Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 16
Virological Non-Suppression, Non-Adherence and the Associated Factors Among People Living with HIV on Dolutegravir-Based Regimens: A Retrospective Cohort Study
Authors Kabiibi F, Tamukong R, Muyindike W, Yadesa TM
Received 15 November 2023
Accepted for publication 13 March 2024
Published 21 March 2024 Volume 2024:16 Pages 95—107
DOI https://doi.org/10.2147/HIV.S449947
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Florence Kabiibi,1 Robert Tamukong,1 Winnie Muyindike,2 Tadele Mekuriya Yadesa1,3
1Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; 2HIV Clinic, Mbarara Regional Referral Hospital, Mbarara, Uganda; 3Department of Clinical Pharmacy and Pharmacy Practice, Kampala International University, Ishaka-Bushenyi, Uganda
Correspondence: Tadele Mekuriya Yadesa, Mbarara University of Science and Technology, P.O Box 1410, Mbarara, Uganda, Tel +256753312571, Email [email protected]
Background: HIV is one of the leading causes of morbidity and mortality, with 39.0 million people living with HIV worldwide, 25.6 million of whom reside in the African region. Highly active anti-retroviral therapy (HAART) has improved survival and quality of life, yet some patients develop viral non-suppression. Dolutegravir (DTG) has been recommended since 2018 as a first-line treatment option in low- and middle-income countries owing to its effectiveness, low cost, and tolerability, but some studies have reported virological non-suppression with its use. This study aims to explore the prevalence and factors associated with virological non-suppression in adults taking DTG-based regimens in Mbarara Regional Referral Hospital.
Methods: A retrospective cohort study was carried out among people living with HIV (PLWHIV) taking DTG-based HAART regimens by way of record review. SPSS was used for analysis, and both binary and multivariate logistic regression analyses were performed to test associated factors.
Results: Among the 422 participants’ records reviewed, 62.8% were female (median age 40 years, IQR=13). The prevalence of virological non-suppression was 4.2%. Poor adherence to HAART was significantly associated with virological non-suppression, with 100.3 increased adjusted odds (95% CI: 28.90– 348.12, p< 0.001) compared to those with a record of good adherence. The reasons for poor adherence included alcohol use, stigma, forgetting to take medication, transport problems, and irregular timing of swallowing.
Conclusion: This study found poor adherence to be associated with a 4.2% prevalence of virological non-suppression among PLWHIV in a large public HIV care clinic. Despite the high suppression rates on DTG-based regimens, adherence counseling and viral load monitoring need to be emphasized at all HIV care centers to mark the trends of virological non-suppression.
Keywords: virological, non-suppression, dolutegravir, regimens
Background
Human immunodeficiency virus (HIV), with its associated opportunistic infections, is one of the world’s leading causes of suffering and death. About 39.0 million people worldwide were living with HIV by 2022, with almost two-thirds of them (25.6 million, 66%) residing in the African region;1 of these, 1.4 million were children aged 0–14 years. Cumulatively, 79.3 million people have been infected since the beginning of the HIV epidemic, with 40.4 million dying from AIDS-related illnesses.1 Uganda, one of the 10 high-burden countries in Sub-Saharan Africa, had a nationwide HIV prevalence of 6.2% among 15–49-year-olds in 2018, with the South-Western region having the country’s second highest HIV prevalence at 7.9%, with 7.6% among females and 4.7% among males.2
Highly active anti-retroviral therapy (HAART) is a medication regimen used to manage and treat HIV, and is made up of various anti-retroviral medicines.3 There has been a significant increase in survival and quality of life among people living with HIV (PLHIV) around the globe since the introduction and expansion of HAART in 1995,4 as well as a reduction in HIV infection rates in areas with high treatment coverage.5 Different HAART drug combinations block HIV replication at various stages of its life cycle,6 thereby suppressing viral replication, restoring the immune system, and improving quality of life.7 However, over time, some individuals on HAART have developed failure on these regimens, necessitating gradual transitions from one line of treatment to the next.
Dolutegravir (DTG) is an integrase strand transfer inhibitor approved for the treatment of HIV infection,8,9 and was recommended by the WHO as a first-line treatment option in combination with other anti-retrovirals for HIV infections in low- and middle-income countries in 2018.10 The national rollout of DTG-based regimens in Uganda began in early 2018, with access being first restricted to women of reproductive age, although this was later expanded to include all adults living with HIV.11,12 The efficacy of DTG has been studied in several studies, whereby it was demonstrated that DTG is superior to both efarvirenz (EFV) and ritonavir-boosted darunavir, and non-inferior to raltegravir,11,13 and it is expected to play a major role in sub-Saharan Africa owing to its barrier to resistance, high potency, good tolerability, and low cost.14 However, although this treatment has generally been accepted and taken up for the treatment of HIV in most of the endemic areas, viral non-suppression by DTG-based regimens has been reported in some studies.15–17
Anti-retroviral therapy success can be diagnosed clinically, immunologically, or virologically, and HAART failure can be classified as virological, immunological, or clinical failure, with virological failure defined as an increase in viral RNA copies to 1000 or more per milliliter of blood, measured consecutively 3–6 months apart.18,19 Virological non-suppression in patients on a DTG-based regimen has strong implications for virological failure, which calls for early detection so that measures can be instituted early enough to prevent possible virological failure,20 along with its related higher second-line treatment costs as well as more adverse drug effects.21,22 Thus, interventions that improve the management of virological non-suppression are urgently needed to maintain control of the global HIV epidemic and ensure attainment of the UNAIDS target of having 95% of patients on treatment virally suppressed.23
Since the introduction of the DTG-based HAART regimens in Uganda in 2018, published information has been scarce on virological non-suppression and associated factors in patients on this regimen in this country. As a result, addressing this information gap would aid healthcare practitioners, local administrators, public health planners, policymakers, and partners in developing and implementing effective intervention measures among HIV patients taking DTG-based regimens.
This study, therefore, attempted to identify the prevalence of virological non-suppression and to explore the factors associated with it among adults taking DTG-based regimens in the HIV Clinic of Mbarara Regional Referral Hospital (MRRH) to ensure better practices and achieve better clinical results from the use of DTG.
Methods
Aim, Design, and Setting of the Study
The aim of this study was to the determine the prevalence of and explore the factors associated with virological non-suppression among people on DTG-based HAART regimens at MRRH, in South-Western Uganda. We employed a retrospective cohort study among adult patients initiated on DTG-based HAART regimens from January 2018 to October 2021.
MRRH is the biggest referral hospital in South-Western Uganda, and is located about 280 km from the Ugandan capital, Kampala. Currently, the HIV clinic serves a total number of 11,218 active clients on anti-retroviral therapy, including 10,619 adults, of whom 9462 clients are on DTG-based regimens.
Study Population
The study population consisted of adults aged 18 years and older living with HIV who had been treated with DTG-based regimens for at least 6 months at the Immune Suppression Syndrome (ISS) clinic of MRRH.
Sample Size Determination
The sample size for this study was determined using Fisher’s formula for the estimation of sample size (Fisher, 1998):
where n is minimum sample size required, 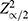 is standard normal variation at the 95% confidence interval corresponding to 1.96, P is estimated prevalence of 50%, and δ is absolute error of 0.05. The addition of 10% to compensate for possible incompleteness of some data gave a total sample size of 422.
is standard normal variation at the 95% confidence interval corresponding to 1.96, P is estimated prevalence of 50%, and δ is absolute error of 0.05. The addition of 10% to compensate for possible incompleteness of some data gave a total sample size of 422.
Sampling Procedures
The study utilized the pre-existing database to determine eligibility based on age, regimen used, viral load results, and duration on DTG since initiation. A systematic random sampling method was then applied to select the 422 client files for the study. Microsoft Excel version 16.0 was used to generate random numbers from the clients’ numbers, which were then used to pick files for enrollment.
Data Collection Tools and Procedures
The study used a document review guide to extract relevant information from the participants’ files. The document review guide was able to gather patient-related and drug-related data, as well as information on adherence to HAART. The data collection tool was pretested on 10 patient files to ensure its reliability. Research assistants were given a two-day training course on data collection and there was close supervision by the principal investigator (PI) throughout the data collection process. Virological non-suppression was established if the plasma viral load was greater than 1000 RNA copies/mL on any viral load assessment results at any time 6 months or longer after the initiation of the DTG-based regimen. Information on drug adherence and opportunistic information was directly obtained from the patient charts. Good adherence referred to taking ≥95% of the doses correctly, whereas fair and poor adherence referred to 80–94.9% and <80%, respectively. The study also reviewed case notes to ensure additional reliability.
Data Analysis
Data were checked for completeness by the PI, entered into Microsoft Excel version 16.0, cleaned, and exported to SPSS version 25 for analysis. For descriptive statistics, frequencies were used to describe the demographic characteristics of patients as well as for the prevalence of virological non-suppression, drug factors, patient factors, and disease factors. Bivariate logistic regression was carried out for all independent variables to indicate their ability to predict the outcome variable (virological non-suppression).
To identify candidate variables for multivariate logistic regression analysis, factors with p<0.25 on bivariate logistic regression analysis were included. Finally, independent variables that had a significant association with virological non-suppression were identified based on the adjusted odds ratio (AOR), 95% confidence interval (95% CI), and p-value <0.05.
Results
Demographic and Clinical Characteristics of the Participants
The majority of participants (62.8%) were female. Participants had a mean age of 41 years, and had attained mostly a secondary level of education (54.9%), mainly earning their living as peasant farmers (53.6%). Furthermore, many of the participants belonged to the Anglican religion (55.1%), and 59.2% were married, with only 7.8% not using any family planning method. About 23.5% had a record of alcohol use (Table 1).
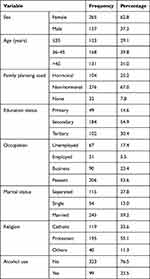 |
Table 1 Sociodemographic Characteristics of People Living with HIV at Mbarara Regional Referral Hospital |
Clinical Characteristics
About 90.6% of the participants had disclosed their serostatus, with 5.2% having a positive history of discordance. TDF/3TC/EFV (62.8%) was the most commonly used HAART regimen before DTG use. The overall duration of HAART for most clients was between 6 and 10 years (44.3%), and only 14% had used HAART for 5 years or less. About 56.2% of the participants had been on a DTG based regimen for more than 2 years, with the biggest number (99.3%) currently using DTG as the first-line regimen. All PLHIV in the sample had used and completed Isoniazid prophylaxis against tuberculosis infection and 2.4% had comorbidities, mostly hypertension and diabetes (Table 2).
 |
Table 2 Clinical Characteristics of People Living with HIV at Mbarara Regional Referral Hospital |
Prevalence of Virological Non-Suppression Among PLHIV on DTG-Based HAART Regimens
Out of 422 patients included, 18 participants had a viral load value greater than 1000 copies/mL, giving an overall prevalence of virological non-suppression of 4.3% (Figure 1).
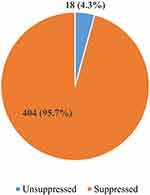 |
Figure 1 Prevalence of virological non-suppression among people living with HIV on dolutegravir-based highly active anti-retroviral therapy regimens. |
Three participants had virological non-suppression 6 months after initiation of the DTG-based regimen, 10 after 12 months, 17 after 24 months, and 18 after 36 months (Figure 2).
 |
Figure 2 Time taken for non-suppression of viral load from the start of the DTG-based regimen. Abbreviations: DTG, dolutegravir; HAART, highly active anti-retroviral therapy. |
Reasons for Non-Adherence
Out of the 422-sample population, 95.8% had a recorded good adherence in their files, while 4.2% were poorly adherent to the HAART medicines. Stigma (46.7%) was the major reason for poor adherence in the study population, followed by travel problems at 33.3% and alcohol use at 20.0% (Figure 3).
 |
Figure 3 Bar chart showing reasons for poor adherence among people living with HIV using dolutegravir-based regimens at Mbarara Regional Referral Hospital. |
Factors Associated with Virological Non-Suppression
A total of 18 variables were considered at univariate level and only four had a p-value <0.25: alcohol use (crude odds ratio [COR] =2.75, 95% CI: 1.06–7.18, p=0.038), ever changed regimen (COR=2.88, 95% CI: 0.65–12.74, p=0.163), greater than 2 years of taking DTG-based regimen (COR=2.84, 95% CI: 0.92–8.78, p=0.07), and poor current adherence (COR=100.31, 95% CI: 28.90–348.12, p<0.001). These were subjected to multivariate analysis and only one variable retained statistical significance, which was poor adherence (AOR=100.30, 95% CI: 28.90–348.12, p<0.001) compared to those with good adherence. Patients with poor adherence had 100.3 higher odds of virological non-suppression compared to those with good adherence (Table 3).
Discussion
The prevalence of virological non-suppression of 4.2% found in the current study is much lower than the assumed prevalence of 50% in our sample size calculation. Thus, our current sample size has achieved a better precision with a two-sided marginal error of 2.5% instead of the initial 5%. The current prevalence of 4.2% is comparable to other previous studies: 3.55% in Cameroon20 and 6% in central Uganda.22 However, our prevalence was lower than the prevalences reported in previous studies: 26.53% in Taiwan,24 21% in a clinical trial conducted in Cameroon,25 and 16% in a clinical trial in South Africa.26 The difference in prevalence can be explained by the high reported tolerability and low toxicity profile of the DTG-based regimen compared to others, which may improve compliance and subsequently lead to better outcomes.27 This could also be due to improvements in the general HIV care service delivery in our setting, which includes intensive adherence counseling, which has been found in previous studies to be associated with enhanced viral load suppression among HIV patients who were previously non-suppressed.28–30 The adoption of the use of viral load monitoring instead of CD4 cell count and clinical monitoring enables early high viremia among patients,31 thereby allowing early intervention to be instituted.
On multivariate logistic regression analysis, the adherence status of the patients was the only independent risk factor associated with virological non-suppression. Patients who had poor adherence to HAART were at about 100.3 times higher odds of having virological non-suppression compared to those with good adherence. This was comparable to other studies.32–35 Poor adherence to HAART has been highly linked to the development of drug resistance among PLHIV,36–39 and this drug resistance leads to virological non-suppression as HIV replication is not well suppressed, which, in turn, leads to an increase in the viral load.40–42 This shows the importance of continuous adherence monitoring and counseling among PLHIV.
Other factors were not found to be significantly associated with virological non-suppression, although they were significant in some previous studies, such as age24,43,44 and duration of DTG regimen.45 This shows that virological non-suppression can be associated with different factors in different populations; therefore, more studies are required.
The reasons for poor adherence identified included alcohol use, stigma, sharing pills, feeling better, forgetting to take medications, the toxicity of HAART, travel problems, running out of pills, and irregular timing of swallowing. These factors were comparable to those found in previous studies. Alcohol use46–50 may reduce adherence because alcohol consumption lowers the concentration and reasoning capacity of the consumer, causing them to miss doses, especially on drinking days.46 Stigma was another reason, as identified in previous studies;51–53 this because it causes a diminished desire to take medication, self-rejection, and depression, leading to poor adherence. Another reason for poor adherence was forgetting to take medication, which was similar to findings in previous studies.54–56 Adherence interventions that focus on assisting PLWHIV to remember their medications should be adopted. Patients who did not adhere properly were also reported to have had issues with transport to the hospital, which led to their missing doses. This problem was also identified in previous studies.57–59 Travel problems were worsened during the COVID-19 pandemic, when there were travel restrictions. Satellite clinics could help to reduce this burden. Other reasons, such as sharing of pills, feeling better after the initial doses, the toxicity of DTG, running out of pills, and irregular timing of swallowing, although identified in a few patients in our study, were reported to significantly affect adherence in previous studies.60–62 This shows that the reasons for poor adherence among PLHIV are diverse, and attention should be drawn to this issue. Similar studies should be conducted in other HIV care centers to provide a more comprehensive understanding of the challenges faced by PLHIV in different settings and inform the development of tailored interventions.
We faced some limitations during this study. Access to all the desired sample records was challenging since the files are not kept in one place, so files in use could only be accessed on another day. The study was conducted in a specific geographic location, and the results may not be generalizable to other populations with different characteristics or HIV care settings.
Conclusion
This study established that the prevalence of virological non-suppression among PLHIV in our setting is comparable to the level that was previously reported. Despite the efforts of the Ugandan government and the global community to control the HIV epidemic using DTG-based regimens for PLHIV, virological non-suppression remains a concern in this population. Failure to address this promptly could result in the emergence of virological resistance, underscoring the importance of broadening and enhancing surveillance through national HIV drug resistance surveys. Poor adherence to HAART remains the most significant independent risk factor for virological non-suppression. Numerous factors contribute to poor adherence, and we suggest implementing technology-based interventions that focus on reminding PLHIV daily to take their medications and addressing the underlying reasons for poor adherence.
We recommend that hospitals explore the possibility of establishing satellite clinics closer to the clients to make it easier for them to access HAART services. This could help to improve adherence and ensure better health outcomes for PLHIV who experience travel problems.
Abbreviations
AE, adverse event; ART, anti-retroviral therapy; DTG, dolutegravir; EFV, efavirenz; FDA, Food and Drug Administration; HAART, highly active anti-retroviral therapy; HIV, human immunodeficiency virus; ISS, immune suppression syndrome; MRRH, Mbarara Regional Referral Hospital; MUST, Mbarara University of Science and Technology; PI, principal investigator; PLHIV, people living with HIV; UNAIDS, Joint United Nations Programme on HIV/AIDS; WHO, World Health Organization.
Data Sharing Statement
The data set and data collection tools used in this study are available upon reasonable request from the corresponding author.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Research and Ethics Committee (REC) of Mbarara University of Science and Technology (MUST); a waiver of informed consent was requested from REC.
Confidentiality was ensured by making sure that patients’ names and any information that could identify them were not included in the data collection tool or were removed from the extracted data. The PI, health professionals on duty, research assistants, patients, and caregivers were protected from any harm, embarrassment, or exposure, especially to COVID-19, through adherence to the standard operating procedures, including social distancing, and the use of face masks, sanitizer, and hand washing. The clinical routines were not interrupted by the study.
Acknowledgments
We would like to thank the staff of Mbarara Regional Referral Hospital who collaborated with us during data collection. We would also like to extend our thanks to the research assistants for their unreserved support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no competing interests in the research, authorship, and publication of this article.
References
1. World Health Organization. People living with HIV People acquiring HIV People dying from HIV-related causes. World Health Organization; 2023:1–8. Available from: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/j0294-who-hiv-epi-factsheet-v7.pdf.
2. Musana H, Ssensamba JT, Nakafeero M, et al. Predictors of failure on second-line antiretroviral therapy with protease inhibitor mutations in Uganda. AIDS Res Ther. 2021;18(1):1–10. doi:10.1186/s12981-021-00338-y
3. Ginat DT, Schaefer PW. Highly Active Antiretroviral Therapy (HAART). Neuroim Pharmacop. 2021;2021:203–212.
4. UNAIDS. UNAIDS Data 2019. UNAIDS; 2019.
5. Ba S, Ba ND, Sembene L, Dia H, Coulibaly M. Prevalence and factors associated with virologic failure among people living with HIV (PLHIV) monitored in a decentralized health care facility. Adv Infect Dis. 2019;2019:226–237.
6. Mwau M, Syeunda CA, Adhiambo M, et al. Scale-up of Kenya’s national HIV viral load program: findings and lessons learned. PLoS One. 2018;13(1):e0190659. doi:10.1371/journal.pone.0190659
7. Günthard HF, Saag MS, Benson CA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society–USA panel. JAMA. 2016;316(2):191. doi:10.1001/jama.2016.8900
8. Park TE, Mohamed A, Kalabalik J, Sharma R. Review of integrase strand transfer inhibitors for the treatment of human immunodeficiency virus infection. Expert Rev Anti Infect Ther. 2015;13(10):1195–1212. doi:10.1586/14787210.2015.1075393
9. Shah BM, Schafer JJ, Desimone JA. Dolutegravir: a new integrase strand transfer inhibitor for the treatment of HIV. Pharmacotherapy. 2014;34(5):506–520. doi:10.1002/phar.1386
10. Mondi A, Cozzi-Lepri A, Tavelli A, et al. Effectiveness of dolutegravir-based regimens as either first-line or switch antiretroviral therapy: data from the Icona cohort. J Int AIDS Soc. 2019;22(1):e25227. doi:10.1002/jia2.25227
11. Alhassan Y, Twimukye A, Malaba T, et al. Community acceptability of dolutegravir-based HIV treatment in women: a qualitative study in South Africa and Uganda. BMC Public Health. 2020;20(1). doi:10.1186/s12889-020-09991-w
12. Rolle CP, Nguyen V, Hinestrosa F, DeJesus E. Virologic outcomes of switching to dolutegravir functional mono- or dual therapy with a non-cytosine nucleoside analog: a retrospective study of treatment-experienced, patients living with HIV. AIDS Res Ther. 2021;18(1):1–11. doi:10.1186/s12981-021-00352-0
13. World Health Organisation. Dolutegravir (DTG) and the fixed dose combination (FDC) of tenofovir/lamivudine/dolutegravir (TLD); 2018.
14. Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV. 2019;6(2):e116–27. doi:10.1016/S2352-3018(18)30317-5
15. Buju RT, Akilimali PZ, Kamangu EN, Mesia GK, Kayembe JMN, Situakibanza HN. Predictors of viral non-suppression among patients living with HIV under dolutegravir in Bunia, Democratic Republic of Congo: a prospective cohort study. Int J Environ Res Public Health. 2022;19:3.
16. Pena MJ, Chueca N, D’avolio A, Zarzalejos JM, Garcia F. Virological failure in HIV to triple therapy with dolutegravir-based firstline treatment. Rare But Possible. 2018;2018:1.
17. Pyngottu A, Scherrer AU, Kouyos R, et al. Predictors of virological failure and time to viral suppression of first-line integrase inhibitor–based antiretroviral treatment. Clin Infect Dis. 2021;73(7):e2134–41. doi:10.1093/cid/ciaa1614
18. World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach. Geneva: World Health Organization; 2021:594.
19. de La Hoz JM, Bolaño L, Cárdenas O, et al. Characterization of treatment failure in HIV positive patients in the Colombian Caribbean region. Colomb Med. 2014;45(4):162–167. doi:10.25100/cm.v45i4.1566
20. Semengue ENJ, Fokam J, Etame N-K, et al. Dolutegravir-based regimen ensures high virological success despite prior exposure to efavirenz-based first-line ART in Cameroon: an evidence of a successful transition model. Viruses. 2022;15(1):18. doi:10.3390/v15010018
21. D’Arminio Monforte A, Lepri AC, Rezza G, et al. Insights into the reasons for discontinuation of the first highly active antiretroviral therapy (HAART) regimen in a cohort of antiretroviral naive patients. Aids. 2000;14(5):499–507. doi:10.1097/00002030-200003310-00005
22. Nabitaka VM, Nawaggi P, Campbell J, et al. High acceptability and viral suppression of patients on Dolutegravir-based first-line regimens in pilot sites in Uganda: a mixed-methods prospective cohort study. PLoS One. 2020;15(5):1–13. doi:10.1371/journal.pone.0232419
23. Siedner MJ, Moosa M-YS, McCluskey S. Resistance testing after virologic failure in sub-Saharan Africa: REVAMP clinical trial project design, methods and implementation status. Ann Internal Med. 2021;2021:1683–1692. doi:10.7326/M21-2229
24. Afrane AKA, Goka BQ, Renner L, et al. HIV virological non-suppression and its associated factors in children on antiretroviral therapy at a major treatment centre in Southern Ghana: a cross-sectional study. BMC Infect Dis. 2021;21(1):1–11. doi:10.1186/s12879-021-06459-z
25. Kouanfack C, Mpoudi-Etame M, Omgba Bassega P, et al. Dolutegravir-based or low-dose efavirenz-based regimen for the treatment of HIV-1. N Engl J Med. 2019;381(9):816–826.
26. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to Treat HIV. N Engl J Med. 2019;381(9):803–815. doi:10.1056/NEJMoa1902824
27. McCluskey SM, Pepperrell T, Hill A, Venter WDF, Gupta RK, Siedner MJ. Adherence, resistance, and viral suppression on dolutegravir in sub-Saharan Africa: implications for the TLD era. AIDS. 2021;35(Suppl 2):S127–35. doi:10.1097/QAD.0000000000003082
28. Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Health. 2016;21(9):1131–1137. doi:10.1111/tmi.12741
29. Pius A, Josephine NN, Erick S, et al. Influence of intensified adherence counselling on viral load suppression of people receiving antiretroviral therapy at a health centre IV in southwestern Uganda: a qualitative study. AIDS Res Ther. 2021;18(1):45. doi:10.1186/s12981-021-00372-w
30. Lukyamuzi Z, Etajak S, Katairo T, et al. Effect and implementation experience of intensive adherence counseling in a public HIV care center in Uganda: a mixed-methods study. BMC Infect Dis. 2021;21(1):1168. doi:10.1186/s12879-021-06862-6
31. Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):169. doi:10.1186/s12879-019-3781-1
32. Byrd KK, Hou JG, Hazen R, et al. Antiretroviral adherence level necessary for HIV viral suppression using real-world data. J Acquir Immune Defic Syndr. 2019;82(3):245–251. doi:10.1097/QAI.0000000000002142
33. Kikaire B, Ssemanda M, Asiimwe A, et al. HIV viral load suppression following intensive adherence counseling among people living with HIV on treatment at military-managed. Int J Infect Dis. 2021;112:45–51. doi:10.1016/j.ijid.2021.08.057
34. Oh KS, Han E. A comparison of medication adherence and viral suppression in antiretroviral treatment-naïve patients with HIV/AIDS depending on the drug formulary. PLoS One. 2021;16(1):e0245185. doi:10.1371/journal.pone.0245185
35. Atnafu GT, Moges NA, Wubie M, Gedif G. Incidence and predictors of viral load suppression after enhanced adherence counseling among HIV-Positive Adults in West Gojjam Zone, Amhara Region, Ethiopia. Infect Drug Resist. 2022;15:261–274. doi:10.2147/IDR.S341392
36. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM. Adherence to antiretroviral therapy and virologic failure: a meta-analysis. Medicine. 2016;95(15):e3361. doi:10.1097/MD.0000000000003361
37. Cheng Y, Sauer B, Zhang Y, et al. Adherence and virologic outcomes among treatment-naïve veteran patients with human immunodeficiency virus type 1 infection. Medicine. 2018;97(2):e9430. doi:10.1097/MD.0000000000009430
38. Bangsberg DR, Acosta EP, Gupta R, et al. Adherence-resistance relationships for protease and non-nucleoside reverse transcriptase inhibitors explained by virological fitness. AIDS. 2006;20(2):223–231. doi:10.1097/01.aids.0000199825.34241.49
39. von Wyl V, Klimkait T, Yerly S, et al. Adherence as a predictor of the development of class-specific resistance mutations: the Swiss HIV Cohort Study. PLoS One. 2013;8(10):e77691. doi:10.1371/journal.pone.0077691
40. Abana CZ, Sagoe KWC, Bonney EY, et al. Drug resistance mutations and viral load in human immunodeficiency virus type 2 and dual HIV-1/HIV-2 infected patients in Ghana. Medicine. 2019;98(6):e14313. doi:10.1097/MD.0000000000014313
41. Mehari Eden AbetuMuche EA, Gonete KA, Gonete KA. Virological suppression and its associated factors of dolutegravir based regimen in a resource-limited setting: an observational retrospective study in Ethiopia. HIV AIDS. 2021;13:709–717. doi:10.2147/HIV.S316776
42. Schramm B, Temfack E, Descamps D, et al. Viral Suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study. Lancet HIV. 2022;9:e544–53. doi:10.1016/S2352-3018(22)00136-9
43. Nabukeera S, Kagaayi J, Makumbi FE, Mugerwa H, Matovu JKB. Factors associated with virological non-suppression among HIV-positive children receiving antiretroviral therapy at the joint clinical research centre in Lubowa, Kampala Uganda. PLoS One. 2021;16(1):e0246140. doi:10.1371/journal.pone.0246140
44. Maena J, Banke-Thomas A, Mukiza N, et al. Determinants of viral load non-suppression among adolescents in Mbale District, Eastern Rural Uganda. AIDS Res Ther. 2021;18(1):91. doi:10.1186/s12981-021-00408-1
45. Berihun H, Bazie GW, Beyene A, Zewdie A, Kebede N. Viral suppression and associated factors among children tested for HIV viral load at Amhara public health institute, Dessie Branch, Ethiopia: a cross-sectional study. BMJ Open. 2023;13(1):e068792. doi:10.1136/bmjopen-2022-068792
46. Braithwaite RS, Bryant KJ. Influence of alcohol consumption on adherence to and toxicity of antiretroviral therapy and survival. Alcohol Res Heal. 2010;33(3):280–287.
47. Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi:10.1097/QAI.0b013e3181b18b6e
48. Kim JY, Yang Y, Kim HK, Kim JY. The impact of alcohol use on antiretroviral therapy adherence in Koreans living with HIV. Asian Nurs Res. 2018;12(4):258–264. doi:10.1016/j.anr.2018.10.002
49. Tran BX, Nguyen LT, Do CD, Le Nguyen Q, Maher RM. Associations between alcohol use disorders and adherence to antiretroviral treatment and quality of life amongst people living with HIV/AIDS. BMC Public Health. 2014;14(1):27. doi:10.1186/1471-2458-14-27
50. Velloza J, Kemp CG, Aunon FM, Ramaiya MK, Creegan E, Simoni JM. Alcohol use and antiretroviral therapy non-adherence among adults living with HIV/AIDS in Sub-Saharan Africa: a systematic review and meta-analysis. AIDS Behav. 2020;24(6):1727–1742. doi:10.1007/s10461-019-02716-0
51. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. doi:10.7448/IAS.16.3.18640
52. Jones HS, Floyd S, Stangl A, et al. Association between HIV stigma and antiretroviral therapy adherence among adults living with HIV: baseline findings from the HPTN 071 (PopART) trial in Zambia and South Africa. Trop Med Int Heal. 2020;25(10):1246–1260. doi:10.1111/tmi.13473
53. Nurfalah F, Yona S, Waluyo A. The relationship between HIV stigma and adherence to antiretroviral (ARV) drug therapy among women with HIV in Lampung, Indonesia. Enfermería Clínica. 2019;29:234–237. doi:10.1016/j.enfcli.2019.04.138
54. Tiyou A, Belachew T, Alemseged F, Biadgilign S. Predictors of adherence to antiretroviral therapy among people living with HIV/AIDS in resource-limited setting of southwest Ethiopia. AIDS Res Ther. 2010;7(1):39. doi:10.1186/1742-6405-7-39
55. Kalichman SC, Kalichman MO, Cherry C. Forget about forgetting: structural barriers and severe non-adherence to antiretroviral therapy. AIDS Care. 2017;29(4):418–422. doi:10.1080/09540121.2016.1220478
56. Freeman R, Gwadz M, Francis K, Hoffeld E. Forgetting to take HIV antiretroviral therapy: a qualitative exploration of medication adherence in the third decade of the HIV epidemic in the United States. SAHARA J J Soc Asp HIV/AIDS Res Alliance. 2021;18(1):113–130. doi:10.1080/17290376.2021.1989021
57. Tuller DM, Bangsberg DR, Senkungu J, Ware NC, Emenyonu N, Weiser SD. Transportation costs impede sustained adherence and access to HAART in a clinic population in southwestern Uganda: a qualitative study. AIDS Behav. 2010;14(4):778–784. doi:10.1007/s10461-009-9533-2
58. Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. doi:10.1371/journal.pmed.0030438
59. Chukwuma A, Oladele Ademola A, Omotoso Ibrahim M, et al. Adherence to combined Antiretroviral therapy (cART) among people living with HIV/AIDS in a tertiary hospital in Ilorin, Nigeria. Pan Afr Med J. 2019;32:10.
60. Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to antiretroviral therapy among people living with HIV. N Am J Med Sci. 2013;5(3):220–223. doi:10.4103/1947-2714.109196
61. Tegegne D, Mamo G, Negash B, Habte S, Gobena T, Letta S. Poor adherence to highly active antiretroviral therapy and associated factors among people living with HIV in Eastern Ethiopia. SAGE Open Med. 2022;10:20503121221104428. doi:10.1177/20503121221104429
62. Mutagonda RF, Mlyuka HJ, Maganda BA, Kamuhabwa AAR. Adherence, effectiveness and safety of dolutegravir based antiretroviral regimens among HIV infected children and adolescents in Tanzania. J Int Assoc Provid AIDS Care. 2022;21:23259582221109612. doi:10.1177/23259582221109613
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.