of the capsular bag and the ease of implantation.
A hydrophobic monofocal or multifocal IOL was placed in the capsular bag where possible. If there was a posterior capsular defect, a 3-piece monofocal IOL was placed in the sulcus, with optic capture when possible. In selected cases, a hydrophilic multifocal IOL designed for sulcus placement (Lentis Mplus LU-814 MF30) was implanted where implantation in the capsular bag was not possible but multifocality was strongly desired by the patient.
Statistical Analysis
Descriptive statistics (mean, standard deviation (SD), minimum, maximum) were calculated for age, time between operations, visual acuity and refraction. An independent t-test was used to compare the values between any 2 groups. A paired t-test was used to compare preoperative and postoperative values. A p value of less than 0.05 was considered statistically significant. Statistical analysis was completed using Microsoft Excel (Version 16.52, Microsoft Corporation) and R (Version 4.1.1, R Core Team 2021) software.
Results
Thirty-seven eyes of 31 patients underwent IOL exchange to replace an opacified multifocal intraocular lens during the study period (Table 1). The mean time from implantation of the primary multifocal IOL until IOL exchange was 7.1 ± 1.6 years. The median CDVA prior to exchange was 0.10 logMAR (−0.14 to 0.46 logMAR). Mean duration of follow-up post-IOL exchange was 6.9 ± 6.0 months.
 |
Table 1 Demographics and Baseline Characteristics |
Ocular and Systemic Comorbidities
The presence of an epiretinal membrane was noted in two eyes prior to IOL exchange. A low endothelial cell count was identified in one eye, and the patient was commenced on a topical Rho kinase inhibitor. One patient underwent further intraocular surgery following the primary IOL implant: repositioning of the IOL 3 weeks postoperatively. No other patients had intraocular surgery between the primary implant and the IOL exchange. One patient underwent photorefractive keratectomy (PRK) 17 months after the primary implant. No patients had a history of uveitis. One patient had type II diabetes mellitus.
Preoperative Capsular Status
Prior to IOL exchange, 9 eyes (24%) had an Nd:YAG posterior capsulotomy. Of those eyes with a posterior capsulotomy, 3 (33%) required an anterior vitrectomy because of vitreous prolapse. The secondary IOL was implanted in the sulcus in 8 eyes (89%) and was iris-fixated in 1 eye (11%).
Of the 28 eyes without a posterior capsulotomy, 6 (21%) required an anterior vitrectomy for vitreous prolapse and 1 case was managed with a planned pars plana vitrectomy. In eyes with an intact posterior capsule preoperatively, the secondary IOL was implanted in the capsular bag in 13 eyes (46%), the sulcus in 14 (50%) and was iris-fixated in 1 (4%).
Capsular hooks were used in 11 eyes (30%). Of these, 10 had an intact posterior capsule preoperatively and 2 cases required an anterior vitrectomy. Of the 26 eyes in which capsular hooks were not used, 18 had an intact posterior capsule preoperatively and anterior vitrectomy was required in 5 of these cases, with another case being managed with a planned pars plana vitrectomy.
Implanted IOL Design and Location
At the time of IOL exchange, 16 eyes (43%) had a multifocal IOL implanted, and 21 eyes (57%) had a monofocal IOL implanted (Table 2). Of the multifocal IOLs, 10 were implanted in the capsular bag and 6 (Lentis Mplus LU-814 MF30) were placed in the sulcus. Monofocal iris-claw lenses were iris-fixated anteriorly in 2 eyes. A 3-piece monofocal IOL was placed in the sulcus in 16 eyes, with optic capture in 5 eyes. A single-piece monofocal IOL was implanted in the capsular bag in 3 eyes.
 |
Table 2 Model, Design and Implant Location of Secondary IOLs |
Intraoperative and Postoperative Complications
Anterior vitrectomy was performed to manage vitreous prolapse in 9 eyes (24%). Iris prolapse was encountered in 2 eyes (5%), and there was an intraoperative iris haemorrhage in 1 eye (3%). Limited zonulodialysis occurred in 3 eyes (8%). A capsular tension ring was inserted in 5 eyes (14%) but was explanted again intraoperatively in 1 case because capsular rupture was noted. In one case, a Lentis Mplus LU-814 MF30 IOL was implanted in the sulcus but appeared unstable and therefore was explanted again intraoperatively, and an Ophtec Artisan IOL was iris-fixated instead.
Postoperatively, 2 eyes (5%) had keratorefractive surgery for residual refractive error: 1 eye underwent laser in situ keratomileusis (LASIK) 6 months post-exchange to correct myopic astigmatism (0.00/-1.25 × 80) and 1 eye had photorefractive keratectomy (PRK) 12 months post-exchange to correct a myopic refractive error (−1.00/-0.25 × 5). Dry eye was noted in 3 eyes (8%). Posterior capsular thickening was present in 1 eye. A borderline elevated intraocular pressure was noted in 1 eye with a 3-piece IOL in the sulcus, and further follow-up was planned. There were no cases of postoperative cystoid macular oedema, uveitis, retinal detachment or endophthalmitis.
Visual and Refractive Outcomes
The mean CDVA improved from 0.11 ± 0.15 logMAR preoperatively to −0.02 ± 0.08 logMAR postoperatively (p = 0.001) in the multifocal-monofocal group (Table 3). Mean CDVA improved from 0.16 ± 0.15 logMAR preoperatively to 0.02 ± 0.08 logMAR postoperatively (p = 0.004) in the multifocal–multifocal group. The mean improvement in CDVA did not differ significantly between the multifocal-monofocal and the multifocal–multifocal groups (−0.12 ± 0.14 logMAR vs −0.15 ± 0.16 logMAR, p = 0.27). Twenty-nine eyes (78%) had a visual acuity of 6/12 or better pre-exchange, and all eyes were 6/12 or better post-exchange.
 |
Table 3 Visual Outcomes Following IOL Exchange |
The mean refractive prediction error was −0.57 ± 0.67 D in the multifocal-monofocal group and −0.33 ± 0.59 D in the multifocal–multifocal group (Table 4). The mean postoperative UDVA was slightly worse for the multifocal-monofocal group, but this did not reach statistical significance (0.20 ± 0.24 logMAR vs 0.16 ± 0.11 logMAR, p = 0.23). The mean absolute refractive prediction error did not differ significantly between the multifocal-monofocal and multifocal–multifocal groups (0.70 ± 0.53 D vs 0.56 ± 0.37 D, p = 0.17). The placement of a multifocal IOL in the sulcus rather than the capsular bag produced a more myopic prediction error (−0.66 ± 0.61 D vs −0.13 ± 0.52 D, p = 0.06) and a greater mean absolute error (0.75 ± 0.45 D vs 0.44 ± 0.27 D, p = 0.08), although these differences were not statistically significant.
 |
Table 4 Refractive Outcomes Following IOL Exchange |
For the multifocal-monofocal and multifocal–multifocal groups, the achieved spherical equivalent (SE) was within ±0.5 D of the attempted SE in 10 eyes (48%) and 8 eyes (50%) and ±1.0 D in 14 eyes (67%) and 15 (94%), respectively. A single patient had a prediction error of more than 1.5 D (PE −2.21, postoperative spherical equivalent −1.75D). This patient had a 3-piece monofocal IOL placed in the sulcus, without optic capture.
Discussion
Patients who request multifocal IOLs have high visual demands and are often very satisfied with their unaided distance, intermediate and near vision post-operatively.14,15 However, some patients may be intolerant of photic phenomena associated with multifocality, such as glare and halos, and this is the most common indication for multifocal IOL exchange.1 A monofocal IOL is typically implanted as the secondary IOL to eliminate these symptoms.16,17 In contrast, a patient who is initially satisfied with the vision achieved with a multifocal IOL but then experiences the late complication of IOL opacification and a reduction in visual acuity, may wish to have multifocality restored. This study is the first to report the visual and refractive outcomes for secondary implantation of a multifocal IOL following explant of an opacified multifocal IOL.
IOL opacification is caused by calcification, and this can be classified as primary or secondary calcification.6 Primary calcification relates to the manufacturing process and occurs when there is a physical feature of the IOL material that allows calcium deposition and crystallisation, such as a porous structure or disruption of the IOL surface by the cleaning process during lens manufacture.6 There is typically no history of other ocular pathology. Secondary calcification occurs when there is crystallisation of calcium on the surface of the IOL due to an alteration of the aqueous milieu.6,16,17 This can occur due to immunologic disruption of the blood-aqueous barrier in uveitis, mechanical disruption from haptic-iris contact with sulcus placement of an IOL, or the use of other intraocular fluids or gas during additional surgical procedures.18–21
The Lentis Mplus IOL (Oculentis GmbH, Berlin, Germany) is a refractive multifocal IOL that incorporates a surface-embedded near section. Excellent visual and refractive outcomes following implantation of this IOL have been reported.22 However, these lenses were included in the urgent recall of Lentis foldable intraocular lenses with model numbers starting with L-, LU- or LS- and having an expiry date between January 2017 and May 2020.10 The manufacturer had identified sporadic cases of opacification of these IOLs. The presence of a phosphate remnant from a detergent product used during cleaning of the IOL was thought to make these IOLs more prone to surface calcification.10
A cross-sectional study of 169 eyes of 154 patients who had received the Lentis LS-502-1 IOL, which uses the same Hydrosmart design as the Lentis Mplus, found a prevalence of IOL opacification of 53.3%.9 The pattern of opacification of these IOLs was classified as peripheral, central, diffuse or superficial.9 The authors propose that these patterns represent different stages of calcification of the IOL: calcium crystals deposit on the lens surface initially, and then they permeate the IOL coating in the central or peripheral optic, ultimately leading to diffuse opacification and a significant decrease in visual acuity.9
In a small case series of opacified multifocal IOLs previously reported, visually significant opacification was noted at a mean time of 4 years postoperatively.12 In our cohort, patients underwent IOL exchange at a mean time of 7 years postoperatively. The time from the development of visually significant opacification to IOL exchange can be variable, with some patients opting for conservative management initially if their visual symptoms are mild.
Monofocal IOLs are a reliable option as a secondary implant during IOL exchange. A variety of designs allow placement in the capsular bag or sulcus or iris fixation with iris-claw lenses. The decision regarding whether a monofocal or multifocal IOL is implanted during IOL exchange can often only be made intraoperatively, once the surgeon ascertains what capsular support is available for the secondary IOL. A detailed discussion preoperatively to understand the patient’s expectations and desired refractive outcome is essential. All patients should be counselled regarding the anticipated surgical challenges and advised that the implantation of multifocal IOL may not be possible.
Previous studies of multifocal IOL exchange have primarily reported outcomes for monofocal IOL implantation as the secondary IOL.16,17 The study by Kamiya et al reviewed the outcomes for 50 eyes undergoing multifocal IOL exchange and included 5 multifocal–multifocal exchanges but did not report the visual or refractive outcomes for these cases separately.17 A recent study by Al-Shymali et al reported the visual and refractive outcomes for multifocal-to-multifocal IOL exchange in 30 eyes of 15 patients who reported poor visual quality following the initial multifocal IOL implant.23 However, the cohort of patients studied by Al-Shymali et al differs from ours in that the IOL exchanges were much earlier (mean time from initial surgery to exchange of 12 months vs 85 months), and none had a prior YAG capsulotomy.23 Despite this, our patients had comparable refractive results (postoperative spherical equivalent of 0.04 ± 0.43D vs −0.35 ± 0.62D) and visual outcomes (UDVA of 0.12 ± 0.77 logMAR vs 0.10 ± 0.15 logMAR; UNVA of 0.28 ± 0.68 logMAR vs 0.25 ± 0.13 logMAR).23
In this study, multifocal IOLs were implanted in 16 eyes (43%): 10 IOLs were placed in the capsular bag and 6 in the sulcus. The placement of a conventional single-piece IOL in the ciliary sulcus is not recommended due to the risk of the optic and haptics causing chafing of the posterior iris surface, leading to pigment dispersion, inflammation and raised intraocular pressure.24 However, the Lentis Mplus LU-814 MF30 is a single-piece multifocal IOL with 4 open-loop haptics and is designed for placement in the capsular bag or sulcus or for scleral fixation (Figure 2). This IOL was placed in the sulcus of 6 eyes and yielded satisfactory distance, intermediate and near visual acuities, with no significant complications at a mean follow-up of 7 months. However, in our experience, the placement of a multifocal IOL in the sulcus yielded less predictable refractive outcomes compared to placement in the bag, with patients being more myopic postoperatively than intended. In our case series, these observations did not reach statistical significance, but a larger study to analyse the refractive outcomes following sulcus placement of the Lentis Mplus LU-814 is warranted.
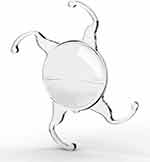 |
Figure 2 Lentis Mplus LU-814 MF30 IOL (image courtesy of Oculentis GmbH). |
The diagnosis of IOL opacification may be challenging, and it can be mistaken for posterior capsule opacification. Patients are more likely to have an Nd:YAG posterior capsulotomy if opacification is misinterpreted as capsular rather than lenticular.25 There is also a higher baseline rate of Nd:YAG posterior capsulotomy for multifocal IOLs because of the additive effect of lens- and capsular-induced reduction in contrast sensitivity, making patients more symptomatic of posterior capsule opacification.26 Anterior segment OCT demonstrates hyperreflectivity of an opacified IOL surface and may be informative in the diagnosis of IOL versus capsular opacification.27
The presence of a posterior capsulotomy prior to IOL exchange has implications for the surgical approach to IOL exchange and the likely anatomical location of the secondary IOL. In this cohort of patients, anterior vitrectomy was performed more frequently in eyes with a pre-existing posterior capsulotomy compared to those with an intact posterior capsule (33% vs 21%). All IOLs implanted in eyes with a pre-existing posterior capsulotomy were placed in the sulcus or iris-fixated, while 46% were implanted in the capsular bag in eyes with an intact posterior capsule preoperatively.
Vitreous prolapse is a common intraoperative complication of IOL exchange and occurred in 24% of eyes in this study. Previous studies have shown that this complication is correlated with preoperative Nd:YAG posterior capsulotomy.3,16 However, eyes with an intact posterior capsule preoperatively may also require anterior vitrectomy for vitreous prolapse if zonulodialysis or capsular rupture is encountered during surgical maneuvers, particularly if the IOL is being removed from a fibrotic and contracted capsular bag.
Capsule retractors are a useful surgical adjunct in cases with frank zonulodialysis or when zonular weakness is suspected. The retractors are similar to iris hooks but are larger and have a curved loop at the distal end to provide better support to the anterior capsule and prevent puncture of the capsule. They stabilize the capsular bag against anteroposterior and rotational forces.28 In our experience, they reduce the likelihood of zonular dehiscence with vitreous prolapse. In eyes that had an intact posterior capsule preoperatively, there was a lower vitrectomy rate in cases when capsule retractors were used compared to when they were not used (20% vs 28%).
The limitations of this study include the fact that the majority of patients referred for IOL exchange had the opacified IOLs implanted in other centers, so the rate of opacification of all implanted IOLs of this model could not be determined. The time from initial surgery to opacification could not be determined as these patients were not under long-term review following the primary procedure. However, the time from initial surgery to IOL exchange is a relevant interval as it reflects the timepoint at which the patient sought medical attention for visual impairment secondary to the IOL opacification.
Conclusion
This study highlights that despite the challenges of exchanging an opacified multifocal IOL, satisfactory visual and refractive outcomes are achievable with a variety of IOL designs including multifocal IOLs in selected cases. Given the high rate of late opacification of a hydrophilic/hydrophobic IOL previously reported and the delay between initial surgery and presentation for IOL exchange observed in our study, it is likely that we will see more of these cases over the coming years.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors did not receive any funding for this work and report no conflicts of interest in this work.
References
1. Davies EC, Pineda R. Intraocular lens exchange surgery at a tertiary referral center: indications, complications, and visual outcomes. J Cataract Refract Surg. 2016;42(9):1262–1267. doi:10.1016/j.jcrs.2016.06.031
2. Abdalla Elsayed MEA, Ahmad K, Al-Abdullah AA, et al. Incidence of intraocular lens exchange after cataract surgery. Sci Rep. 2019;9(1):12877. doi:10.1038/s41598-019-49030-2
3. Leysen I, Bartholomeeusen E, Coeckelbergh T, Tassignon MJBR. Surgical outcomes of intraocular lens exchange: five-year study. J Cataract Refract Surg. 2009;35(6):1013–1018. doi:10.1016/j.jcrs.2009.01.024
4. Neuhann T, Yildirim TM, Son HS, Merz PR, Khoramnia R, Auffarth GU. Reasons for explantation, demographics, and material analysis of 200 intraocular lens explants. J Cataract Refract Surg. 2020;46(1):20–26. doi:10.1016/j.jcrs.2019.08.045
5. Goemaere J, Trigaux C, Denissen L, et al. Fifteen years of IOL exchange: indications, outcomes, and complications. J Cataract Refract Surg. 2020;46(12):1596–1603. doi:10.1097/j.jcrs.0000000000000349
6. Neuhann IM, Kleinmann G, Apple DJ. A new classification of calcification of intraocular lenses. Ophthalmology. 2008;115(1):73–79. doi:10.1016/j.ophtha.2007.02.016
7. Werner L. Causes of intraocular lens opacification or discoloration. J Cataract Refract Surg. 2007;33(4):713–726. doi:10.1016/j.jcrs.2007.01.015
8. Bompastor-Ramos P, Póvoa J, Lobo C, et al. Late postoperative opacification of a hydrophilic-hydrophobic acrylic intraocular lens. J Cataract Refract Surg. 2016;42(9):1324–1331. doi:10.1016/j.jcrs.2016.06.032
9. Costa JF, Bompastor-Ramos P, Marques M, et al. Large-scale opacification of a hydrophilic/hydrophobic intraocular lens. Eur J Ophthalmol. 2020;30(2):307–314. doi:10.1177/1120672119830581
10. Oculentis BV. Urgent – field safety notice: recall lentis foldable intraocular lenses. Medicines & Healthcare products Regulatory Agency; 2017. Available from: https://mhra-gov.filecamp.com/s/rPGukaTBxpBa5Oia/fo/tbcqXSosX9c44xpp/fi/Nx0nPUHTINxNIv8B.
11. Elgohary M, Zaheer A, Werner L, Ionides A, Sheldrick J, Ahmed N. Opacification of Array SA40N silicone multifocal intraocular lens. J Cataract Refract Surg. 2007;33(2):342–347. doi:10.1016/j.jcrs.2006.09.034
12. Bang SP, Moon K, Lee JH, Jun JH, Joo CK. Subsurface calcification of hydrophilic refractive multifocal intraocular lenses with a hydrophobic surface: a case series. Medicine. 2019;98(50):e18379. doi:10.1097/MD.0000000000018379
13. Yamashita K, Hayashi K, Hata S. Toric Lentis Mplus intraocular lens opacification: a case report. Am J Ophthalmol Case Rep. 2020;18:100672. doi:10.1016/j.ajoc.2020.100672
14. McNeely RN, Pazo E, Spence A, et al. Visual quality and performance comparison between 2 refractive rotationally asymmetric multifocal intraocular lenses. J Cataract Refract Surg. 2017;43(8):1020–1026. doi:10.1016/j.jcrs.2017.05.039
15. de Vries NE, Nuijts RMMA. Multifocal intraocular lenses in cataract surgery: literature review of benefits and side effects. J Cataract Refract Surg. 2013;39(2):268–278. doi:10.1016/j.jcrs.2012.12.002
16. Kim EJ, Sajjad A, Montes de Oca I, et al. Refractive outcomes after multifocal intraocular lens exchange. J Cataract Refract Surg. 2017;43(6):761–766. doi:10.1016/j.jcrs.2017.03.034
17. Kamiya K, Hayashi K, Shimizu K, et al. Multifocal intraocular lens explantation: a case series of 50 eyes. Am J Ophthalmol. 2014;158(2):215–220.e1. doi:10.1016/j.ajo.2014.04.010
18. Skip Nichamin LD. Effect of repositioning on IOL opacification. J Cataract Refract Surg. 2009;35(6):965. doi:10.1016/j.jcrs.2009.01.043
19. Gurabardhi M, Häberle H, Aurich H, Werner L, Pham DT. Serial intraocular lens opacifications of different designs from the same manufacturer: clinical and light microscopic results of 71 explant cases. J Cataract Refract Surg. 2018;44(11):1326–1332. doi:10.1016/j.jcrs.2018.07.026
20. Darcy K, Apel A, Donaldson M, et al. Calcification of hydrophilic acrylic intraocular lenses following secondary surgical procedures in the anterior and posterior segments. Br J Ophthalmol. 2019;103(12):1700–1703. doi:10.1136/bjophthalmol-2018-313385
21. Oner FH, Ozturk T, Yaman A, Werner L. Intraocular lens opacification following silicone oil endotamponade. Ophthalmic Surg Lasers Imaging Retina. 2021;52(1):37–43. doi:10.3928/23258160-20201223-07
22. McAlinden C, Moore JE. Multifocal intraocular lens with a surface-embedded near section: short-term clinical outcomes. J Cataract Refract Surg. 2011;37(3):441–445. doi:10.1016/j.jcrs.2010.08.055
23. Al-Shymali O, McAlinden C, Alio Del Barrio JL, Canto-Cerdan M, Alio JL. Patients’ dissatisfaction with multifocal intraocular lenses managed by exchange with other multifocal lenses of different optical profiles. Eye Vis. 2022;9(1):8. doi:10.1186/s40662-022-00280-8
24. Chang DF, Masket S, Miller KM, et al. Complications of sulcus placement of single-piece acrylic intraocular lenses: recommendations for backup IOL implantation following posterior capsule rupture. J Cataract Refract Surg. 2009;35(8):1445–1458. doi:10.1016/j.jcrs.2009.04.027
25. Haymore J, Zaidman G, Werner L, et al. Misdiagnosis of hydrophilic acrylic intraocular lens optic opacification: report of 8 cases with the MemoryLens. Ophthalmology. 2007;114(9):1689–1695. doi:10.1016/j.ophtha.2006.12.024
26. Shah VC, Russo C, Cannon R, Davidson R, Taravella MJ. Incidence of Nd: YAGcapsulotomy after implantation of AcrySof multifocal and monofocal intraocular lenses: a case controlled study. J Refract Surg. 2010;26(8):565–568. doi:10.3928/1081597X-20100303-01
27. Choudhry S, Goel N, Mehta A, Mahajan N. Anterior segment optical coherence tomography of intraocular lens opacification. Indian J Ophthalmol. 2018;66(6):858–860. doi:10.4103/ijo.IJO_1172_17
28. Grove K, Condon G, Erny BC, Chang DF, Kim T. Complication from combined use of capsule retractors and capsular tension rings in zonular dehiscence. J Cataract Refract Surg. 2015;41(11):2576–2579. doi:10.1016/j.jcrs.2015.10.012
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
