Back to Journals » Infection and Drug Resistance » Volume 15
Virulence Characteristics, Antibiotic Resistance Patterns and Molecular Typing of Enteropathogenic Producing Escherichia coli (EPEC) Isolates in Eastern Province of Saudi Arabia: 2013–2014
Received 6 September 2022
Accepted for publication 15 November 2022
Published 23 November 2022 Volume 2022:15 Pages 6763—6772
DOI https://doi.org/10.2147/IDR.S388956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Lamya Zohair Yamani, Nasreldin Elhadi
Department of Clinical Laboratory Science, College of Applied Medical Sciences, Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia
Correspondence: Nasreldin Elhadi, Department of Clinical Laboratory Science, Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia, Email [email protected]
Introduction: The Kingdom of Saudi Arabia has limited data on enteropathogenic Escherichia coli (EPEC). Therefore, this study was undertaken to contribute to EPEC surveillance and investigate the molecular epidemiology of EPEC strains that have been implicated in human infection in King Fahd Hospital of the University (KFHU) between 2013 and 2014 in the Eastern Province of Saudi Arabia.
Methods: A total of 60 non-duplicate E. coli isolates associated with human gastroenteritis were included in this study. They were characterized using PCR to determine virulence genes, antimicrobial resistance patterns, and enterobacterial repetitive intergenic consensus (ERIC-PCR).
Results: Among the 60 strains, 20% of those examined were positive for the intimin eae and bfpA genes and identified as typical EPEC (tEPEC). Furthermore, 44 of E. coli strains tested positive for the eae gene only and revealed a high occurrence rate of 73.3% of atypical EPEC (aEPEC) within the overall examined strains. All strains were positive for the EAST1 gene, and none tested positive for the stx gene. More than 70% of EPEC strains were multi-drug resistant (MDR) and aEPEC strains with the highest proportion of this feature of MDR. ERIC-PCR fingerprint revealed a total of 19 ERIC types with eight related distinct clusters and a similarity rate cut-off with ≥ 90% homology from the identified isolates.
Conclusion: A high antibiotic resistance rate was reported for first-line antibiotics, such as ampicillin, tetracycline, nalidixic acid, trimethoprim-sulfamethoxazole, noroxin, and ciprofloxacin.
Keywords: EPEC, virulence, antimicrobial resistance, MDR, ERIC-PCR
Introduction
The uniqueness of Escherichia coli (E. coli) lies in its versatile nature compared to many microorganisms. It is an important member of the normal intestinal flora, where the colonization of the gastrointestinal tract begins within a few hours after birth.1 The apparent coexistence between these commensals and hosts has led to mutual benefits. They have been observed to colonize the mucosal membranes of the colon, forming niches in that area. Their presence has rarely been shown to cause disease unless the host was immunocompromised or in instances where the barriers of the intestinal tract have been defective or breached.2 E. coli strains that have been known to cause infections were found to possess highly potent virulence attributes that support their adaptation within new niches causing extensive complications, leading to a diseased state. Around the world, deaths of children under the age of 5 years have been credited to diarrheal diseases in underdeveloped countries, where the highest implication has been due to diarrheagenic E. coli (DEC).3–5 DEC strains were quite difficult to detect as they had major similarities with their normal counterpart and were unable to be distinguished from one another until PCR techniques made it possible to detect virulence genes found only within the pathogenic strains.6 Extraintestinal pathogenic E. coli (ExPEC) has been observed to cause infections related to diarrheal/enteric disease, meningitis or sepsis, and urinary tract infections.7 These ExPEC are results of successful combinations with diverse virulence factors that persist within the host system.7 All E. coli pathotypes can be grouped together based on shared O lipopolysaccharide (LPS) and H flagellar antigens, which make up the serogroup O antigen, or individual serotypes related to O and H antigens.3,8
Enteropathogenic E. coli (EPEC) falls under the pathotype category of intestinal pathogens. It was the first to be discovered, as there was a large outbreak of infant diarrhea in the United Kingdom. The causative agent for the outbreak was not well understood, but strain isolation was performed, and since then, many advances have been made pertaining to EPEC mode of transmission and pathogenesis cycles.5,7,9 EPEC infections have characteristic intestinal histology that is known as “attaching and effacing”. The strains are observed to attach themselves to the epithelial cells of the intestine, causing drastic cytoskeletal changes leading to polymerization of the actin filaments.10 The intestinal microvilli become effaced, and the pathogenic bacteria will be able to move, causing infectious spread. The ability of this strain to have such characteristic histology lies within its pathogenicity island, locus of enterocyte effacement (LEE). LEE encodes a protein intimin, which is needed for EPEC attachment. EPEC attaches to epithelial cells within the intestine.7,11 This site also encodes the eae gene. EPEC strains can be divided into either “typical” or “atypical” strains. This division is based on whether they have the EPEC adherence factor (EAF) plasmid.12 This plasmid encodes a type IV pilus named the bundle-forming pilus (BFP) needed for bacterial strain adherence to intestinal epithelial cells.13 Atypical EPEC (aEPEC) does not have this EAF plasmid. Thereby, strains that possess both eae+ bfpA+ are classified under typical EPEC (tEPEC), while those that are eae+ but lack bfpA- are said to be aEPEC. aEPEC has been isolated more frequently in diarrheal cases from developed countries, whereas; tEPEC isolation has been found more often in developing countries.4,14
An increasing concern relates to antibiotic resistance with increased use as a method of treatment, as there have been clinical reports of an increased occurrence of resistance to third-generation cephalosporin bacteria in Saudi Arabia.15,16 One study looking at drug resistance foretold that approximately ten million people would suffer fatally from antibiotic-resistant diseases annually by the year 2050. E. coli infections are among the bacterial infections that have high death predictions by antibiotic-resistance.17 Another group advised that infections caused by E. coli strains should not be limited to antibiotic usage as a treatment method to prevent emerging antibiotic resistance with high risks of severe complications leading to hemolytic uremia. E. coli strains that are either pathogenic or non-pathogenic have developed ways to assist in their resistance to antibiotics by utilizing diverse mechanisms. In Saudi Arabia, EPEC outbreaks have been very limited or seldom reported.18 Studies describing the clinical prevalence of diarrhea among Hajj pilgrims have reported infectious spread and diarrhea that were associated with E. coli strains. EPEC attributed to diarrheal during the 2013 and 2016 Hajj pilgrimages.19 Therefore, this study was undertaken to contribute to EPEC surveillance and investigate the molecular epidemiology of EPEC isolates that have been implicated in human infection between 2013 and 2014 in the Eastern Province of Saudi Arabia.
Materials and Methods
Bacterial Strains Al-Khobar, Saudi Arabia
A total of 60 non-duplicate E. coli isolates which were previously isolated from humans showing gastroenteritis symptoms of diarrhea between 2013 and 2014 were characterized in this study. These isolates were collected from the culture collection of the microbiology laboratory at KFHU, Al-Khobar, Saudi Arabia. Clinical information on patients from which the isolates of EPEC were isolated was unavailable. The collected bacterial isolates were further inoculated onto MacConkey agar medium and followed by 18–24 h incubation at 37°C in order to isolate pure E. coli colonies. Isolated colonies of EPEC isolates were further tested using standard biochemical tests and API20E (BioMérieux, France) for validation. Isolates were further examined for EPEC virulence gene signatures using PCR.
DNA Extraction and PCR Assay
Pure isolated colonies of EPEC isolates were cultured in 5 mL of Luria Bertani (LB) broth and incubated overnight at 37°C. Then, 1.0 mL of incubated cultures were centrifuged at 10,000 × g for 2 min, and pellets were re-suspended in 0.5 mL sterilized distilled water. All isolate pellets were vortexed for 2 min and boiled at 100°C for 15 min. Furthermore, the boiled tubes were centrifuged at 12,000 × g for 5 min, and supernatant DNA was transferred into 1.2 mL sterilized tubes and stored at −20°C until used for PCR assay of virulence genes and molecular typing of EPEC isolates. The extracted DNA was used as a template in all PCR reactions to screen EPEC virulence gene markers using the previously described protocol,20,21 as presented in Table 1, and positive controls were included in each PCR run.
 |
Table 1 Primers Used in This Study |
Antimicrobial Susceptibility Testing
The antimicrobial agent susceptibility testing for all EPEC isolates was examined following the Kirby-Bauer disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.22 The antimicrobial disks used were ampicillin (AM, 25 µg), aztreonam (ATM 30 µg), augmentin (AUG 30 µg), cefotaxime (CTX 30 µg), chloramphenicol (C 30 µg), cephalothin (KF 30 µg), ciprofloxacin (CIP 5 µg), cefepime (FEP 30 µg), gentamicin (GM 5 µg), nalidixic acid (NA 30 µg), noroxin (NOR 10 µg), piperacillin PRL (100 µg), streptomycin (S 10 µg), trimethoprim-sulfamethoxazole (SXT 25 µg) and tetracycline (TE 30 µg). All the antibiotic disks were obtained from Oxoid (Basingstoke, Hampshire, UK). The zone of inhibition diameter was measured in mm using Vernier calipers and results were interpreted as sensitive (S), intermediate (I) and (R) resistant according to CLSI breakpoints and for pathogenic E. coli with the help of protocol published elsewhere.22 The strain of E. coli ATCC 25922 was used as a control for antibiotic susceptibility testing.
ERIC-PCR Typing
The ERIC2 primer (‘5-AAGTAAGTGACTGGGGTGAGCG-3’) was used for typing EPEC isolates as described previously.23,24 The Gelj software25 was used for genetic similarity analysis. The construction of the dendrogram clusters and dice correlation coefficient calculations were obtained using the unweighted average pair group method (UPGMA). Optimization and band position tolerance were adjusted to 1%. The similarity coefficient of 90% cut-off was used for cluster groups.
Statistical Analysis
The antibiotic susceptibility testing results for resistance, intermediate, and sensitivity were observed, and the difference between the groups (tEPEC, aEPEC and non-EPEC) was investigated using the Kruskal–Wallis test. Following the test, post hoc comparisons between groups were performed using the Mann–Whitney U-test with Bonferroni corrected p-values. The analysis was completed using PAST statistical software.26
Results
Occurrence of Virulence Genes
As presented in Table 2, 12 (20%) and 4 (6.7%) of the examined isolates tested positive for intimin eae and bfpA genes and were identified as tEPEC and negative isolates for both genes were identified as non-EPEC, respectively. The most prevalent among the examined E. coli isolates was aEPEC, with occurrence rate of 44 (73.3%), as shown in Table 2. Despite the fact that all 60 isolates tested positive for the EAST1 gene, no stx gene was found positive (Table 2).
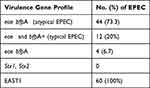 |
Table 2 Distribution of Virulence Genes in EPEC Isolates |
Antibiotic Resistance Patterns
Results of antibiotic susceptibility testing for 15 types of antibiotics are presented in Table 3. The overall antibiotic resistance prevalence rates among the 60 strains of E. coli were: ampicillin (63.3%), tetracycline (60%), nalidixic acid (55%), trimethoprim-sulfamethoxazole (48.3%), noroxin (36.7%), and ciprofloxacin (35%). Among the categories of EPEC, aEPEC isolates were observed with high resistance > 35% for ampicillin, tetracycline, nalidixic acid, and trimethoprim-sulfamethoxazole (Table 3). Findings of these studies demonstrated that 70% of investigated isolates were found to be MDR (Figure 1). Table 3 shows the resistance of the three groups of tEPEC, aEPEC, and non-EPEC isolates that were compared using the Kruskal–Wallis test. The test showed significant differences between the three groups of tEPEC, aEPEC, and non-EPEC strains. Similarly, for intermediate and sensitive, the difference between the three groups was significant. For resistance, the post hoc comparison showed that Bonferroni corrected p-values were significant for tEPEC vs aEPEC and tEPEC vs non-EPEC. For intermediate strains, the post hoc comparison showed that only tEPEC vs non-EPEC was significant. For sensitive isolates, all three post hoc tests were significant (Table 3).
 |
Table 3 Antibiotic Susceptibility of Typical, Atypical EPEC and Non-EPEC Isolated from Patients with Diarrhea |
ERIC Typing
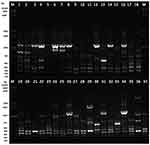
The ERIC products ranged in size between 250 to 2500 bp, and DNA fingerprint patterns ranged between 5 to 13 bands among analyzed isolates (Figure 1). The ERIC typing method was able to genotype all isolates from humans between 2013 and 2014 of tEPEC, aEPEC, and non-EPEC into detailed cluster groups generated by the Gelj software (Figure 1). Before clustering isolates with a high degree of DNA fingerprints homology can be identified and labeled, a cut-off value of 90% genetic similarity was defined (Figure 2). Based on the ERIC typing dendrogram, 19 ERIC types and eight related clusters (A to H) with ≥ 90% homology of isolates were identified (Figure 2). At the same time, 11 (18.3%) isolates had individual, different ERIC fingerprint type lineages (1 to 7 and 9 to 12). Cluster F, G, and H comprised 58.3% of examined isolates of EPEC in this study. The highest number of tEPEC and aEPEC isolates of the year 2013 were grouped in cluster H, and two isolates of non-EPEC were grouped together in cluster A. The ERIC-PCR dendrogram in this study showed high genetic diversity, and there was no correlation observed between the phylogenetic relationship among the 60 isolates of EPEC. However, the result indicates a variation in tEPEC, aEPEC, and non-EPEC isolates is implicated in the human infection between 2013 and 2014 in Eastern Province of Saudi Arabia. Based on the obtained results, ERIC-PCR is a more suitable discriminative DNA fingerprinting method to analyze the genetic diversity of investigated EPEC isolates in this study.
Discussion
EPEC is an important pathogen among the DEC pathotypes and is a known causative agent of persistent diarrhea in children and adults worldwide.4,9 Strains of EPEC isolated from fecal specimens during sporadic and emerging cases are divided into tEPEC and aEPEC.14,27,28 The tEPEC is usually responsible for causing acute diarrhea in infants and children in developing countries, while the aEPEC is a causative agent of infection in children and adults worldwide.4,29,30 In this study, we investigated the frequency of EPEC categories, virulence genes, antibiotic resistance patterns, and genetic relationships among EPEC isolates implicated in the human infection between 2013 and 2014 in Eastern Province of Saudi Arabia. Few studies conducted in Saudi Arabia have been documented regarding DEC pathotypes implicated in human infections.31 Our study is consistent with several recent epidemiological studies that have reported an increased emergence and identification of aEPEC among EPEC infections, and there is an association with diarrheal outbreaks in both developing and developed countries.30,32–38
All strains in this study were positive for the EAST1 gene and negative for the stx gene (Table 2). The EAST1 gene is originally recognized in strains of an enteroaggregative Escherichia coli (EAEC) isolated from a Chilean children’s stool specimen while suffering diarrhea.39 Our study is in concordance with several studies that have proven that gene encoding EAST1 is not restricted to EAEC and broadly distributed among DEC pathotypes and other human enteric pathogens.40–43 Strains expressing the EAST1 gene have been associated with inducing diarrhea in children and adults.41,44 Although some strains of E. coli isolated from human diarrheal cases were lacking the known virulence determinants other than the EAST1 gene thus making it a potential virulence factor.41,45 During the second international symposium on EPEC conducted in 1995 in São Paulo, Brazil, most of the participants had agreed and defined EPEC as DEC that produces characteristic histopathology known as attaching and effacing (A/E) on intestinal cells and not expressing Shiga-toxin genes.14 However, the tEPEC strains harbor a virulence plasmid known as the EPEC adherence factor (EAF) plasmid.30 This plasmid is encoded by the bundle forming pilus (bfp), which is responsible for its adherence to intestinal epithelial cells. The presence of this EAF plasmid is an important marker to differentiate between strains of tEPEC and aEPEC.14,27,30
Among overall isolates of EPEC analyzed in this study, more than 80% were resistant to at least one of the commonly prescribed antibiotics. These isolates were more often resistant to the first-line antibiotics such as ampicillin, tetracycline, nalidixic acid, and sulfamethoxazole-trimethoprim, and this might indicate the misuse of prescribed antibiotics in some countries for the treatment of enteric infections caused by Gram-negative bacteria.35 Moreover, 70% of EPEC isolates in this study (Figure 2) were classified and identified as MDR based on the most frequent method used to characterize bacterial strains as MDR if they are resistant to three or more antimicrobial agents.46–48 Similarly, a hospital-based study from China and Mexico reported a high prevalence of drug resistance among aEPEC isolated from children and adult patients with acute diarrhea, and the majority of strains were resistant to cephalosporins, fluoroquinolones and trimethoprim-sulfamethoxazole.35,37,49,50
The analysis of ERIC fingerprinting patterns results by using Dice coefficient and UPGMA revealed that the genetic similarity relationship rate among EPEC isolates rate was 81.7% when clustered with the similarity between 90 to 100% (Figure 2). Whereas 11 (18.3%) isolates of EPEC had less than 90% similarity, as presented in ERIC dendrogram analysis (Figure 2). Our study agreed with a study conducted elsewhere,51 showing that strains with a similarity rate below 90% were considered genetically unrelated. The results of ERIC fingerprinting patterns generated using the ERIC2 primer in this study have indicated the potential and usefulness of ERIC-PCR as a molecular typing tool method for epidemiological investigation and genetic analysis of EPEC isolates. Overall, ERIC-PCR DNA fingerprinting has good discriminatory power and revealed genetic diversity among EPEC isolates investigated from humans in Eastern Province of Saudi Arabia.
Conclusion
To our knowledge, this is the first study to demonstrate detailed characterization of human EPEC isolates in Saudi Arabia. Genotyping for virulence gene signatures of EPEC revealed that most of the isolates were positive for eae gene and very few isolates were concurrently positive for eae and bfp genes. The majority of EPEC infections which occurred in Eastern Province of Saudi Arabia between 2013 and 2014 were caused by subtype “aEPEC” and these findings are in agreement with global trends of EPEC human infections. A high antibiotic resistance rate was reported for first-line antibiotics, such as ampicillin (63.3%), tetracycline (60%), nalidixic acid (55%), trimethoprim-sulfamethoxazole (48.3%), noroxin (36.7%), and ciprofloxacin (35%). The majority of EPEC isolates were MDR and aEPEC with the highest proportion of this feature of MDR. The results of the ERIC-PCR revealed high genetic similarity among the EPEC strains and demonstrated that ERIC-PCR is a rapid method for studying the clonal variation within EPEC associated with human gastroenteritis. This study recommends further future molecular epidemiology studies of EPEC to understand the epidemiology of EPEC by comparing EPEC isolates of human and food origin to trace the sources of human infection in Saudi Arabia.
Abbreviations
DEC; diarrheagenic E. coli, LPS; lipopolysaccharide, LEE; locus of enterocyte effacement, EAF; E. coli adherence factor, eae; E. coli attaching and effacing, bfp; bundle-forming pilus, stx; Shiga toxin, EAST-1; enteroaggregative heat stable enterotoxin 1, EPEC; enteropathogenic E. coli, aEPEC; atypical EPEC, tEPEC; typical EPEC, ERIC-PCR; enterobacterial repetitive intergenic consensus, PCR; polymerase chain reaction, MDR; multi-drug resistant, KFHU; King Fahd Hospital of the University, CLSI; Clinical and Laboratory Standards Institute, LB; Luria Bertani, AM; Ampicillin, ATM; Aztreonam, AUG; Augmentin, CTX; Cefotaxime, C; Chloramphenicol, KF; Cephalothin, CIP; Ciprofloxacin, FEP; Cefepime, GM; Gentamicin, NA; Nalidixic acid, NOR; Noroxin, PRL; Piperacillin, S; Streptomycin, SXT; Trimethoprim-sulfamethoxazole, TE; Tetracycline, S; sensitive, I; intermediate, R; resistant, CLSI; Clinical Laboratory Standards Institute, ATCC; American type culture collection, UPGMA; unweighted pair group method with arithmetic average.
Ethical Approval
This study was approved by the Institutional Review Board (IRB) of Imam Abdulrahman Bin Faisal University (IRB approval number: IRB – 2022 −03 −220).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. The infant microbiome development: mom matters. Trends Mol Med. 2015;21(2):109–117. doi:10.1016/j.molmed.2014.12.002
2. Sweeney NJ, Klemm P, McCormick BA, et al. The Escherichia coli K-12 gntP gene allows E. coli F-18 to occupy a distinct nutritional niche in the streptomycin-treated mouse large intestine. Infect Immun. 1996;64(9):3497–3503. doi:10.1128/iai.64.9.3497-3503.1996
3. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2(2):123–140.
4. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. doi:10.1016/S0140-6736(13)60844-2
5. Mare AD, Ciurea CN, Man A, et al. Enteropathogenic Escherichia coli - A summary of the literature. Gastroenterol Insights. 2021;12(1):28–40. doi:10.3390/GASTROENT12010004
6. Bischoff C, Lüthy J, Altwegg M, Baggi F. Rapid detection of diarrheagenic E. coli by real-time PCR. J Microbiol Methods. 2005;61(3):335–341. doi:10.1016/j.mimet.2004.12.007
7. Croxen MA, Law RJ, Scholz R, Keeney KM, Wlodarska M, Finlay BB. Recent advances in understanding enteric pathogenic. Escherichia Coli Clin Microbiol Rev. 2013;26(4):822–880.
8. Donnenberg MS, Whittam TS. Pathogenesis and evolution of virulence in enteropathogenic and enterohemorrhagic Escherichia coli. J Clin Invest. 2001;107(5):539–548. doi:10.1172/JCI12404
9. Pakbin B, Brück WM, Rossen JWA. Virulence factors of enteric pathogenic Escherichia coli: a review. Int J Mol Sci. 2021;22(18):9922. doi:10.3390/ijms22189922
10. McDaniel TK, Jarvis KG, Donnenberg MS, Kaper JB. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci. 1995;92(5):1664–1668. doi:10.1073/pnas.92.5.1664
11. Jerse AE, Yu J, Tall BD, Kaper JB. A genetic locus of enteropathogenic Escherichia coli necessary for the production of attaching and effacing lesions on tissue culture cells. Proc Natl Acad Sci. 1990;87(20):7839–7843.
12. Tobe T, Hayashi T, Han C-G, Schoolnik GK, Ohtsubo E, Sasakawa C. Complete DNA sequence and structural analysis of the enteropathogenic Escherichia coli adherence factor plasmid. Infect Immun. 1999;67(10):5455–5462.
13. Giron JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254(5032):710–713.
14. Trabulsi LR, Keller R, Tardelli Gomes TA. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8(5):508–513. doi:10.3201/eid0805.010385
15. Al-Tawfiq JA. Increasing antibiotic resistance among isolates of Escherichia coli recovered from inpatients and outpatients in a Saudi Arabian hospital. Infect Control Hosp Epidemiol. 2006;27(7):748–753. doi:10.1017/S0195941700044933
16. Abo-Amer AE, Shobrak MY, Altalhi AD. Isolation and antimicrobial resistance of Escherichia coli isolated from farm chickens in Taif, Saudi Arabia. J Glob Antimicrob Resist. 2018;15:65–68.
17. Mea DK, Stewardson AJ, Will HS. 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016;13(11):e1002184.
18. Abubakar I, Gautret P, Brunette GW, et al. Global perspectives for prevention of infectious diseases associated with mass gatherings. Lancet Infect Dis. 2012;12(1):66–74. doi:10.1016/S1473-3099(11)70246-8
19. Sow D, Dogue F, Edouard S, et al. Acquisition of enteric pathogens by pilgrims during the 2016 Hajj pilgrimage: a prospective cohort study. Travel Med Infect Dis. 2018;25:26–30. doi:10.1016/j.tmaid.2018.05.017
20. Vidal M, Kruger E, Durán C, et al. Single multiplex PCR assay to identify simultaneously the six categories of diarrheagenic Escherichia coli associated with enteric infections. J Clin Microbiol. 2005;43(10):5362–5365. doi:10.1128/JCM.43.10.5362-5365.2005
21. Müller D, Greune L, Heusipp G, et al. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl Environ Microbiol. 2007;73(10):3380–3390. doi:10.1128/AEM.02855-06
22. CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing. Thirty-First Information Supplement M100. CLSI (Clinical and Laboratory Standards Institute); 2021.
23. Hulton CSJ, Higgins CF, Sharp PM. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991;5(4):825–834.
24. Versalovic J, Koeuth T, Lupski R. Distribution of repetitive DNA sequences in eubacteria and application to finerpriting of bacterial genomes. Nucleic Acids Res. 1991;19(24):6823–6831. doi:10.1093/nar/19.24.6823
25. Heras J, Domínguez C, Mata E, et al. GelJ - a tool for analyzing DNA fingerprint gel images. BMC Bioinform. 2015;16(1):1–8. doi:10.1186/s12859-015-0703-0
26. Ø H, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4(1):9.
27. Carlino MJ, Kralicek SE, Santiago SA, Sitaraman LM, Harrington AT, Hecht GA. Quantitative analysis and virulence phenotypes of atypical enteropathogenic Escherichia coli (EPEC) acquired from diarrheal stool samples from a Midwest US hospital. Gut Microbes. 2020;12(1):1824562. doi:10.1080/19490976.2020.1824562
28. Lee JB, Kim SK, Yoon JW. Pathophysiology of enteropathogenic Escherichia coli during a host infection. J Vet Sci. 2022;23(2). doi:10.4142/JVS.21160
29. Gomes TAT, Irino K, Girão DM, et al. Emerging enteropathogenic Escherichia coli strains? Emerg Infect Dis. 2004;10(10):1851.
30. Hernandes RT, Elias WP, Vieira MAM, Gomes TAT. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol Lett. 2009;297(2):137–149.
31. Elhadi N, Aljindan R, Alsamman K, Alomar A, Aljeldah M. Antibiotic resistance and molecular characterization of enteroaggregative Escherichia coli isolated from patients with diarrhea in the Eastern Province of Saudi Arabia. Heliyon. 2020;6(4):e03721.
32. Nakhjavani FA, Emaneini M, Hosseini H, et al. Molecular analysis of typical and atypical enteropathogenic Escherichia coli (EPEC) isolated from children with diarrhoea. J Med Microbiol. 2013;62(2):191–195. doi:10.1099/jmm.0.046516-0
33. Foster MA, Iqbal J, Zhang C, et al. Enteropathogenic and enteroaggregative E. coli in stools of children with acute gastroenteritis in Davidson County, Tennessee. Diagn Microbiol Infect Dis. 2015;83(3):319–324. doi:10.1016/j.diagmicrobio.2015.07.016
34. Hu J, Torres AG. Enteropathogenic Escherichia coli: foe or innocent bystander? Clin Microbiol Infect. 2015;21(8):729–734.
35. Canizalez-Roman A, Flores-Villaseñor HM, Gonzalez-Nuñez E, et al. Surveillance of diarrheagenic Escherichia coli strains isolated from diarrhea cases from children, adults and elderly at Northwest of Mexico. Front Microbiol. 2016;7:1924.
36. Natarajan M, Kumar D, Mandal J, Biswal N, Stephen S. A study of virulence and antimicrobial resistance pattern in diarrhoeagenic Escherichia coli isolated from diarrhoeal stool specimens from children and adults in a tertiary hospital, Puducherry, India. J Heal Popul Nutr. 2018;37(1):1–11.
37. Zhou Y, Zhu X, Hou H, et al. Characteristics of diarrheagenic Escherichia coli among children under 5 years of age with acute diarrhea: a hospital based study. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-017-2936-1
38. Zhou SX, Wang LP, Liu MY, et al. Characteristics of diarrheagenic Escherichia coli among patients with acute diarrhea in China, 2009‒2018. J Infect. 2021;83(4):424–432. doi:10.1016/j.jinf.2021.08.001
39. Levine MM, Prado V, Robins-Browne R, et al. Use of DNA probes and HEp-2 cell adherence assay to detect diarrheagenic Escherichia coli. J Infect Dis. 1988;158(1):224–228.
40. Savarino SJ, McVeigh A, Watson J, et al. Enteroaggregative Escherichia coli heat-stable enterotoxin is not restricted to enteroaggregative E. coli. J Infect Dis. 1996;173(4):1019–1022.
41. de Sousa CP, Dubreuil JD. Distribution and expression of the astA gene (EAST1 toxin) in Escherichia coli and Salmonella. Int J Med Microbiol. 2001;291(1):15–20.
42. Konno T, Yatsuyanagi J, Saito S. Virulence gene profiling of enteroaggregative Escherichia coli heat-stable enterotoxin 1-harboring E. coli (EAST1EC) derived from sporadic diarrheal patients. FEMS Immunol Med Microbiol. 2012;64(3):314–320.
43. Dubreuil JD. EAST1 toxin: an enigmatic molecule associated with sporadic episodes of diarrhea in humans and animals. J Microbiol. 2019;57(7):541–549. doi:10.1007/s12275-019-8651-4
44. Vila J, Gene A, Vargas M, Gascon J, Latorre C, De Anta MTJ. A case-control study of diarrhoea in children caused by Escherichia coli producing heat-stable enterotoxin (EAST-1). J Med Microbiol. 1998;47(10):889–891. doi:10.1099/00222615-47-10-889
45. Rahman MM, Ahmed P, Kar A, et al. Prevalence, Antimicrobial Resistance, and Pathogenic Potential of Enterotoxigenic and Enteropathogenic Escherichia coli Associated with Acute Diarrheal Patients in Tangail, Bangladesh. Foodborne Pathog Dis. 2020;17(7):434–439. doi:10.1089/fpd.2019.2741
46. Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55(12):1619–1629. doi:10.1099/jmm.0.46747-0
47. Kallen AJ, Hidron AI, Patel J, Srinivasan A. Multidrug resistance among gram-negative pathogens that caused healthcare-associated infections reported to the National Healthcare Safety Network, 2006–2008. Infect Control Hosp Epidemiol. 2010;31(5):528–531. doi:10.1086/652152
48. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi:10.1111/j.1469-0691.2011.03570.x
49. Yang CM, Lin MF, Lin CH, Huang YT, Hsu CT, Liou ML. Characterization of antimicrobial resistance patterns and integrons in human fecal Escherichia coli in Taiwan. Jpn J Infect Dis. 2009;62(3):177–181.
50. Silva SS, Monfardini MV, Scaletsky ICA. Large plasmids encoding antibiotic resistance and localized-like adherence in atypical enteropathogenic Escherichia coli strains. BMC Microbiol. 2020;20(1):1–8. doi:10.1186/s12866-020-01809-4
51. Szczuka E, Kaznowski A. Typing of clinical and environmental Aeromonas sp. strains by random amplified polymorphic DNA PCR, repetitive extragenic palindromic PCR, and enterobacterial repetitive intergenic consensus sequence PCR. J Clin Microbiol. 2004;42(1):220–228. doi:10.1128/JCM.42.1.220-228.2004
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.