Back to Journals » Journal of Multidisciplinary Healthcare » Volume 14
Virtual Multidisciplinary Review of a Complex Case Using a Digital Clinical Decision Support Tool to Improve Workflow Efficiency
Authors Soo KC, Al Jajeh I, Quah R , Seah HKB, Soon S, Walker E
Received 19 February 2021
Accepted for publication 30 April 2021
Published 20 May 2021 Volume 2021:14 Pages 1149—1158
DOI https://doi.org/10.2147/JMDH.S307470
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Khee Chee Soo,1 Issam Al Jajeh,2 Raymond Quah,3 Hoe Kuen Brandon Seah,4 Sharon Soon,4 Espen Walker4
1General Surgery Department, Farrer Park Hospital, Singapore; 2Department of Pathology, Farrer Park Hospital, Singapore; 3Department of Diagnostic Radiology, Farrer Park Hospital, Singapore; 4Roche Diagnostics Asia Pacific, Ltd, Singapore
Correspondence: Espen Walker
Roche Diagnostics Asia Pacific, Ltd, 8 Kallang Avenue #10-01/09 Aperia Tower 1, 339509, Singapore
Tel +65 8428 0386
Email [email protected]
Objective: Integration of distinct clinical perspectives in multi-disciplinary tumor board meetings is critical to determine optimal patient care. Digital tools can support the data consolidation needed for meeting preparation and data sharing during complex case reviews. In this paper, we assessed the value of a clinical decision support tool on workflow efficiency and conducting a complex case review of a dermatofibrosarcoma protuberans (DFSP) tumor.
Methods: Case presentation was performed by each unique clinical specialty that had relevant information about the patient; an oncologist, a pathologist, and a radiologist. Virtual discussion was completed online with case presentation and documentation with NAVIFY Tumor Board. Workflow efficiency assessment was done through interviews and observation of the # of steps across different team members involved in preparing and conducting cancer multidisciplinary team (MDT) meetings before and after the implementation of the NAVIFY Tumor Board solution.
Results: Case review consisted of surgical and therapeutic intervention history, distinct histological and sequencing patterns representative of DFSP, with radiological review to determine areas for surgical intervention. Consolidation of clinical input led to a recommendation of a formal external hemipelvectomy with potential chemotherapy. Workflow assessment demonstrated a 46% total reduction in the # of steps for meeting preparation (from 69 to 37), with specific changes based on role: data manager (33 to 15), pathologist (26 to 13), radiologist (no change), and logistics (5 to 4). There was a 31% total reduction in the # of steps for conducting the meeting (from 51 to 35).
Conclusion: Utilizing a digital clinical decision support tool helped to consolidate patient data and improved case presentation through workflow efficiency. This allowed for improved interdisciplinary discussion on a complex DFSP case and supported the determination of a clinical decision.
Keywords: multidisciplinary team meetings, clinical decision support, digital tool, workflow efficiency, virtual MDT
Introduction
Making the right clinical decision for cancer treatment is critical for optimal patient outcomes. With the complexity of factors involved in each patient’s case, it is necessary to be able to review the massive amount of information available in a targeted manner relevant to the patient. Cancer multidisciplinary team (MDT) meetings bring together experts in each specialty to contribute to the clinical decision making.1,2 With a blend of pathology, radiology, and surgical expertise available, the key clinicians can provide their input to drive the best decision for the patient and are now considered best practice in clinical care.2,3
Distinct clinical settings across public and private care settings require different levels of involvement from the primary care provider. In an academic medical center, there are often residents or junior physicians to assist in the preparation of case material. Some institutions employ nurse navigators that are responsible for collating and bringing together case information for physician review, bringing increased efficiency and better service integration to patient management.4,5 In private and non-academic settings, the primary care provider must bring together all the case information, in addition to any relevant publications or trials that may relate to the case to prepare a presentation for the MDT meeting. MDT meetings are limited by the capacity of the information to be collated and presented well.6 There is a need for tools that can allow easy case preparation and seamless presentation of cases to ensure appropriate diagnostic and therapeutic options are discussed and implemented for each patient.
Recent advances in technology have enabled quicker access to patient information and potential treatment options. Navigating the massive amount of data available can be challenging. Selection of the patients to include in an MDT must be prioritized to those that have a complex history and need for a variety of expertise.7 It is necessary to bring the relevant information quickly to the clinician to make the best decision for the patient. Virtual MDT meetings have been emerging as essential to deliver optimal healthcare for complex cases in a remote setting,8 with examples of clinicians stating that virtual MDTs provide the same standard of care as face-to-face MDTs.9
Digital MDT tools, such as NAVIFY Tumor Board can support operational details and improve efficiency in MDT preparation.5,10,11 With the recent need for virtual connectivity following the covid-19 pandemic, digital engagement has become necessary to bring clinicians together online. Transition of the traditional MDT into a “Smart-MDT” has allowed continuity of care for oncology patients,12 with clinicians easily adapting to the virtual setting.11
In this publication, we describe the implementation of NAVIFY Tumor Board into the Farrer Park Hospital MDT meeting and the clinical application on a complex case reviewed during a virtual MDT meeting. We also demonstrate improvements to the workflow of the MDT meetings using this digital clinical decision support tool. Despite the remote settings imposed due to the covid-19 pandemic, the clinicians were able to discuss the case, reach a decision with confidence and suggest treatment.
Materials and Methods
Patient Case Review Process in MDT
Case review at the Farrer Park Hospital MDT incorporated various specialties and comprehensive review of relevant information. It started with the oncologist (surgical, radiation or medical), presenting the patient clinical summary and previous treatments. The radiologist then shared the imaging results and potential changes in tumor size or shape over time in response to previous treatments. This included each lesion of interest, and assessment whether it has responded to previous treatments. Details assessed include the extent of the tumor and tumor spread, whether by direct invasion of adjacent structures, lymphatic spread, metastatic deposits and perineural extension. The pathologist then reviewed the histological and molecular findings to describe the recurrent tumor’s histological type and grade to relate findings to the first resection and intervening recurrences. The gross photographs of various resection specimens were presented to demonstrate the extent of disease and to address the differential diagnosis. Finally, the team discussed relevant publications and potential treatment options, aligned on and documented the decisions and next steps (Figure 1).
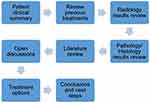 |
Figure 1 Flowchart of Multidisciplinary (MDT) meeting at Farrer Park Hospital. |
Digital MDT Process
The digital MDT is similar to the standard case review with some exceptions. To comply with social distancing regulations due to covid-19 pandemic in Singapore and minimize face-to-face interactions, physicians dialed in to a video call using Zoom Enterprise software (San Jose, California, USA) to discuss the case. As each presenter must share their unique perspective of the case through different tools available, various systems were utilized to share each data point to the MDT group. For case presentation, NAVIFY Tumor Board (Roche Diagnostics Information Systems, Belmont, California, USA) presentation mode was used to display patient case history information, histopathology images, and immunohistochemistry results via screen sharing. The NAVIFY Tumor Board solution is a cloud-based workflow product that can help facilitate MDT meetings by displaying relevant clinical data per patient. It supports the preparation, presentation and documentation of MDT meeting by displaying a holistic patient dashboard for oncology teams to make clinical decisions.5,10,11 The radiologist then took over screen sharing to display MRI images available on his computer. Each attending physician was able to provide input on the various aspects of the case, utilizing various hospital data management systems viewed sequentially. Documentation of the clinical decision made was done in NAVIFY Tumor Board for the patient case.
Workflow Assessment of Digital Clinical Decision Support Tool
Assessment on the MDT meeting workflow was done before and after implementation of NAVIFY Tumor Board to determine how this digital tool could improve efficiency. Interviews were completed with members of the MDT meeting including those setting up the meeting as well as clinicians conducting the meeting to gather feedback on how a digital clinical decision support tool may improve workflow efficiency. Informants were selected based on their involvement in preparation of conduct of the MDT meeting. Individuals supporting the logistics of the meeting included the data manager, medical affairs/quality department manager, clinical assistant, and pathology lab assistant. Clinicians interviewed were the pathologist, radiologist, and surgical oncologist.
By reviewing the MDT workflow, identification of the process steps involved in the preparation and conduction of the MDT meeting was done. Each action completed by the various team members (such as scheduling a meeting or preparing a slide to present) was designated as a distinct step in the process.
Observations of the MDT meetings at FPH hospital were completed to review any changes to the workflow before and after implementation of NAVIFY Tumor Board. Various aspects of the meeting were reviewed, including qualitative assessment of the effectiveness of the digital clinical decision support tool to support workload distribution and patient file compilation. Documentation of the number of process steps required to prepare and conduct the MDT meeting was done to quantify the effectiveness of the digital clinical decision support tool.
Results
Oncologist Case Overview
The oncologist shared the patient background and history of treatments with the dashboard view in NAVIFY Tumor Board that included the relevant information for the virtual discussion. A 48-year-old female with a history of multiple surgeries starting in 2009 for a right groin tumor was discussed. At the first surgery, the groin tumor was identified as a dermatofibrosarcoma protuberans (DFSP). At this time, the margins were determined to be negative (3mm). In 2014, recurrence was noted locally, and the second surgery was done. The surgical margins were involved, and at the third surgery, negative margins were finally obtained.
In 2016, on CT surveillance a soft tissue mass was noted just above the previous surgical site. After resection, the tumor was reported as a high-grade sarcoma. Radical adjuvant radiation therapy was given post operatively. In 2017, another soft tissue mass was noted adjacent to the psoas muscle, above the site of the pelvic resection. Further resection (5th) was done. Clear surgical margins were obtained but follow-up CT scan showed further pelvic recurrence. This was treated with pazopanib, temozolomide then bevacizumab respectively. However, tumor was noted to be progressing on radiological surveillance.
Next Generation Sequencing (NGS) testing done with Foundation One CDx (Foundation Medicine, Cambridge, Massachusetts, USA) and the report results were viewed within the NAVIFY case specific dashboard under biomarker status. The report showed a COL1A1-PDGFB gene fusion supporting a diagnosis of DFSP. Imatinib was started and over the year, the dose had to be escalated as there was radiological evidence of tumor progression. Her sixth surgery was in 2019 (ten years after initial diagnosis). This time the psoas muscle was resected on bloc with the right colon. Latest CT scan however had shown pelvic recurrence with tumor involving the pelvic acetabulum and bladder.
Radiologist Case Review
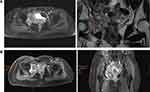
Assessment of key organs and vascularisation surrounding the surgical site is critical for surgical planning. Preservation of specific structures is vital to retaining function, whether for pre-operative surgical planning, or in pre-radiation treatment planning. Medical imaging allows for non-invasive viewing of these structures. The MRI image of 1 June 2020 was presented to the MDT attendees from the radiologist’s computer using the hospital’s image viewing system. As this was a virtual meeting, all attendees could easily view the image on their computer. It demonstrated a heterogeneously enhancing mass arising from the right acetabulum, extending into the right hip joint (Figure 2B). Compared to the earlier MRI of 4 January 2020 (Figure 2A), this mass demonstrated interval increase in size. The mass was close to the right external and internal iliac arteries and veins, as well as closely abutted the urinary bladder. While there may be a need to partially resect the urinary bladder, this may be repaired, without significant loss of function. As such, the possibility of limb preservation hinges on being able to achieve clear surgical margins while being able to preserve the right external iliac arteries and veins.
Pathologist Case Review
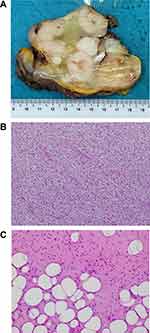
Utilizing presentation mode in NAVIFY Tumor Board, the pathologist was able to display the histological images along with customized text to emphasize areas of interest during the virtual online meeting. The gross anatomical findings include a recurrent dominant pelvic mass, spatially nearest to the primary groin tumor, that consisted of fish-flesh-like nodules set in a fibromyxoid lipomatous tumor-like mass (Figure 3). The peritoneal lesions comprised small nodules and plaques with rather similar composition.
The tumor portrayed several architectural profiles mirroring its variegated gross appearance. The pelvic mass fish-flesh-like nodules comprised spindle cell sarcoma with a lipomatous background and low-grade, atypical lipomatous patterns (Figure 3A). The colonic peritoneal nodules comprised variegated tumor with circumscribed nodule and infiltrating areas of spindle cell sarcoma and sclerosing lipomatous tumor (Figure 3B). Such morphology in a retroperitoneal sarcoma would be fitting of well-differentiated liposarcoma, sclerosing lipoma-like, with areas of de-differentiated liposarcoma with high-grade spindle cell sarcoma and low-grade dermatofibrosarcoma (DFSP)-like patterns with pericellular fat infiltration (Figure 3C).13 The primary tumor had a morphology of slender spindle cells with cart-wheel arrangement and was the basis for rendering a diagnosis of DFSP.
This appears to be a rare case of DFSP of skin and soft tissues with multiple local recurrences and resections. Spatial extension into adjoining intra-abdominal soft tissues and peritoneum; with morphological transformation in intermediate grade spindle cell sarcoma. The most fitting tumor type of intra-abdominal recurrence is DFSP, based on pericellular fat infiltration, agreeable immunohistochemistry and typical COL1A1-PDGFB gene fusion.
MDT Meeting Outcomes
The MDT meeting members discussed the care after the respective presentations. As the tumor had shown resistance to imatinib after 1 year of treatment, it was concluded that the tumor would now be quite resistant to further chemotherapy. With no other targeted therapy recommended, it was decided further surgery was the treatment of choice. However, there was a recognition that adequate surgical margins might be difficult to obtain, especially as the tumor had extended to the region adjacent to the lower pole of the right kidney and intra-peritoneally into the pelvis including into the wall of the urinary bladder.
While it may be possible to do an internal hemipelvectomy, sparing the lower limb, a formal external hemipelvectomy would be the soundest option from the oncological perspective. These decisions were documented in the patient’s case in NAVIFY Tumor Board. The findings and recommendations were discussed with the patient; however, she did not wish further surgery so instead elected for palliative chemotherapy.
Workflow Assessment Results
Utilisation of a digital clinical decision support tool in a virtual MDT can allow for improved cross functional discussion and efficient case review to allow optimal clinical decisions to be made. Assessment of the MDT meeting workflow at Farrer Park identified potential areas that could be improved with the preparation and conduction of the MDT meeting at Farrer Park Hospital, as well as steps that had potential for optimization with a clinical decision support digital tool. Prior to the implementation of NAVIFY Tumor Board, preparation for the meeting included manual data collection and preparation activities, such as patient file collection and pathology image collation. Conducting the MDT meetings, there was difficulty in standardising tumor board files across different laboratory systems and it required coordination across many different departments.
Implementation of NAVIFY Tumor Board was done for the MDT meeting at Farrer Park Hospital for both in person and virtual meetings. During the transition to using NAVIFY Tumor Board, there was an increase in the work required to prepare and conduct the meeting, including learning how to use the system and manual input of data. After the transition period however, workload was re-distributed across individuals and preparation was streamlined. Having a single platform allowed for a common data repository and documentation of next steps for patients.
For meeting preparation, the total # of process steps went from 69 before, 72 during the transition, to 37 after implementation (Figure 4A). Specific roles in the MDT meeting had different changes in the # of process steps for preparation. Data manager went from 33 steps before to 15 steps after implementation, pathologist went from 26 steps before to 13 steps after, radiologist remained the same with 5 steps both before and after, while logistics went from 5 steps before to 4 steps after (Figure 4B).
For conducting the meeting, the # steps went from 51 before, 52 during the transition, to 35 after implementation. There was a temporary increase in the # of steps during the transition due to change management that was resolved. Overall, there was a 46% reduction in preparation, and 31% reduction in conducting the MDT meeting (Figure 4A).
Discussion
Continuity of cancer care is critical and cannot be interrupted based on restrictions in social interaction. Virtual MDTs have emerged as a solution to allow multidisciplinary discussion and collaborative decision-making in the age of covid-19.9 In this study, we describe an improved efficiency in the MDT meeting at Farrer Park Hospital with the implementation of the NAVIFY Tumor Board solution. Specific roles, such as data manager and pathologist saw an improvement in the preparation process as compared to other roles. Utilizing this digital clinical decision support tool, the oncologist, pathologist, and radiologist were able to share and discuss their findings regarding a complex DFSP case and propose a surgical intervention.
At daily encounters with cancer patients, new diagnoses are made, responses to treatment are noted and recurrences are detected. MDT meetings provide physicians an opportunity to present such patients for discussion with their peers.14 Inputs and insights from other clinicians provide great benefit for patients. However, there is a need to improve MDT meetings in order to keep up with the increasing complexity of healthcare information15–17 and maintain efficiency with patient care management.18
When patients are discussed at MDT meetings, their clinical decisions are protected from the vagaries of individual professional preferences. Appropriate clinical trials are suggested that can allow otherwise hopeless patients the opportunity to receive the latest medical advances. MDT meetings also help to cross-educate physicians19 and trainees20 involved and are the cornerstone of sound cancer medicine.
Complexity of cancer biology and therapies has gone beyond the capacity for an individual to keep up to date with all the information and options available. Clinical decision support tools, such as NAVIFY Tumor Board, aim to support clinicians to review the comprehensive diagnostic data, patient history, and additional information needed for a clinical decision.11 NAVIFY has the capacity to manage meeting logistics as well as improve data collection and review. With the potential to view relevant publications, clinical trials, and treatment guidelines that match the patient’s profile, this can help streamline overall patient review. Documenting decisions made and next steps with a clinical decision support tool like NAVIFY can improve accessibility to patient information for future monitoring.
As the diagnostic data available to review patients increase, tools to organize and support clinicians to determine what is clinically relevant are needed.6 Workflow assessments of the various processes that take place in the preparation and conduct of an MDT meeting can help make them more efficient. With the increased use of virtual MDTs,8 there has been an increase in MDT functionality that allows for improved patient care.
At Farrer Park Hospital, the use of NAVIFY Tumor Board tool has allowed for an improvement in the workflow efficiency by reducing the # of steps needed in the preparation and conduct of MDT meeting. However, as the tool was being implemented, there was an increase in the # of steps due to challenges in adopting a new technology into the standard clinical workflow. As seen from the pre and post data, this increase in # of steps was only temporary, largely attributed to the challenges of a change management process in adopting a new technology in the MDT meeting workflow. These challenges were quickly resolved, resulting in a final reduction in steps needed to prepare and conduct the MDT meeting.
Tracking the steps of the distinct roles involved in meeting preparation gave insights into who may benefit most from a digital clinical decision support tool. In our assessment, the data manager and pathologist saw their steps cut in half with the use of a digital tool, while the radiologist and logistics function did not see any change. These results are in line with studies that show digital tools can improve overall preparation, but may be different across multiple users.5 However, in a pilot study for NAVIFY Tumor Board;10 both pathologist and radiologist preparation were not affected by a digital preparation tool. The improvement in pathologist preparation we observed may be due to the pathologist in our study using the NAVIFY Tumor Board presentation mode to discuss the DFSP case, as opposed to a different image display tool as the radiologist did. Both the radiologist and pathologist used independent viewing tools in the Krupinski study.
While we were able to separate out the specific roles that benefitted from a digital clinical decision support tool for meeting preparation, we did not stratify the role type when collecting the # of steps for meeting conduct. Further studies would be needed to determine which users would benefit from a digital clinical decision support tool in the conduct of an MDT.
For the case of DFSP reviewed, it was critical to have the various experts available to provide their input. The oncologist reviewed the patient history, including the therapy and surgeries done with what options were available. The pathologist provided histological assessment and comparison to previous case history. The radiologist described the extent of the tumor, and potential regions of resection needed. All together, these experts suggested a viable surgical option for this patient using a digital clinical decision support tool to enable better cross-functional collaboration. Despite the thorough historical review, pathologist testing, and radiological examination pointing to a formal external hemipelvectomy as the best option, the patient decided another surgery was not her preference. Palliative chemotherapy was the patient’s decision despite clinical recommendation. This demonstrates the various influencing factors that go into clinical decision, with the patient being the final decision maker.
The consideration of including the patient in MDT meetings must take into account various factors. These include the healthcare provider’s view and what they are willing to discuss in front of the patient,7 as well as how much the patient may contribute or hinder the conversation. A potential benefit to including the patient would be to allow them to ask questions or share their treatment preferences.21 Knowing that surgical intervention was not preferred by the DFSP patient may have influenced the treatment suggest outcomes from the clinicians at the MDT meeting.
This study continues to build the collection of evidence demonstrating the value of digital clinical decision support tools in MDTs and virtual engagement. The case study described is an example of how complex case can benefit from various clinical expertise to understand the patient background and clinical findings. As virtual MDTs become more and more common9 and healthcare delivery is happening from different locations,22 a deeper understanding of the benefits and limitations of the digital tools and processes involved is needed.
Conclusion
As digitization becomes ingrained in the healthcare processes, we need to determine what tools can bring value to impact clinical care as well as the process to adopt these tools. In identifying and adopting the right clinical decision support tools, we should always maintain the emphasis on the patient pathways and decisions that can improve patient outcomes. With a complex DFSP case as described here, it was valuable to bring together insights from the oncologist, pathologist, and radiologist to provide optimal care planning virtually, especially with the social distancing regulations from the covid-19 pandemic. As such, clinical decision support digital tools, like NAVIFY Tumor Board, were beneficial in allowing for continuity of cancer care management at Farrer Park Hospital.
Abbreviations
MDT, multidisciplinary tumor board; DFSP, dermatofibrosarcoma protuberans; FPH, Farrer Park Hospital; MRI, magnetic resonance imaging; NGS, next generation sequencing.
Ethical Approval and Consent to Participate
Ethical approval and consent to review cases has been completed by the ethics committee at Farrer Park Hospital. Study data were stored safely, personal data were removed and the identities of participants remained confidential.
Consent to Publish
Consent to publish case information has been obtained with written informed consent.
Acknowledgments
The authors would like to acknowledge the Medical Affairs team at Farrer Park Hospital for the support and planning of the multidisciplinary team meetings at Farrer Park Hospital. We would also like to acknowledge the support of the NAVIFY clinical decision support team and the Roche Healthcare Consulting team in the evaluation and processing of data for the workflow assessment at Farrer Park Hospital.
Author Contributions
All authors have contributed to this manuscript according to the IMCJE authorship guidelines and have been involved in the primary five criteria for authorship: (1) Made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. (2) Have drafted or written, or substantially revised or critically reviewed the article. (3) Have agreed on the journal to which the article will be submitted. (4) Reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. (5) Agree to take responsibility and be accountable for the contents of the article.
Funding
This work was supported financially by Roche Diagnostics, Ltd.
Disclosure
Khee Chee Soo has received honorarium and held consultant and advisory roles for Roche. Hoe Kuen Brandon Seah, Sharon Soon, and Espen Walker are employees of Roche Diagnostics and have stock and ownership interests in Roche. The authors report no other conflicts of interest in this work.
References
1. Lamb BW, Green JS, Benn J, Brown KF, Vincent CA, Sevdalis N. Improving decision making in multidisciplinary tumor boards: prospective longitudinal evaluation of a multicomponent intervention for 1421 patients. J Am Coll Surg. 2013;217(3):412–420. doi:10.1016/j.jamcollsurg.2013.04.035
2. Specchia ML, Frisicale EM, Carini E, et al. The impact of tumor board on cancer care: evidence from an umbrella review. BMC Health Serv Res. 2020;20(73).
3. Ruhstaller T, Roe H, Thurlimann B, Nicoll JJ. The multidisciplinary meeting: an indispensable aid to communication between different specialities. Eur J Cancer. 2006;42(15):2459–2462. doi:10.1016/j.ejca.2006.03.034
4. McMurray A, Cooper B. The nurse navigator: an evolving model of care. Collegian. 2017;24(2):205–212. doi:10.1016/j.colegn.2016.01.002
5. Hammer RD, Fowler D, Sheets LR, Siadimas A, Guo C, Prime MS. Digital tumor board solutions have significant impact on case preparation. JCO Clin Cancer Inform. 2020;4:757–768. doi:10.1200/CCI.20.00029
6. Berardi R, Morgese F, Rinaldi S, et al. Benefits and limitations of a multidisciplinary approach in cancer patient management. Cancer Manag Res. 2020;12:9363–9374. doi:10.2147/CMAR.S220976
7. Heuser C, Diekmann A, Schellenberger B, et al. Patient participation in multidisciplinary tumor conferences from the providers’ perspective: is it feasible in routine cancer care? J Multidiscip Healthc. 2020;13:1729–1739. doi:10.2147/JMDH.S283166
8. Aston SJ, Reade S, Petersen B, Ward C, Duffy A, Nsutebu E. Extraordinary virtual multidisciplinary team meetings – a novel forum for the coordinated care of patients with complex conditions within a secondary care setting. Future Healthc J. 2018;5(3):218–223. doi:10.7861/futurehosp.5-3-218
9. Sidpra J, Chhabda S, Gaier C, Alwis A, Kumar N, Mankad K. Virtual multidisciplinary team meetings in the age of COVID-19: an effective and pragmatic alternative. Quant Imaging Med Surg. 2020;10(6):1204–1207. doi:10.21037/qims-20-638
10. Krupinski EA, Comas M, Gallego LG. A new software platform to improve multidisciplinary tumor board workflows and user satisfaction: a pilot study. J Pathol Inform. 2018;9(1):26. doi:10.4103/jpi.jpi_16_18
11. Hammer RD, Prime MS. A clinician’s perspective on co-developing and co-implementing a digital tumor board solution. Health Informatics J. 2020;26(3):2213–2221. doi:10.1177/1460458219899841
12. Mercantini P, Lucarini A, Mazzuca F, Osti MF, Laghi A. How technology can help in oncologic patient management during COVID-19 outbreak. Eur J Surg Oncol. 2020;46(6):1189–1191. doi:10.1016/j.ejso.2020.04.050
13. Okumura N, Nonaka K, Takahashi T, et al. A case of retroperitoneal recurrence of dermatofibrosarcoma protuberans developed 7 years after radical excision. J Jpn Surg Assoc. 2010;71(12):3232–3236.
14. Soukup T, Lamb BW, Arora S. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49–61. doi:10.2147/JMDH.S117945
15. Baker M. Next-generation sequencing: adjusting to data overload. Nat Methods. 2010;7:495–499. doi:10.1038/nmeth0710-495
16. Patkar V, Acosta D, Davidson T, Jones A, Fox J, Keshtgar M. Cancer multidisciplinary team meetings: evidence, challenges, and the role of clinical decision support technology. Int J Breast Cancer. 2011;831605.
17. Lippi G, Plebani M. Integrated diagnostics: the future of laboratory medicine? Biochem Med. 2020;30(1):18–30. doi:10.11613/BM.2020.010501
18. Falk S. Making MDTs better. National Institute for Health Research; 2018. Available from: https://www.england.nhs.uk/south/wp-content/uploads/sites/6/2018/11/12-MDT-rforms-0618.pptx.
19. Rosell L, Alexandersson N, Hagberg O, Nilbert M. Benefits, barriers and opinions on multidisciplinary team meetings: a survey in Swedish cancer care. BMC Health Serv Res. 2018;18(249). doi:10.1186/s12913-018-2990-4
20. Trivedi DB. Educational value of surgical multidisciplinary team meetings for learning non-technical skills - a pilot survey of trainees from two UK deaneries. J Surg Educ. 2019;76(4):1034–1047. doi:10.1016/j.jsurg.2019.02.001
21. Bohmeier B, Schellenberger B, Diekmann A, Ernstmann N, Ansmann L, Heuser C. Opportunities and limitations of shared decision making in multidisciplinary tumor conferences with patient participation - a qualitative interview study with providers. Patient Educ Couns. 2020;S0738–3991(20):30482.
22. Vaartio‐Rajalin H, Ngoni K, Fagerström L. Balancing between extremes- work in hospital‐at‐home. Nurs Open. 2020;7(1):398–410. doi:10.1002/nop2.402
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.