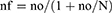Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Virological Suppression and Its Associated Factors of Dolutegravir Based Regimen in a Resource-Limited Setting: An Observational Retrospective Study in Ethiopia
Authors Mehari EA , Muche EA , Gonete KA
Received 20 April 2021
Accepted for publication 9 June 2021
Published 29 June 2021 Volume 2021:13 Pages 709—717
DOI https://doi.org/10.2147/HIV.S316776
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Eden Abetu Mehari,1 Esileman Abdela Muche,1 Kedir Abdela Gonete2
1Department of Clinical Pharmacy, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia; 2Department of Human Nutrition, Institute of Public Health, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Eden Abetu Mehari Email [email protected]
Background: Toxicity, treatment failure, and resistance to existing HIV treatment regimens have become a challenge in resource-limited settings. As a result, a dolutegravir based regimen has recently been utilized. However, there is a paucity of evidence in sub-Saharan countries regarding its virological suppression. Thus, this study aimed to assess virological suppression and associated factors of dolutegravir based regimen.
Methods: A retrospective follow-up study was conducted on 349 individuals. They were selected using a systematic random sampling technique among all treatment-experienced adult HIV patients who were on a dolutegravir based regimen. From this, 81.4% of them were virologically suppressed before the initiation of dolutegravir based regimen. The study was carried out at twelve months of therapy after shifting to dolutegravir based regimen (TDF-3TC-DTG) during the period May 2018–August 2020 at Debre Markos referral hospital. Retrospective data before and after dolutegravir based regimen initiation were collected from their medical records. The time on dolutegravir based regimen was one year. Bivariable and multivariable logistic regression was used to identify factors. Variables with p < 0.05 were considered statistically significant.
Results: From a total of 359, 349 participated (97.2%) in the study, and the mean age of the participants was 40.28 ± 11.6 years. Totally, 192 (55.0%) of them were female. The proportion of virological suppression was 92%. Good adherence (participants who reported an intake of ≥ 95% of the prescribed medication) (AOR=6.2, 95% CI: 1.93, 20.11) and overall duration of ART (AOR=1.02, 95% CI: 1.01, 1.04) were associated with virological suppression.
Conclusion: Dolutegravir based regimen maintains high virological suppression. Adherence and duration of ART were associated with virological suppression. Therefore, designing effective mechanisms to maintain virological suppression is important.
Keywords: dolutegravir, virological suppression, HIV, Debre-Markos
Introduction
Antiretroviral therapies (ART) are important to reduce viral load levels, improve survival, and also reduce human immunodeficiency virus (HIV) transmission.1 Since the advent of combination antiretroviral therapies, the survival and quality of life of people living with HIV have steadily improved.2 Until mid 2018, the World Health Organization (WHO) recommended ART regimen for HIV-1 infection which comprised a combination of two nucleoside reverse-transcriptase inhibitors (NRTIs) combined with a non-nucleoside reverse-transcriptase inhibitor (NNRTI), namely Efavirenz (EFV) at a dose of 600 mg daily (known as EFV 600).3 The Efavirenz-based regimen was then challenged by the landmark Study ING11446 (SINGLE trial), which showed that the dolutegravir-based regimen had a more sustained viral suppression and immunologic recovery than the efavirenz-based regimen.4 Studies4–6 suggested that dolutegravir (DTG) has potent antiviral activity, high barrier to resistance, and better safety profile.4,7,8 In settings with a high prevalence of transmitted resistance to non-nucleosides (NNRTIs), DTG might be a preferred first-line option to EFV.9,10 However, this prediction has not been validated in our setting. Thus, to support the use of DTG based regimens in mass treatment programs; real-world studies are needed to assess virological suppression of these new antiretroviral drugs.11 Viral load monitoring is the preferred approach to monitor treatment outcome, reducing the accumulation of drug-resistance mutations and improving clinical outcomes because of its early and more accurate indication of treatment failure compared to immunological and clinical monitoring.12 Therefore; this study aimed to assess virological suppression and its associated factors of dolutegravir based regimen among HIV patients at Debre Markos referral hospital, Ethiopia.
Methods
Study Setting
The study was conducted at Debre Markos Referral Hospital which is located in Debre Markos town, Ethiopia. Debre Markos town is located in the East Gojjam Zone of Amhara Nation Regional State. The distance from Addis Ababa, the capital city of Ethiopia is 299 km. More than five million people in the East and West Gojjam Zone are expected to be served by the hospital. Starting from 2005, ART service has been initiated in the hospital and around 6350 patients received ART service and from this 5839 were adults. In this study area, around 2151 patients received dolutegravir based regimen.
Study Design and Population
A retrospective follow-up study was carried out starting from May 2018 – August 2020 on treatment-experienced adult HIV patients on dolutegravir based regimen. Ethiopia adopted dolutegravir (DTG) containing regimen into the 2018 edition of the national comprehensive HIV prevention, care and treatment guideline and all eligible existing first-line clients were shifted to DTG containing regimens and their baseline viral load had to be <1000 to be eligible for shifting. Due to this reason, 81.4% of the study participants had a viral load level of < 50 copies/mL prior to the initiation of dolutegravir based regimen. These patients were on a dolutegravir based regimen for a year. Retrospective data before (within 12 months before DTG) and after initiation of dolutegravir based regimen were collected from June 1 to August 30, 2020. Participants had to have at least one-year data before dolutegravir based regimen initiation and time on dolutegravir based regimen was similar. Those participants who had incomplete viral load information on their medical charts were excluded. In addition, those with interruptions and loss to follow-ups were withdrawn from the study as they would not have complete viral load information.
Sample Size Determination and Sampling Procedure
The sample size was calculated using a single population proportion formula with a 95% confidence level, the proportion of viral suppression on dolutegravir=50% since there was no previous study done in this study population and relative precision was considered to be 5%. The calculated sample size was 384. The total number of HIV patients on the dolutegravir based regimen was 2151. Since it was less than 10,000 the sample size was reduced using a correction formula mentioned below.
Where nf= final sample size, no=sample size calculated (384), N=total population (2151)
nf ≈326
A non-response rate of 10% was added on 326 to obtain the final sample size for this study and it was found to be 359. The study participants were selected from HIV patients who visited the ART clinic during the data collection period using a systematic random sampling technique. The sampling fraction (k) was found by dividing the projected number of HIV patients on ART for three months by the total sample size. Every kth participant was selected using their medical record order. If the patient was not available, the next immediate medical record was selected and the sampling fraction was added to get the next patient. An identification number was given for each medical record to avoid duplication.
Data Collection Instrument and Procedure
The data collection instrument used in this study was a data abstraction format that was used to extract data from the medical charts. A structured interviewer-administered questionnaire was used for socio-demographic characteristics which was designed by reviewing relevant literature. The data collection tool had three parts.
The first section contained questions about the participant’s socio-demographic characteristics which were obtained via interviewer-administered questionnaire. The second section was about clinical and treatment-related factors pre- and post-DTG initiation which were extracted from medical charts. The clinical and treatment-related factors consisted of factors such as the clinical stage of the participants, the ART regimen, co-morbidities and concurrent medications. The third part was about the adherence level which was measured by pill count. The pre- and post-dolutegravir based regimen adherence level was extracted from the medical charts. Then, based on WHO guidelines, reported intake of ≥ 95% of the prescribed medication was categorized as “good adherence”; and 85–95% intake as “fair adherence” and “poor adherence” was <85% intake.13,14 Data were collected from their medical charts and socio-demographic characteristics were obtained from the patients. The study participants visited the hospital every one to three months. Annual viral load monitoring is implemented at Debre-markos hospital.
Data Quality Control and Assurance
Training was delivered to data collectors for two days. The training focused on data handling, participants’ approach and also on ethical aspects of data collection. The trained data collectors were subjected to supervision by a single supervisor. A pre-test was done on 5% of adult HIV patients on dolutegravir based regimen at Felege hiwot comprehensive and specialized Hospital to assess the understandability and availability of the data.
Daily close supervision was done during the time of data collection. The questionnaire was reviewed and checked for completeness, accuracy, and consistency by the supervisor.
Data Processing and Analysis
Virological suppression was defined as a plasma viral load of less than 50 copies/ mL as determined by the Food and Drug Administration Snapshot algorithm.15
Epi Info 7 was used to enter the data. After data entry, the data were transferred to Statistical Package for Social Science (SPSS) version 23 for analysis. Descriptive statistics (frequencies, percentages, mean, median, SD, and IQR) were calculated for variables.
Besides, after the assumption was satisfied, a Wilcoxon signed-rank test at a 95% confidence interval and a 5% significance level was used to determine the significance of median virologic suppression difference between pre- and post-DTG based regimen initiation and the rank difference between pre- and post-DTG adherence. A paired sample t-test at a 95% confidence interval and a 5% significance level was used to determine the significance of the mean weight difference between pre- and post-DTG based regimen initiation.
A binary logistic regression model was fitted to identify factors associated with the dependent variable. Variables with a p-value of less than 0.2 in the bivariable binary logistic regression were run in the multivariable logistic regression. Adjusted odds ratio (AOR) with a p-value <0.05 and 95% CI was used to express the strength of association between the dependent variable and the independent variables. Multicollinearity was checked and the result disclosed that all the variance inflation factor (VIF) values were less than three and tolerance less than ten, which confirmed the absence of multicollinearity.16
Box plot was used and no outshining outlier effect was observed. The Omnibus test result was significant with p-value =0.001, and the Hosmer and Lemeshow test showed a good model fit with p-value=1.00, which signifies the goodness fit of the model.17
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the Ethical Review Committee of the School of Pharmacy, University of Gondar. Necessary permission was gained from the Debre Markos referral hospital. The respondents were informed about the purpose of the study and their consent to participate was obtained. Data were collected after written informed consent was obtained and confidentiality of the information was maintained. The study was conducted according to the criteria set by the declaration of Helsinki.
Result
Socio-Demographic Characteristics
In this study, 359 patients were approached and 349 participants agreed to take part in the study, giving a response rate of 97.2%. The mean age of the participants was 40.28±11.6 years and 192 (55.0%) of them were female. The majority of the participants, 297 (85.1%), were urban dwellers and 98 (28.1%) participants had an average monthly income of ≥3500 Ethiopian Birr (86.48 USD). Among the study participants, 109 (31.2%) had primary school education. The mean overall duration on ART was 8.3 ±3.6 years (Table 1).
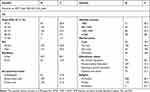 |
Table 1 Descriptive Statistics of Socio-Demographic Characteristics of HIV Patients on Dolutegravir Based Regimen at Debre Markos Referral Hospital 2020 |
Clinical and Treatment-Related Factors
Prior to the initiation of dolutegravir based regimen, TDF+3TC+EFV were prescribed to half of the participants, 175 (50.1%). All study participants were taking TDF-3TC-DTG. Almost 90% (312) of the study participants had no co-morbidity. Hypertension was the most common co-morbidity, 14 (58.3%) (Table 2).
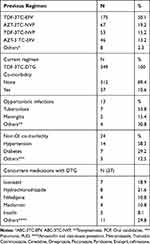 |
Table 2 Clinical Characteristics of HIV Patients on Dolutegravir Based Regimen at DRH 2020 |
Clinical Characteristics Pre- and Post-Dolutegravir Based Regimen
In this study, before the initiation of the dolutegravir based regimen, mean weight of the participants was 52.47 ±12.2 kg which increased to 55.29 ±12.3 kg after dolutegravir based regimen (p < 0.001). The mean weight gain among female participants was 55.48 ± 12.5 Kg whereas, the highest mean weight gain was seen in the age group 45–57 which was found to be 57.15 ± 12.6 kg (Table 3). The proportion of patients with hypertension and diabetes post-dolutegravir based regimen did not significantly differ from the pre-dolutegravir based regimen (McNemar’s test, p=0.061). Regarding adherence, a Wilcoxon signed rank test indicated that post-DTG adherence ranks were not statistically different from pre-DTG adherence ranks (p-value = 0.538). Pre-dolutegravir based regimen viral load suppression was 284 (81.4%) whereas; post-dolutegravir based regimen viral load suppression was 321 (92%) (Table 4).
 |
Table 3 Mean Weight Gain Stratified by Sex and Age of HIV Patients on Dolutegravir Based Regimen at Debre Markos Referral Hospital 2020 |
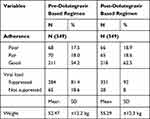 |
Table 4 Clinical Characteristics Pre- and Post-Dolutegravir Based Regimen at Debre Markos Referral Hospital 2020 |
Virological Suppression of Dolutegravir Based Regimen
Overall, virological suppression increased after dolutegravir based regimen, 321 (92%) (95% CI 89.1–94.8), from pre-dolutegravir based regimen viral load suppression of 284 (81.4%). The data were skewed and the Wilcoxon signed rank test was performed which reported that the pre-dolutegravir based regimen median viral load score and post-dolutegravir based regimen median viral load score were significantly different (p-value = 0.001) with a small effect size (r =0.18).
Factors Associated with Virological Suppression of Dolutegravir Based Regimen
In the bivariate logistic regression analysis, it was illustrated that adherence, pre-dolutegravir based regimen viral load, WHO clinical stage, educational status, marital status, and duration of ART were significantly associated with virological suppression (Table 5). A cutoff point of p value < 0.2 was used to select variables for the multivariable binary logistic regression. Consequently, the multivariable binary logistic regression analysis revealed that adherence and duration of ART were found to be significantly associated with virological suppression. The odds of having virological suppression were six times (AOR=6.2, 95% CI: 1.93, 20.11, P= 0.002) higher among participants with good adherence than those with poor adherence. With regard to the overall duration of ART, the odds of having virologic suppression were about one time (AOR=1.02, 95% CI: 1.01, 1.04, P=0.001) higher for a year increase in duration of ART (Table 5)Table4.
 |
Table 5 Factors Associated with Virological Suppression of Dolutegravir Based Regimen at DRH 2020 |
Discussion
This study attempted to elucidate the virological suppression and associated factors of dolutegravir based regimen among HIV patients attending Debre Markos referral hospital. The virological suppression and factors associated with the virological suppression of dolutegravir based regimen were identified using the viral load level. It was found that 321 [(92%), (95% CI 89.1–94.8)] of the participants maintained viral suppression. This percentage of viral suppression was in line with the study done in Uganda which showed a high virologic suppression with 94% of patients maintaining virologic suppression.18 Since there is limited evidence in resource limited settings, it is difficult to compare the result with other studies. This study revealed that there is a better virological suppression after the initiation of dolutegravir based regimen than pre-dolutegravir based regimen initiation (Z scores = −4.825; p-value <0.001), with a small effect size (r =0.18). This result indicated that dolutegravir based regimen maintained high virological suppression but the magnitude of this difference was small. This might be due to the fact that the current study participants were treatment-experienced. Previously, 81.4% of them were virologically suppressed and in this case, the magnitude of treatment differences may not weigh more than the previous regimen, which causes a small effect size.
After the initiation of dolutegravir based regimen significant weight gain was reported, ie, 55 Kg post-DTG compared to 52 Kg in the pre-DTG regimen (p = 0.001). Substantial weight gain was reported in studies done in Africa such as NAMSAL, and ADVANCE trials.19,20 The same results were reported from previous studies.19,21–23 On the contrary, some studies reported comparable weight gain24 and no significant difference in the rate of weight gain with dolutegravir based regimen.25
The proportion of patients with hypertension and diabetes post-dolutegravir based regimen did not significantly differ from the pre-dolutegravir based regimen. This may be due to the short duration of follow-up post-DTG regimen. Those diseases which are associated with weight gain may take a long period until they are evident in patients, but one year follow-up is not sufficient to show disorders such as hypertension and diabetes thus, these results suggest that follow-up of these participants is critical and needs further investigation. Though weight gain after starting antiretroviral treatment is common and beneficial to HIV patients, substantial weight gain may increase the risk of adverse birth outcomes, diabetes, cardiovascular disease, and some cancers.26,27
In this study, the virological suppression was about six times higher among participants with good adherence as compared to participants with poor adherence. This result is in line with Ethiopian studies28,29 and a Kenyan study30 done on other ART regimens where good adherence was a significant predictor of viral load suppression. This association was also reported by other similar studies.31,32 This could lend weight to the concept that poor adherence is associated with virological non-suppression or good adherence is associated with virological suppression. This could be due to the fact that, as the drug concentration decreases in the body fluids, HIV replication might not be suppressed, which in turn leads to an increase in viral load.28
Another predictor of viral load suppression found by this study was duration of ART regimen. This result is in harmony with previous research works which found improved virological suppression with time on ART.33–35 These findings can be explained by the evidence from clinical practice, which suggested prolonged ART can reduce viral load below the limit of detection for a long-term period.36
Limitation of the Study
The limitation of this study was that secondary data (data abstracted from medical charts) were used to measure the virological suppression and important variables might have been missed such as CD4 count, clinical stage, and treatment stage. It would be better if a prospective cohort study could be conducted in the future, and also this study is not generalizable to treatment-naïve patients, and treatment interruptions and loss to follow-up were not included.
Conclusion
This study identified that, dolutegravir based regimen maintains virologic suppression among treatment-experienced HIV patients. Moreover, good ART adherence and duration of ART were significantly associated with viral suppression.
Practical Implications
Increasing the level of virological suppression is the preliminary step toward improving HIV patients’ health conditions. Dolutegravir based regimen maintains virologic suppression but the weight gain after its use needs further research to check whether it is beneficial or harmful. The limitation behind this study is that it is not possible to generalize the findings to treatment-naïve participants. Therefore, future research shall be conducted on treatment-naïve HIV patients on dolutegravir based regimen.
Abbreviations
ART, antiretroviral therapy; DRH, Debre Markos Referral Hospital; EPI Info, Epidemiological Information; HIV, Human immune deficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; WHO, World Health Organization.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on request.
Ethical Approval and Consent to Participate
Ethical clearance was obtained from the Ethical Review Committee of School of Pharmacy, University of Gondar. Necessary permission was gained from the Debre Markos referral hospital. The respondents were informed about the purpose of the study and their consent to participate was obtained. Data were collected after written informed consent was obtained and confidentiality of the information was maintained. All methods were performed in accordance with the relevant guidelines and regulations. The study was conducted according to the criteria set by the declaration of Helsinki.
Consent for Publication
Participants’ consent was obtained to publish this work.
Acknowledgments
We would like to acknowledge the University of Gondar for funding this research project. We are also greatful to study participants, data collectors, and supervisor for their contribution to this research.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article and revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This research was done with the financial support of University of Gondar. The funding offices had no direct or indirect involvement in the study write up and analysis.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi:10.1056/NEJMoa1105243
2. Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV/AIDS. 2012;4:117.
3. Organization WH.. Updated Recommendations on First-Line and Second-Line Antiretroviral Regimens and Post-Exposure Prophylaxis and Recommendations on Early Infant Diagnosis of HIV: Interim Guidelines: Supplement to the 2016 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. World Health Organization; 2018.
4. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir–lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807–1818. doi:10.1056/NEJMoa1215541
5. Oliveira M, Mesplede T, Quashie PK, Moïsi D, Wainberg MA. Resistance mutations against dolutegravir in HIV integrase impair the emergence of resistance against reverse transcriptase inhibitors. AIDS. 2014;28(6):813–819. doi:10.1097/QAD.0000000000000199
6. Treviño A, Cabezas T, Lozano AB, et al. Dolutegravir for the treatment of HIV-2 infection. J Clin Virol. 2015;64:12–15. doi:10.1016/j.jcv.2015.01.001
7. Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700–708. doi:10.1016/S0140-6736(13)61221-0
8. Trottier B, Lake JE, Logue K, et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, phase IIIb study. Antivir Ther. 2017;22(4):295–305. doi:10.3851/IMP3166
9. Snedecor SJ, Radford M, Kratochvil D, Grove R, Punekar YS. Comparative efficacy and safety of dolutegravir relative to common core agents in treatment-naïve patients infected with HIV-1: a systematic review and network meta-analysis. BMC Infect Dis. 2019;19(1):484. doi:10.1186/s12879-019-3975-6
10. Cruciani M, Parisi SG. Dolutegravir based antiretroviral therapy compared to other combined antiretroviral regimens for the treatment of HIV-infected naive patients: a systematic review and meta-analysis. PLoS One. 2019;14(9):e0222229. doi:10.1371/journal.pone.0222229
11. McCormack PL. Dolutegravir: a review of its use in the management of HIV-1 infection in adolescents and adults. Drugs. 2014;74(11):1241–1252. doi:10.1007/s40265-014-0256-y
12. HIV W. AIDS. Monitoring Response to ART and Diagnosis of Treatment Failure.
13. Organization WH. Adherence to Long-Term Therapies: Evidence for Action. World Health Organization; 2003.
14. Sangeda RZ, Mosha F, Prosperi M, et al. Pharmacy refill adherence outperforms self-reported methods in predicting HIV therapy outcome in resource-limited settings. BMC Public Health. 2014;14(1):1035. doi:10.1186/1471-2458-14-1035
15. Evaluation FD. Human Immunodeficiency Virus-1 Infection: Developing Antiretroviral drugs for Treatment. Guidance for industry; 2015.
16. O’brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quant. 2007;41(5):673–690. doi:10.1007/s11135-006-9018-6
17. Hosmer DW
18. Nabitaka VM, Nawaggi P, Campbell J, et al. High acceptability and viral suppression of patients on dolutegravir-based first-line regimens in pilot sites in Uganda: a mixed-methods prospective cohort study. PLoS One. 2020;15(5):e0232419. doi:10.1371/journal.pone.0232419
19. Group NAS. Dolutegravir-based or low-dose efavirenz–based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826. doi:10.1056/NEJMoa1904340
20. Venter W, Moorhouse M, Sokhela S, et al. The ADVANCE trial: phase 3, randomized comparison of TAF/FTC/DTG, TDF/FTC/DTG or TDF/FTC/EFV for first-line treatment of HIV-1 infection.
21. Norwood J, Turner M, Bofill C, et al. Weight gain in persons with HIV switched from efavirenz-based to integrase strand transfer inhibitor-based regimens. J Acquir Immune Defic Syndr. 2017;76(5):527. doi:10.1097/QAI.0000000000001525
22. Bourgi K, Rebeiro PF, Turner M, et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267–1274. doi:10.1093/cid/ciz407
23. Rizzardo S, Lanzafame M, Lattuada E, et al. Dolutegravir monotherapy and body weight gain in antiretroviral naive patients. AIDS. 2019;33(10):1673–1674. doi:10.1097/QAD.0000000000002245
24. Wohl DA, Yazdanpanah Y, Baumgarten A, et al. Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 2019;6(6):e355–e363. doi:10.1016/S2352-3018(19)30077-3
25. Burns JE, Stirrup OT, Dunn D, et al. No overall change in the rate of weight gain after switching to an integrase-inhibitor in virologically suppressed adults with HIV. AIDS. 2020;34(1):109–114. doi:10.1097/QAD.0000000000002379
26. Blair SN, Church TS. The fitness, obesity, and health equation: is physical activity the common denominator? JAMA. 2004;292(10):1232–1234. doi:10.1001/jama.292.10.1232
27. Bodnar LM, Siega-Riz AM, Simhan HN, Himes KP, Abrams B. Severe obesity, gestational weight gain, and adverse birth outcomes. Am J Clin Nutr. 2010;91(6):1642–1648. doi:10.3945/ajcn.2009.29008
28. Desta AA, Woldearegay TW, Futwi N, et al. HIV virological non-suppression and factors associated with non-suppression among adolescents and adults on antiretroviral therapy in northern Ethiopia: a retrospective study. BMC Infect Dis. 2020;20(1):1–10. doi:10.1186/s12879-019-4732-6
29. Bayu B, Tariku A, Bulti AB, Habitu YA, Derso T, Teshome DF. Determinants of virological failure among patients on highly active antiretroviral therapy in University of Gondar Referral Hospital, Northwest Ethiopia: a case–control study. HIV/AIDS. 2017;9:153.
30. Maina E, Mureithi H, Adan A, Muriuki J, Lwembe R, Bukusi E. Incidences and factors associated with viral suppression or rebound among HIV patients on combination antiretroviral therapy from three counties in Kenya. Int J Infect Dis. 2020. doi:10.1016/j.ijid.2020.05.097
31. Kiweewa F, Esber A, Musingye E, et al. HIV virologic failure and its predictors among HIV-infected adults on antiretroviral therapy in the African Cohort Study. PLoS One. 2019;14(2):e0211344. doi:10.1371/journal.pone.0211344
32. Casado JL, Sabido R, Perez-Elías MJ, et al. Percentage of adherence correlates with the risk of protease inhibitor (PI) treatment failure in HIV-infected patients. Antivir Ther. 1999;4:157–162.
33. Omooja J, Nannyonjo M, Sanyu G, et al. Rates of HIV-1 virological suppression and patterns of acquired drug resistance among fisherfolk on first-line antiretroviral therapy in Uganda. J Antimicrob Chemother. 2019;74(10):3021–3029. doi:10.1093/jac/dkz261
34. Hanna DB, Felsen UR, Ginsberg MS, et al. Increased antiretroviral therapy use and virologic suppression in the bronx in the context of multiple HIV prevention strategies. AIDS Res Hum Retroviruses. 2016;32(10–11):955–963. doi:10.1089/aid.2015.0345
35. Lokpo SY, Ofori-Attah PJ, Ameke LS, et al. Viral suppression and its associated factors in HIV patients on Highly Active Antiretroviral Therapy (HAART): a Retrospective Study in the Ho Municipality, Ghana. AIDS Res Treat. 2020;2020:1–7. doi:10.1155/2020/9247451
36. Resino S, Resino R, Micheloud D, et al. Long-term effects of highly active antiretroviral therapy in pretreated, vertically HIV type 1-infected children: 6 years of follow-up. Clin Infect Dis. 2006;42(6):862–869. doi:10.1086/500412
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.