Back to Journals » Journal of Pain Research » Volume 15
ViphyllinTM, a Standardized Black Pepper Seed Extract Exerts Antinociceptive Effects in Murine Pain Models via Activation of Cannabinoid Receptor CB2, Peroxisome Proliferator-Activated Receptor-Alpha and TRPV1 Ion Channels
Authors Venkatakrishna K, Sundeep K, Sudeep HV , Gouthamchandra K, Shyamprasad K
Received 3 December 2021
Accepted for publication 25 January 2022
Published 5 February 2022 Volume 2022:15 Pages 355—366
DOI https://doi.org/10.2147/JPR.S351513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qi Fang
Karempudi Venkatakrishna, Kuppam Sundeep, Heggar Venkataramana Sudeep, Kuluvar Gouthamchandra, Kodimule Shyamprasad
R&D Center for Excellence, Vidya Herbs Pvt Ltd, Bangalore, 560 105, Karnataka, India
Correspondence: Heggar Venkataramana Sudeep, Tel +91 80-42094158
, Email [email protected]
Purpose: Plant-based natural products as anti-nociceptors have enormous potential as safer alternatives to conventional opiates and NSAIDS. Piper nigrum (black pepper) is one of the major culinary spices with medicinal attributes.
Methods: In the present study, the antinociceptive activity of a standardized black pepper seed extract (Viphyllin) containing not less than 30% β-caryophyllene (BCP) was evaluated using pain models in mice, namely acetic acid-induced writhing test, formalin-induced paw licking test, hot plate test and tail flick test. Further, the antagonists SR141716A (0.1 mg/kg i.p.), AM630 (5 mg/kg i.p.), capsazepine (0.1 mg/kg body weight i.p.), and GW6471 (1 mg/kg i.p.) were used to evaluate the involvement of cannabinoid receptors CB1 and CB2, TRPV1 ion channel and PPARα receptor, respectively. Molecular docking (AutoDock 4.2) was used to study the interaction of BCP with the agonist-binding sites of the selected pain receptors.
Results: Viphyllin at 10 mg, 25 mg and 50 mg/kg (i.p.) significantly inhibited the writhings in mice as compared to untreated control group (p < 0.001). Further, Viphyllin at 50 mg/kg showed strong antinociceptive effect in formalin-induced paw licking test (p < 0.05). Pretreatment of mice with AM630 significantly reversed the antinociceptive activity of Viphyllin in both early and late phases of formalin test (p < 0.05). Administration of Viphyllin markedly increased the latency time of mice in hot plate test (p < 0.001). Further, Viphyllin markedly increased the latency time of tail flick compared to control group from 30 min to 90 min after treatment. AM630, Capsazepine, and GW6471 abolished the analgesic effect of Viphyllin. These findings clearly suggest the involvement of CB2 receptor, TRPV1 ion channel and PPARα receptor activation in Viphyllin-mediated antinociceptive activity. Docking score predictions further supported the possible involvement of BCP in the antinociceptive mechanism of Viphyllin.
Conclusion: In conclusion, Viphyllin could be a natural pain-relieving agent involving safer pain signaling mechanisms, unlike conventional opiates and NSAIDs.
Keywords: Piper nigrum, β-caryophyllene, pain, nociceptive receptors
Introduction
Pain is a serious health issue with notable socioeconomic concerns affecting work productivity.1,2 Pain is associated with inflammation which can lead to tissue damage and loss of functionality at times.3 Nevertheless, a good number of analgesics such as opioids and non-steroidal anti-inflammatory drugs (NSAIDS) are available in the market, the associated side effects cannot be ignored.1,4 Prolonged usage of NSAIDS may lead to gastrointestinal and cardiovascular complications.5,6 This necessitates the search for complementary and alternative medicine, which can improve the quality of life, relieving the pain and inflammation. In this direction, dietary supplements and herbal extracts have gained significant attention, several of them being used since ages in ethnomedicine.7
Several culinary spices including clove, turmeric, ginger, and black pepper (Piper nigrum L.) have been reported to possess antinociceptive activity.8–11 Different parts of P. nigrum such as seeds, leaves and fruit have been used in traditional medicine, mostly in the Asian subcontinent. Preparations of black pepper have been used folklorically to cure gastrointestinal disorders, menstrual complications including menstrual pain, throat infection, cough, fever and skin diseases.12 The seeds of the plant have been used in ethnomedicinal preparations to relieve pain including headache, body ache, ear ache and throat pain.13–15 Black pepper extracts have been evaluated to possess several pharmacological activities, viz. antioxidant, anticonvulsant, antimicrobial, anticancer, antiinflammatory, analgesic and neuroprotective activities.11,16–19
The presence of piperine and essential oils impart pungency to black pepper. The essential oil contains terpenes such as β-caryophyllene (BCP), limonene, β-pinene and sabinene.20 BCP is a selective CB2 receptor agonist with health benefits such as neuroprotective and anti-inflammatory effects as established in preclinical models.21–23 The molecule is reported to exert antinociceptive effect in mouse model of pain via activation of cannabinoid receptor-2 (CB2).24 Here we have used a black pepper seed extract (Viphyllin) with a standardized content of BCP to investigate the potential mechanism of analgesic activity in mouse pain models. The involvement of endocannabinoid receptors CB1, CB2, transient receptor vanilloid 1 (TRPV1) ion channel and peroxisome proliferator-activated receptor α (PPARα) in Viphyllin-mediated antinociceptive activity is reported in this study. Further, in silico docking studies were performed to predict the interaction of BCP with the agonist-binding sites of the pain receptors.
Materials and Methods
Plant Extract
ViphyllinTM, black pepper extract was procured from the Department of Phytochemistry, R&D Center, Vidya Herbs Pvt Ltd. The black pepper seeds were harvested during winter (December–February). The oil fraction from the seeds was extracted using supercritical fluid extraction, and formulated to form the powder. Viphyllin was characterized using GCMS to contain not less than 30% BCP.25
Drugs
β-Caryophyllene (90% pure) was procured from Sigma-Aldrich. Diclofenac sodium (PubChem CID: 5018304), capsazepine (selective TRPV1 antagonist, PubChem CID: 2733484), GW6471 (PPARα antagonist, PubChem CID: 446738) and SR141716A (CB1 antagonist, PubChem CID: 104850) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). AM630 (selective CB2 antagonist, PubChem CID: 4302963) was procured from Tocris Bioscience, UK. The antagonists were reconstituted in dimethyl sulphoxide (DMSO). The concentration of DMSO in the final dose formulations did not exceed 0.5%.
Animals
Male Balb/c mice weighing 25–30 g (7–8-weeks-old) procured from the authorized animal supplier Biogen Laboratory Animal Facility, Bangalore, India, were used for the animal experiments. The animals were housed in the animal facility of R&D Center, Vidya Herbs Pvt Ltd under controlled environmental conditions (temperature: 23±2 °C, relative humidity: 35–50%, 12 h light/dark cycle). The animals were given commercial pellet diet and water ad libitum. All the animals were allowed to acclimatize to the environmental conditions before the start of experiments. The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Vidya Herbs Pvt Ltd., Bangalore, India (VHPL/PCL/IAEC/01/2021). The animals were maintained throughout the study in accordance with the CPCSEA (The Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India) guidelines. All the animal experiments were conducted with care considering the humane end points.
In vivo Antinociceptive Activity of Viphyllin
Acetic Acid-Induced Writhing Test
Viphyllin at different doses was screened for antinociceptive activity using acetic acid-induced writhing test as described by Koster et al26 with slight modifications. Thirty-six male mice were randomized into six groups (n=6 in each group): Control group were given normal saline solution while positive control group received reference drug diclofenac sodium (30 mg/kg). Three test doses of Viphyllin (10, 25 and 50 mg/kg body weight) were used in the experiment. Another group of mice were administered with 15 mg/kg of pure BCP. All the treatments were intraperitoneally injected. Thirty minutes after the respective treatments, the animals were given 10 mL/kg body weight of 1% acetic acid intraperitoneally. The number of writhes were recorded after 10 min of acetic acid administration.
Formalin-Induced Paw Licking Test
Formalin-induced paw licking test was performed in the presence and absence of selective antagonists of pain receptors. Forty-two male mice were divided into seven groups (6/group). Control group received vehicle while the treatment groups received the effective dose of Viphyllin (50 mg/kg body weight) and the reference compound BCP (15 mg/kg body weight). Other groups were intraperitoneally injected with different antagonists, viz. capsazepine (0.1 mg/kg body weight), GW6471 (1 mg/kg), AM630 (5 mg/kg), and SR141716A (0.1 mg/kg). The control group was administered intraperitoneally with 0.5% DMSO in saline. Thirty minutes after the antagonist administration, the mice were given Viphyllin or BCP intraperitoneally. Formalin test was conducted 30 min after the vehicle/extract treatment.27 Briefly, the animals were given 20 µL intraplantar injection of a 2.5% formalin solution. The injected paw-licking time (in seconds) was recorded during early (0–5 min) and late phase (25–30 min) after formalin injection.
Hot Plate Test
Mice were divided into different groups with six animals in each group. Control group was injected with vehicle and the treatment groups received Viphyllin (50 mg/kg body weight) and reference compound BCP (15 mg/kg body weight). The other groups were injected with the respective doses of antagonists as mentioned in previous experimental section. The mice were given Viphyllin 50 mg/kg body weight i.p. 30 min after the antagonist administration. Thirty minutes later, the animals were placed on a thermostatic hot plate with a temperature maintained at 55 ± 2 °C. The latency time ie, the time taken by the mice for initial jump was recorded. To prevent tissue damage, a cutoff time of 60s was used.28
Tail Flick Test
The antinociceptive activity of Viphyllin was further evaluated using tail flick test.29 Eighteen male mice were randomized to three groups with six animals in each group. The control group animals were intraperitoneally given the vehicle (0.5% DMSO) while the other two groups received 15 mg/kg i.p. of BCP and 50 mg/kg i.p. of Viphyllin, respectively. The tail flick (immersion) test was performed at 0, 30, 60, 90 and 120 min after the respective treatments. In brief, tail of each animal (5 cm) was immersed in a water bath maintained at 55 ± 2 °C. The reflex time of animal to withdraw the tail was recorded using a stopwatch. The cut-off time was kept as 15s to avoid tissue injury.
Further, to investigate the role of specific pain receptors, the tail flick test was performed in the presence or absence of selective antagonists of PPARα, TRPV1, CB1 and CB2 receptors. The randomization of animals and the respective antagonist treatments were done as mentioned in the previous sections. Mice were injected with the vehicle/Viphyllin (50 mg/kg body weight) 30 min after intraperitoneal administration of different antagonists as described previously. After 30 min of extract treatment, tail flick test was conducted.
Molecular Docking
The crystal structures of agonist-bound CB1 (PDB ID: 5XR8), CB2 (PDB ID: 6PT0), PPARα (PDB ID: 6KXY) receptors and TRPV1 ion channel (PDB ID: 3J5R) were retrieved from PDB database (http://www.rcsb.org/). Chain A of CB1 and PPARα receptors, chain R of CB2 receptor, and chain B of TRPV1 were used for macromolecule preparation. The coordinates of PDB structures were prepared for molecular docking by removing the water ions and ligands using Python molecule viewer.
The 3D structures of BCP were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov). The drug-like properties of the molecules were determined using SWISSADME prediction (http://www.swissadme.ch/). The OpenBabel tool was used to minimise energy of natural compounds and 3D coordinates were prepared (http://www.cheminfo.org/).
The crucial amino acid residues actively involved in the agonist binding of the nociceptive receptors were retrieved from the literature.30–33 AutoDock tool was utilized to generate grids, calculate dock score, and evaluate the conformers of BCP interacting in the binding sites of receptors. The grid map parameters were generated with AutoGrid (Table 1). As per genetic algorithm, all the torsions could rotate during docking. The Lamarckian genetic algorithm and the pseudo-Solis and Wets methods were applied for minimization, using default parameters.34
 |
Table 1 Grid Point Parameters Used for Molecular Docking of β-Caryophyllene with Nociceptive Receptor-Agonist Binding Sites |
Statistical Analysis
All the experimental data were analyzed by one way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism 9.0 (GraphPad Software, Inc.). The data were presented as mean ± SEM. p<0.05 were considered statistically significant.
Results
Viphyllin Exerts Significant Anti-Nociceptive Effects Against Visceral Pain in Mice
In this study, the antinociceptive effect of Viphyllin was initially screened using acetic acid-induced writhing test in mice. Figure 1 shows the number of writhings recorded in different treatment groups. Viphyllin at the doses of 10, 25 and 50 mg/kg body weight showed significant antinociceptive activity in dose-dependent fashion [F(5,30) = 22.27, p<0.001]. Viphyllin showed 38.4%, 61.2% and 69.97% inhibition in number of writhes at 10, 25 and 50 mg/kg respectively, as compared to control mice. The reference standard diclofenac sodium exhibited the writhing inhibition of 86.55% with respect to control group. Interestingly, the reference compound BCP at 15 mg/kg showed the highest antinociceptive activity (95.71%). Except for Viphyllin at 10 mg/kg [F(5,30) = 22.27, p<0.05], the extract treatment groups showed insignificant difference in the number of writhes as compared to diclofenac sodium treated mice. Based on these data, we have used 50 mg/kg of Viphyllin for subsequent experiments.
Viphyllin-Mediated Analgesic Action Involves Multiple Pain Receptors
The antinociceptive action of Viphyllin was investigated in the presence or absence of selective antagonists of different pain receptors. The extract at 50 mg/kg body weight exhibited a 2.18-fold [F(6,35) = 12.40, p<0.001] and 1.81-fold [F(6,35) = 8.32, p<0.01] reduction in the paw licking time compared to the vehicle treated group during early and late phases of formalin test respectively. The antinociceptive effect of Viphyllin was comparable to the reference compound BCP. BCP at 15 mg/kg reduced the paw licking time to 3.26-fold [F(6,35) = 12.40, p<0.001] and 3.19-fold [F(6,35) = 8.32, p<0.001] during early and late phases of the test respectively, compared to the control group.
The reduction in the paw licking time of Viphyllin-treated mice was reversed to a significant extent by the selective CB2 receptor antagonist (AM630) in both early [F(6,35) = 12.40, p<0.05] and late phases [F(6,35) = 8.32, p<0.05] of formalin test (Figure 2A and B), as compared to Viphyllin-treated mice. However, there was no significant change in the effect of Viphyllin observed following treatments with antagonists of TRPV1 (capsazepine), PPARα (GW6471) and CB1 (SR141716A).
In the hot plate test, Viphyllin treatment markedly enhanced the latency time of mice compared to the vehicle control group [F(6,35) = 15.25, p<0.001]. The antinociceptive activity of Viphyllin was comparable to reference compound BCP. The extract-mediated antinociceptive action was significantly reduced by the selective TRPV1, PPARα [F(6,35) = 15.25, p<0.05] and CB2 [F(6,35) = 15.25, p<0.001] receptor antagonists. CB1 antagonist did not have any significant influence on the effect of Viphyllin (Figure 3).
In tail flick test, Viphyllin at 50 mg/kg demonstrated significantly higher latency time compared to control group after 30, 60 [F(1.30,6.49) = 143.0, p<0.01] and 90 min [F(1.30,6.49) = 143.0, p<0.05] of extract treatment. The reference compound BCP also showed strong antinociceptive effect by significantly enhancing the latency time compared to control group at the respective time points after treatment [F(1.30,6.49) = 143.0, p<0.001 at 30 min; p<0.01 at 60 and 90 min; p<0.05 at 120 min] (Figure 4A). Interestingly, the action of Viphyllin was reversed to a significant extent by antagonists of PPARα [F(6,35) = 17.34, p<0.01], TRPV1, CB2 and CB1 receptors [F(6,35) = 17.34, p<0.001] (Figure 4B). All the pain models included a normal control group, the data of which were out of scale to present in the results (data not shown).
β-Caryophyllene, Prinicipal Component of Viphyllin Interacts with the Agonist-Binding Sites of Pain Sensors
In this study, we have predicted the interactive mode of BCP, the standardized content of Viphyllin, with the different nociceptive receptors. The drug likeliness of BCP was determined using SWISSADME (Table 2). The molecule satisfied the Lipinski’s rule of five (molecular weight, polar surface area, lipophilicity, hydrogen bonding and charge) with one violation (MLogP >4.15).
 |
Table 2 Drug Likeliness of β-Caryophyllene |
Figure 5 shows the three-dimensional view of the pain receptors retrieved from PDB. Chain A of CB1 and PPARα receptors, chain R of CB2 receptor, and chain B of TRPV1 were used for docking studies.
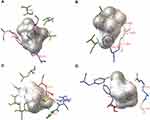
Table 3 shows the docking scores of BCP bound in the agonist-binding sites of the pain receptors. BCP showed an appreciable interaction pattern in the orthosteric pocket of CB2 with a binding energy of −7.83 kJ/mol and Ki of 1.81 µM. Due to the structural constraints from the nine-membered ring, BCP demonstrated only four geometries during docking. The molecule closely interacted with hydrophobic residues Trp194, and Phe183 and Ile186 of ECL2 (Figure 6A). These residues have been reported to be crucial to function in ligand binding.35,36 In addition, BCP had profound interactions with Ile110, Thr114, Val113 and Phe281. BCP had a moderate binding with CB1 receptor binding site. The best docking pose showed a binding energy of −5.16 kJ/mol (Ki = 165.22 µM). BCP did not interact with any of the key residues of CB1 (Figure 6B). The best scoring pose of BCP with PPAR-α putative binding site (Ki = 13.05 µM) showed key interactions including Ile241, Ile272, and Ala333, and the lowest binding energy of −6.66 kJ/mol. BCP showed several molecular interactions with the amino acid residues of the binding pocket (Figure 6C). The docking results of BCP against TRPV1 ion channel showed the lowest binding energy value of −7.58 kJ/mol (Ki = 2.8 µM). BCP engaged in interaction with Phe488, Arg491, Gly492, Glu513, Phe516, and Tyr554 residues in the capsaicin-binding pocket of TRPV1 (Figure 6D).
 |
Table 3 Docking Score of Interaction of β-Caryophyllene with Agonist Binding Sites of Different Pain Receptors |
Discussion
Plants in general are a good source of compounds with analgesic action.37 Black pepper oil has several terpene compounds with pain relieving attributes involving peripheral pain receptors.38 BCP is a cannabinoid analogue that selectively activates the CB2 receptor and has potential anti-nociceptive effects in inflammatory and neuropathic pain models.24 In this study, Viphyllin, extract from the seeds of black pepper with a standardized content of BCP, not less than 30% was used to evaluate the potential antinociceptive activity.
The antinociceptive action of Viphyllin was initially evaluated with 10mg, 25mg and 50mg/kg doses using acetic acid–induced writhing test. There was a dose-dependent response of Viphyllin-treated mice to exert antinociceptive effect in writhing test. The extract at 50 mg/kg showed 69.97% inhibition of inflammatory pain, comparable to the reference drug diclofenac sodium. Previously, Jeena et al demonstrated the antinociceptive activity of black pepper oil using inflammatory pain models. It was reported that black pepper oil could substantially reduce the nociception via inhibition of lipoxygenases and cyclooxygenases.16 Our results are in line with these findings, and suggest the inhibitory effects of Viphyllin on inflammatory mediators of nociception. Further to evaluate the mode of action of Viphyllin, we used 50 mg/kg as the test dose in other murine models of pain viz., formalin-induced paw licking, hot plate, and tail flick tests.
Several pain sensors other than the opioid receptors play crucial role in the antinociceptive action of compounds. The activation of cannabinoid receptors CB1 and CB2 predominantly subsides the chronic and inflammatory pain.39 In the peripheral sensory neurons, the activated PPARα diminishes pain by desensitizing the TRPV1 ion channels.40 Development of analgesics involving these receptors seem to be the safer anti-nociceptive treatment strategies compared to the conventional opiates and NSAIDS. Hence, in this study, we have investigated the role of CB1, CB2, PPARα and TRPV1 receptors in Viphyllin-mediated analgesic effects.
As like the writhing test, Viphyllin showed significant analgesic activity in another inflammatory pain model, formalin test. This effect was reversed by AM630 (selective CB2 antagonist) in both early and late phases of formalin test. This explains the role of CB2 receptor in the antinociceptive effect of Viphyllin. The presence of BCP in the extract might have substantially contributed to the activation of CB2 receptor.24
Further, the extract was evaluated for thermal analgesia using hot plate and tail flick tests. Hot plate test is a simple and extensively used method to investigate the central antinociceptive activity of drugs.41 Both hot plate and tail flick models have been implicated, assessing the antinociceptive action of drugs in modulating acute thermal pain. However, the neurological mechanism of pain modulation is different in these models. Tail flick test uses radial heat to measure the spinal refluxes, while hot plate test involves a more complex supraspinal reflux action.42 Viphyllin treated mice showed a significantly higher latency time compared to control animals. This antinociceptive effect was inhibited to a significant extent by the pretreatment of mice with capsazepine (TRPV1 antagonist), GW6471 (PPARα antagonist) and AM630 (CB2 antagonist) before Viphyllin administration. Similar observations were made in tail flick test. However, there was also an involvement of CB1 receptor in Viphyllin-mediated analgesic effect in tail flick test. It can be speculated that the active constituents, including BCP of Viphyllin, may activate one or more of these receptors to modulate pain.
TRPV1 transmembrane ion channels transduce pain signals in response to noxious stimuli.43 Activation of TRPV1 leads to a cascade of calcium-dependent processes to desensitize the channel.44 Endocannabinoids and synthetic cannabinoid ligands have been reported to modulate the TRPV1 channel.45,46 BCP is a selective CB2 agonist and can thus contribute significantly to the desensitization of TRPV1 channel, as observed in the present study. There exists a close link between endogenous PPARα signaling in sensory neurons and TRPV1 desensitization to control excess pain. Ambrosino et al reported that palmitoylethanolamide, a PPARα agonist activated the TRPV1 channel in sensory neurons.47 Our results are in line with these findings. Viphyllin-mediated activation of PPARα and TRPV1 receptors in thermal antinociceptive tests could be attributed majorly to the presence of BCP. However, the activation of TRPV1 ion channel might have also involved other constituents of black pepper. Okumura et al have reported previously the involvement of several phytoconstituents such as piperine, piperoline A, isopiperine, isochavisine, piperoline B and dihydropipernonaline in the activation of TRPV1.48 Overall, the data obtained from the in vivo pain models strongly indicates the involvement of CB2, PPARα receptors and TRPV1 ion channel.
The interaction of BCP, the characterized constituent of Viphyllin with the agonist-binding sites of pain receptors was studied using molecular docking simulation. BCP showed an obvious stronger binding affinity with the orthosteric site of CB2 receptor. Alongside, the molecule also interacted well with the agonist-binding sites of PPARα and TRPV1. The docking predictions agree with the experimental data and further support the involvement of BCP in the activation of the pain receptors contributing to the analgesic activity of Viphyllin. The outcome of the study highlights the possible role of BCP as a major contributor to the observed antinociceptive effects of Viphyllin. The Viphyllin-mediated antinociceptive activity was comparable to that of BCP in pure form. It can be thus speculated that BCP could act in synergy with other phytoconstituents to ameliorate the pain in animals.
The results of the present study shows that Viphyllin could act as antinociceptive agent by selectively activating the pain receptors CB2 and PPARα in peripheral tissues to desensitize the TRPV1 ion channel. Further, the phytoconstituents of Viphyllin including BCP could possibly reduce the release of endogenous PGE2 via inhibition of inflammatory factors such as lipoxygenase and cyclooxygenases, to ameliorate the inflammatory pain.
Conclusion
The present study findings demonstrate the efficacy of Viphyllin, alleviating the peripheral and central pain in mice. The extract was found to exert antinociceptive action via activation of endocannabinoid receptor CB2, PPARα and TRPV1 ion channels. β-Caryophyllene, the characterized active constituent of Viphyllin was further demonstrated to have considerable interaction with the agonist-binding sites of the nociceptive receptors. This study strongly recommends Viphyllin as potential candidate for reducing pain and inflammation.
Acknowledgment
The authors thank Dr Mounisha R. for technical assistance in the animal experiments.
Disclosure
All the authors are employed by Vidya Herbs Pvt Ltd., and hence declare potential conflicts of interest. The authors report no other potential conflicts of interest for this work.
References
1. Cazacu I, Mogosan C, Loghin F. Safety issues of current analgesics: an update. Clujul Med. 2015;88:128–136.
2. Fongang ALM, Laure Nguemfo E, Djouatsa Nangue Y, et al. Antinociceptive and anti-inflammatory effects of the methanolic stem bark extract of Antrocaryon klaineanum Pierre (Anacardiaceae) in mice and rat. J Ethnopharmacol. 2017;203:11–19. doi:10.1016/j.jep.2017.03.036
3. Furtado AA, Torres-Rego M, Lima MC, et al. Aqueous extract from Ipomoea asarifolia (Convolvulaceae) leaves and its phenolic compounds have anti-inflammatory activity in murine models of edema, peritonitis and air-pouch inflammation. J Ethnopharmacol. 2016;192:225–235. doi:10.1016/j.jep.2016.07.048
4. Tacconelli S, Bruno A, Grande R, et al. Nonsteroidal anti-inflammatory drugs and cardiovascular safety - translating pharmacological data into clinical readouts. Expert Opin Drug Saf. 2017;16:791–807. doi:10.1080/14740338.2017.1338272
5. Nagata N, Niikura R, Aoki T, et al. Effect of proton-pump inhibitors on the risk of lower gastrointestinal bleeding associated with NSAIDs, aspirin, clopidogrel, and warfarin. J Gastroenterol. 2015;50:1079–1086. doi:10.1007/s00535-015-1055-2
6. Moore N, Salvo F, Duong M, et al. Cardiovascular risks associated with low-dose ibuprofen and diclofenac as used OTC. Expert Opin Drug Saf. 2014;13:167–179. doi:10.1517/14740338.2014.846324
7. Jahromi B, Pirvulescu I, Candido KD, et al. Herbal medicine for pain management: efficacy and drug interactions. Pharmaceutics. 2021;13(2):251. doi:10.3390/pharmaceutics13020251
8. El-Saber Batiha G, Alkazmi LM, Wasef LG, Beshbishy AM, Nadwa EH, Rashwan EK. Syzygium aromaticum L. (Myrtaceae): traditional uses, bioactive chemical constituents, pharmacological and toxicological activities. Biomolecules. 2020;10(2):202.
9. Razavi BM, Rahbardar G, Hosseinzadeh H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother Res. 2021;35(12):6489–6513. doi:10.1002/ptr.7224
10. Pagano E, Souto EB, Durazzo A, et al. Ginger (Zingiber officinale Roscoe) as a nutraceutical: focus on the metabolic, analgesic, and anti-inflammatory effects. Phytother Res. 2021;35(5):2403–2407. doi:10.1002/ptr.6964
11. Tasleem F, Azhar I, Ali SN, et al. Analgesic and anti-inflammatory activities of Piper nigrum L. Asian Pac J Trop Med. 2014;7:S461–8. doi:10.1016/S1995-7645(14)60275-3
12. Takooree H, Aumeeruddy MZ, Rengasamy KRR, et al. A systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Crit Rev Food Sci Nutr. 2019;59(sup1):S210–S243. doi:10.1080/10408398.2019.1565489
13. Francis Xavier T, Kannan M, Auxilia A. Observation on the traditional phytotherapy among the Malayali tribes in Eastern Ghats of Tamil Nadu, South India. J Ethnopharmacol. 2015;165:198–214.
14. Bhuyan B, Baishya K. Ethnomedicinal value of various plants used in the preparation of traditional rice beer by different tribes of Assam, India. Drug Invent Today. 2013;5(4):335–341. doi:10.1016/j.dit.2013.09.002
15. Ayyanar M, Ignacimuthu S. Traditional knowledge of Kani tribals in Kouthalai of Tirunelveli hills, Tamil Nadu, India. J Ethnopharmacol. 2005;102(2):246–255. doi:10.1016/j.jep.2005.06.020
16. Jeena K, Liju VB, Umadevi NP, et al. Antioxidant, anti-inflammatory and antinociceptive properties of black pepper essential oil (Piper nigrum Linn). J Essent Oil-Bear Plants. 2014;17(1):1–12. doi:10.1080/0972060X.2013.831562
17. Belemkar S, Kumar A, Pata MK. Pharmacological screening of herbal extract of Piper nigrum (Maricha) and Cinnamomum zeylanicum (Dalchini) for anticonvulsant activity. Invent Rapid Ethnopharmacol. 2013;2:1–5.
18. Prashant A, Rangaswamy C, Yadav AK, et al. In vitro anticancer activity of ethanolic extracts of Piper nigrum against colorectal carcinoma cell lines. Int J Appl Basic Med Res. 2017;7(1):67–72. doi:10.4103/2229-516X.198531
19. Hritcu L, Noumedem JA, Cioanca O, et al. Anxiolytic and antidepressant profile of the methanolic extract of Piper nigrum fruits in beta-amyloid (1–42) rat model of Alzheimer’s disease. Behav Brain Funct. 2015;11:13. doi:10.1186/s12993-015-0059-7
20. Wang M, Chittiboyina A, Parcher J, et al. Piper nigrum oil – determination of selected terpenes for quality evaluation. Planta Med. 2018;17:84.
21. Ojha S, Javed H, Azimullah S, et al. beta-caryophyllene, a phytocannabinoid attenuates oxidative stress, neuroinflammation, glial activation, and salvages dopaminergic neurons in a rat model of Parkinson disease. Mol Cell Biochem. 2016;418:59–70. doi:10.1007/s11010-016-2733-y
22. Guo K, Mou X, Huang J, et al. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-kappa B activation in microglia. J Mol Neurosci. 2014;54:41–48. doi:10.1007/s12031-014-0243-5
23. Viveros-Paredes JM, Gonzalez-Castaneda RE, Gertsch J, et al. Neuroprotective effects of beta-caryophyllene against dopaminergic neuron injury in a murine model of Parkinson’s disease induced by MPTP. Pharmaceuticals. 2017;10:60. doi:10.3390/ph10030060
24. Klauke AL, Racz I, Pradier B, et al. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur Neuropsychopharmacol. 2014;24:608–620. doi:10.1016/j.euroneuro.2013.10.008
25. Sudeep HV, Venkatakrishna K, Gouthamchandra K, et al. A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins. J Food Biochem. 2021;45(12). doi:10.1111/jfbc.13994
26. Koster R, Anderson M, De Beer EJ. Acetic acid for analgesic screening. Fed Proc. 1959;18:412–418.
27. Jaffal SM, Abbas MA. Antinociceptive action of Achillea biebersteinii methanolic flower extract is mediated by interaction with cholinergic receptor in mouse pain models. Inflammopharmacol. 2018;27:961–968. doi:10.1007/s10787-018-0524-7
28. Farsam H, Amanlou M, Deppour AZ, et al. Anti-inflammatory and analgesic activity of Biebersteinia multifida. DC root extract. J Ethnopharmacol. 2000;55:93–98.
29. Langerman L, Zakouski MI, Piskoun B, et al. Hot plate versus tail flick evaluation of acute tolerance to continuous morphine infusion in the rat model. J Pharmacol Toxicol Methods. 1995;34:23–28. doi:10.1016/1056-8719(94)00077-H
30. Hua T, Vemuri K, Nikas SP, et al. Crystal structures of agonist-bound human cannabinoid receptor CB1. Nature. 2017;547:468–471. doi:10.1038/nature23272
31. Gertsch J, Leonti M, Raduner S, et al. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci USA. 2008;105:9099–9104. doi:10.1073/pnas.0803601105
32. Yang F, Zheng J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell. 2017;8:169–177.
33. Yoshida T, Oki H, Doi M, et al. Structural basis for PPARα activation by 1H-pyrazolo-[3,4-b]pyridine derivatives. Sci Rep. 2020;10:7623. doi:10.1038/s41598-020-64527-x
34. Rodriguez A, Infante D. Characterization in silico of flavonoids biosynthesis in Theobroma cacao L. Net Biol. 2011;1:34–45.
35. Xing C, Zhuang Y, Xu TH, et al. Cryo-EM structure of the human cannabinoid receptor CB2-Gi signaling complex. Cell. 2020;180(4):645–654.e13. doi:10.1016/j.cell.2020.01.007
36. Feng Z, Alqarni MH, Yang P, et al. Modeling, molecular dynamics simulation, and mutation validation for structure of cannabinoid receptor 2 based on known crystal structures of GPCRs. J Chem Inf Model. 2014;54:2483–2499. doi:10.1021/ci5002718
37. de Cássia da Silveira ESR, TC Lima, da Nóbrega FR, et al. Analgesic-like activity of essential oil constituents: an update. Int J Mol Sci. 2017;18(12):2392. doi:10.3390/ijms18122392
38. Sakurada T, Mizoguchi H, Kuwahata H, et al. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol Biochem Behav. 2011;97:436–443. doi:10.1016/j.pbb.2010.09.020
39. Anthony A, Rahmat S, Sangle P, et al. Cannabinoid receptors and their relationship with chronic pain: a narrative review. Cureus. 2020;12:e10436.
40. Giniatullin R, Bart G, Tavi P. Complex role of peroxisome proliferator activator receptors (PPARs) in nociception. Scand J Pain. 2015;9:70–71. doi:10.1016/j.sjpain.2015.08.006
41. de Carvalho PR, Ropero DR, Pinheiro MM, et al. Quinoline alkaloids isolated from Choisya Aztec-pearl and their contribution to the overall antinociceptive activity of this plant. PLoS One. 2016;11:e0164998. doi:10.1371/journal.pone.0164998
42. Santenna C, Kumar S, Balakrishnan S, et al. A comparative experimental study of analgesic activity of a novel non-steroidal anti-inflammatory molecule – zaltoprofen, and a standard drug – piroxicam, using murine models. J Exp Pharmacol. 2019;11:85–91. doi:10.2147/JEP.S212988
43. Caterina MJ. TRP channel cannabinoid receptors in skin sensation, homeostasis, and inflammation. ACS Chem Neurosci. 2014;5:1107–1116.
44. Iannotti FA, Hill CL, Leo A, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5:1131–1141. doi:10.1021/cn5000524
45. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163:1479–1494. doi:10.1111/j.1476-5381.2010.01166.x
46. Soethoudt M, Grether U, Fingerle J, et al. Cannabinoid CB2 receptor ligand profiling reveals biased signalling and off-target activity. Nat Commun. 2017;8:13958.
47. Ambrosino P, Soldovieri MV, Russo C, et al. Activation and desensitization of TRPV1 channels in sensory neurons by the PPAR agonist palmitoylethanolamide. Br J Pharmacol. 2013;168:1430–1444. doi:10.1111/bph.12029
48. Okumura Y, Narukawa M, Iwasaki Y, et al. Activation of TRPV1 and TRPA1 by black pepper components. Biosci Biotechnol Biochem. 2010;74(5):1068–1072. doi:10.1271/bbb.90964
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.