Back to Journals » Journal of Pain Research » Volume 15
Vessel-Plasty Using Bone-Filling Mesh Container for Treatment of Malignant Severe Compression Fractures in Cervical Vertebrae
Authors Yang Y, Tian Q , Wang T, Lu Y, Li W, Wu C
Received 28 January 2022
Accepted for publication 6 April 2022
Published 21 April 2022 Volume 2022:15 Pages 1173—1182
DOI https://doi.org/10.2147/JPR.S360195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Krishnan Chakravarthy
Yue Yang, Qinghua Tian, Tao Wang, Yingying Lu, Wenbin Li, Chungen Wu
Department of Diagnostic and Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, Shanghai, People’s Republic of China
Correspondence: Chungen Wu; Wenbin Li, Department of Diagnostic and Interventional Radiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital, 600 Yi Shan Road, Shanghai, People’s Republic of China, Tel +8618930177559 ; +8618930177524, Email [email protected]; [email protected]
Objective: To evaluate the feasibility, safety, and efficacy of vessel-plasty using bone-filling mesh container (BFMC) for malignant severe compression fractures of cervical vertebra.
Methods: This study prospectively recruited fifteen consecutive patients (eight men, seven women; mean age, 57.4 years) with severe malignant compression fractures of cervical vertebrae for vessel-plasty. Procedure duration, incidence of cement leakage and other complications, pain relief and improvement of neck function were analyzed. Pain was assessed using a visual analog scale (VAS) and function by the neck disability index (NDI), with scores recorded before the procedure and at 3 days and 1, 3, 6 and 12 months after the procedure.
Results: A total of 16 vertebrae were treated. All vertebrae had destruction of bone in more than one place as well as broken bone walls. Mean procedure duration was 42.9± 13.6 minutes. Bone cement leakage occurred in two vertebrae without any symptoms. No procedure-related complications occurred. Mean VAS and NDI declined from 7.1 ± 1.4 and 63.6 ± 16.3, respectively, before the procedure to 3.5 ± 1.1 and 37.4 ± 11.0, respectively, at three days after the procedure (P < 0.01). CT images at three months after the procedure confirmed that there were no cases of refractures at the treated or adjacent levels, recurrence of vertebral collapse and mobilization of bone cement block.
Conclusion: Vessel-plasty using BFMC appears to be effective and safe for malignant severe compression fractures in cervical vertebrae. It is effective in stabilizing vertebral body, relieving pain.
Keywords: vessel-plasty, bone-filling mesh container, cervical spine, severe, compression fractures, metastasis, percutaneous vertebroplasty
Introduction
More than two-thirds of patients with advanced malignancy will develop spinal metastasis.1 Spinal metastases with pathological fractures need immediate treatment due to severe pain, destruction of spinal stability and potential compression of spinal cord.2 The treatment methods mainly include open spinal surgery and minimal access spinal surgery. Open spinal surgery is beneficial in terms of better local control, but it is a highly demanding and risky procedure. It may involve more postoperative complications with a trend of lower survival rates and higher recurrence rates compared to minimal access spinal surgery.3,4 Percutaneous vertebroplasty (PVP), as a minimally invasive procedure, has been recognized as an effective method to relieve pain and stabilize the spine.5,6 There are several patterns of vertebral fractures. Severe compression fractures refer to fractures in which the height of the vertebral body collapses less than one-third of its original height.7 Such fractures are considered contraindications due to technical difficulty and high risk of cement leakage.8,9 In recent years, PVP and vertebral augmentation with auxiliary device is gradually applied to severe compression fractures and extensive lytic lesions of thoracic and lumbar spine with the progress of minimally invasive procedure.7,10–14 However, severe compression fractures of the cervical spine have always been considered high-risk fractures.15–17 The main reason is that they are located in the upper vertebrae, close to the main blood vessels and nerve structures.18,19 The diameter of the puncture needle is similar to the height of the severely compressed vertebral body, so it is difficult to penetrate into the vertebral body. In addition, metastatic fractures are accompanied by serious collapse and destruction of bone wall, and there is no “bed” to hold bone cement during injection and bone cement may easily enter the spinal canal along the posterior wall, aggravating the compression of the spinal cord originally caused by compression fractures.
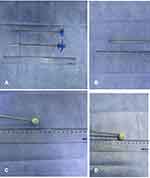
The treatment of such high-risk cases is very difficult. Studies specifically addressing to severe compression fractures of the cervical spine are quite rare and only one article once reported five cervical vertebrae to be best knowledge of the authors.20 There is an urgent need for more experience and technology modification and updating of cementoplasty.15 In recent years, a new minimally invasive approach of vertebral augmentation using Vessel-X Bone-Filling Mesh Container System has been developed and applied.21–23 This technology has two theoretical advantages over PVP. First, an expander raises the collapsed endplate, forms a cavity in the bone lesion area, and increases the filling space of bone cement. Second, the injection of bone cement is performed through a bone-filling mesh container (BFMC), which creates the “bone wall” of the vertebral body and reduces the risk of cement leakage (Figure 1). Due to technical difficulties, there is yet no reported application in cervical spine. In view of the innovation of this new technology, we applied it to malignant severe compression fracture of cervical spine, and reported the feasibility, safety and effectiveness of its application in such high-risk cases.
Materials and Methods
Patients
This study was a prospective, single-arm, observational study. From June 2019 to June 2021, fifteen consecutive patients (eight males and seven females; mean age: 57.4±10.9 years) with malignant osteolytic lesions of the cervical spine and severe compression fractures were treated with vessel-plasty using BFMC at our hospital.
The inclusion criteria were: 1) The primary malignant tumor with cervical metastasis or primary malignant tumor of cervical spine diagnosis was clear; 2) patients presented with severe pain in the neck and functional impairment for at least 1 month; 3) Preoperative CT images indicated osteolytic lesions of cervical spine with severe compression fractures. The measurement of the vertebral body height is determined by the largest collapse layer on the sagittal CT image, and the original height is estimated by the height of the adjacent vertebral body. When its height is less than one-third of the original height, it is determined as a severe compression fracture; 4) ineffective treatments with opioids, chemotherapy or radiotherapy; 5) A multidisciplinary team of orthopaedic specialists, neurosurgeons and interventional physicians believed that the patient would benefit from the expected procedure and determined the treatment decision according to the spinal instability neoplastic score (SINS) and Tomita score.24,25 The SINS is a practical classification system, which aims to guide clinicians to determine the stability of spinal tumors for patients. The Tomita score is used to determine the treatment plan accordingly for patients.
The exclusion criteria were: 1) persistent systemic infection; 2) uncorrectable coagulation disorders (platelet count <90,000 mm3, international normalized ratio >1.5).
Enter patient information (age, sex, primary tumor, and so on) into the database. Severity and duration of pain and functional impairment were assessed via a questionnaire. Computed tomography (CT) and contrast-enhanced magnetic resonance imaging (MRI) were performed before the procedure to determine the characteristics, shape of the lesions and the presence of bone wall damage and spinal canal invasion.
Procedure
The procedure was performed under strict aseptic conditions, with continuous monitoring of the electrocardiogram, oxygen saturation, and blood pressure.
The puncture was performed under biplane fluoroscopic (Innova IGS 630, GE Medical Systems Scs, American) guidance. The vertebral bodies were punctured through an anterolateral approach with the patient in the supine position. The preoperative unenhanced CT and enhanced MRI images were used to determine the entry point and the angle and depth of needle insertion.
The procedure was performed under local anesthesia and conscious sedation (mix of 2% lidocaine and ropivacaine).26 Disinfect the puncture site and anesthetize the puncture approach. The needle was advanced slowly, with intermittent aspiration performed to prevent accidental injection of the anaesthetic into a blood vessel.
Because of the proximity of the cervical spine to vital structures, all punctures were performed using a 17G coaxial biopsy needle (Bard Peripheral Vascular Inc., Arizona, USA), which consists of an additional blunt tip stylet and a conventional puncture needle. The index and middle fingers were placed between the trachea and carotid sheath, and pressure was applied so that the carotid artery was pushed posterolaterally and the trachea was pushed contralaterally. The puncture needle was slowly passed along the medial border of the carotid artery at an angle of approximately 15°-25° under fluoroscopic guidance monitoring. The blunt tip stylet helped avoid damage to important blood vessels and nerves. The patient’s general condition, blood pressure, respiration, articulation, swallowing function and sensory motor function of limbs were continuously monitored during the procedure.
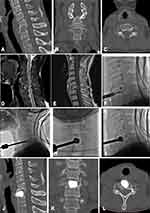
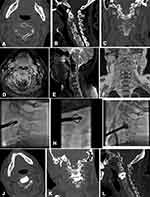
After the 17G puncture needle safely reached the target site, exchange 11G trocar through the expansion tube to establish a working path with the same diameter as an expander. The solid core vertebral drill (Shandong Guanlong Medical Products Co., Shandong, China) was used to gradually rotate and cut surrounding bone tissue to establish the channel under fluoroscopy. The expander (Shandong Guanlong Medical Products Co., Shandong, China) was inserted to expand the vertebral body in multiple directions and establish a cavity. The collapsed endplates and vertebral body were restored, and the expander was removed. Then, the BFMC (Shandong Guanlong Medical Products Co., Shandong, China) was placed in the created cavity. Bone cement was prepared and loaded into the screw-syringe, which was connected to the BFMC. The bone cement (OSTEOPAL® V, Heraeus Medical GmbH, Hanau, Germany) —was then injected in very small increments under fluoroscopy. As the cement was injected, the BFMC expanded and small amounts of bone cement slowly exuded through the mesh into the vertebral body. When the bone cement had spread to the edge of the vertebral body, the injection was stopped. The BFMC was released and the procedure was terminated (Figures 2 and 3).
Data Collection
Patient Characteristics
Site of primary tumor, level of involved cervical spine, and lesion characteristics (bone wall breakage, involvement of the spinal canal) were recorded.
Procedure-Related Variables
Technical success, duration of the procedure, volume of cement injected into each vertebral body, and distribution of cement were recorded. Cement leakage was evaluated and documented with nonenhanced CT scan in the immediate postoperative period. The leakage sites were classified as prevertebral area, paravertebral area, puncture path, intervertebral disc area, paravertebral vein, and epidural area. Procedure-related complications were recorded and graded according to the Cardiovascular and Interventional Radiological Society of Europe and Society of Interventional Radiology guidelines.27 Non-enhanced CT examination was performed at three months after the procedure. Imaging data were used to evaluate new spinal events such as refractures at the treated or adjacent levels, recurrence of vertebral collapse and mobilization of bone cement block. When the patient has sudden neck dysfunction, urgentclinical and imaging examination should be performed to exclude any recurrent or new spinal events.
Clinical Evaluation
Visual analog scale (VAS) scores were used to assess the severity of subjective pain. The VAS score could range from 0 to 10, with 0 indicating no pain and 10 indicating the most severe pain. Neck Disability Index (NDI) were used to assess neck dysfunction.28 The NDI comprises 10 items, each with six possible responses. The total score of NDI ranges from 1 to 100. The higher the score, the more serious the neck dysfunction. VAS and NDI scores were recorded before the procedure (baseline) and at 3 days, 1 month, 3 months, 6 months and 12 months after the procedure. Scores at baseline and at 3 days after the procedure were recorded via face-to-face interview with a clinician. Scores at 1 month and thereafter were recorded by the same clinician via postal questionnaires and telephone interviews.
Statistical Analysis
Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Data were expressed as means ± standard deviations. The paired t-test was used to compare differences between the preoperative and postoperative vertebral body heights and the differences in VAS and NDI scores between time points. Statistical significance was at P < 0.05.
Results
Patient Characteristics
Of the fifteen patients, fourteen had primary tumor metastasis to cervical spine, with the primary tumors being in lung (seven patients), liver (three patients), stomach (two patient), breast (one patient), and thyroid (one patient). One patient had multiple myeloma involving the cervical spine. A total of 16 vertebral bodies were treated. The involved vertebrae were C3 (n = 2), C4 (n = 1), C5 (n = 6), C6 (n = 4), and C7 (n = 3). All 16 vertebral bodies had malignant severe fractures with broken bone walls; in thirteen cases there was associated spinal canal stenosis. There was rupture of the anterior bone wall in ten cases, the posterior wall in twelve cases, the upper endplate in twelve cases, the lower endplate in eleven cases, and the lateral wall in ten cases. Table 1 summarizes the patient demographics and lesion characteristics.
 |
Table 1 Characteristics of the Patients |
Procedure-Related Variables
Vessel-plasty was technically successful in all sixteen vertebral bodies in the study group, with a success rate of 100%. Outside the study group of vessel-plasty, there are three vertebral bodies (one C4 and two C7) with osteolytic metastatic were treated with traditional PVP.
The mean duration of the procedure was 42.9±13.6 minutes (range, 24–67 minutes). Each vertebral body was injected with a mean of 2.8 ± 0.6 mL (range, 1.8–3.4 mL) of bone cement. Cement distribution was satisfactory in all cases. Cement leakage occurred in two cases (12.5%), with the sites of leakage being to the paravertebral vein (one case) and the paravertebral area (one case); however, there were no associated symptoms in these cases. No neurological or severe vascular injuries were recorded. No pulmonary embolism, infection or other systemic complications occurred. No patient needed additional surgical procedures such as spinal fixation or surgical decompression.
All patients underwent CT scanning at 3 months after the procedure. The images confirmed that there were no cases of refractures at the treated or adjacent levels, recurrence of vertebral collapse and mobilization of bone cement block.
Clinical Evaluation
Mean follow-up was for 5.9 ± 3.1 months (range, 3–12 months). Two patients died of their primary tumor during the follow-up period.
Mean VAS score decreased from 7.1 ± 1.4 before the procedure to 3.5 ± 1.1 at 3 days after the procedure and to 2.4 ± 0.7 at 1 month after the procedure. Mean NDI score decreased from 63.6 ± 16.3 before the procedure to 37.4 ± 11.0 at 3 days after the procedure and to 24.8 ± 7.5 at 1 month after the procedure. The decreases in the scores were statistically significant (P < 0.01; Table 2).
 |
Table 2 Clinical Outcomes as Assessed by Changes in VAS and NDI Scores |
As of the last follow-up, no patients in the study group underwent urgent clinical and imaging examination due to sudden neck dysfunction.
Discussion
Malignant severe compression fractures of the cervical spine have rarely been reported due to the challenging nature of treatment in this region. In this study, 16 vertebral bodies of 15 patients were successfully treated with vessel-plasty. The success rate of this technology was 100% with remarkable effect of pain alleviation and vertebral body stabilization.
At present, there are no comparable relevant researches on the same subject. Our results showed that, in terms of pain relief and recovery of function, this approach is as effective as PVP in the treatment of cervical spine metastases.15,18,29 However, Bone cement leakage occurred in two of sixteen vertebral bodies with a leakage rate of 12.5%, which is lower than the rates reported with PVP of cervical spine metastases.18,30 In particular, all vertebrae had multiple bone wall destruction and collapse in this study. In clinical practice, such cases are vulnerable to multiple leaks along the bone wall cracks while injecting, including epidural leakage, which is the main risk of traditional PVP.31 This leakage will aggravate compression symptoms, and emergency surgical decompression is required in case of spinal cord or nerve root injury. In our study, there is only one case of cement leakage into the paravertebral vein and another case into the paravertebral area, but no leakage into the epidural space. This reflects the superior anti-leakage performance of BFMC.For cases of extreme osteolysis and severe compression fractures, there are reports of some new technologies, such as stent assisted cementoplasty.12–14 Our results seem to have a lower leakage rate than some reports.13,14 However, the sites involved in these reports are the thoracic and lumbar vertebrae. In addition, the auxiliary devices used in these reports are metallic stent, and the BFMC used in this study is made of Polyethylene terephthalate. To the knowledge of the authors, there is no biomechanical study on the effects of vessel-plasty on refractures at the treated or adjacent levels.The follow-up period of this study was 1–11 months, with a mean period of 5 months.During the follow-up period, no fractures or loosening of bone cement blocks in the vertebral body secondary to this procedure was recorded, which seems to indicate its good medium and long-term effect, but this result may be limited by the small number of cases. Larger sample size studies may be required to confirm our result. In terms of the injection volume of bone cement, the only study on the same subject reported that the mean injection volume of bone cement was 1.8mL,17 which was quite small due to severe compression of the cervical vertebral body. In this study, the mean volume of cement per cervical vertebral body reached 2.8 mL, which was a relatively large volume, ensuring more sufficient augmentation. We believe that this result is achieved through the repair and elevation of the vertebral collapse endplate by the expander. In our experience, good coverage of the lesion area by bone cement ensures better long-term results. Our view supports that it is a oversimplification to assume that any minimal amount of injected bone cement will have a clinical effect.32 However, there are different opinions in the literature.22 The relationship between the amount of bone cement injection and long-term efficacy needs to be further studied.
The volume of cervical vertebra is smaller than that of thoracic vertebra and lumbar vertebra. The model of puncture needle of BFMC system is 11G. When the vertebral body is seriously compressed fracture, it will be not possible for the puncture needle to penetrate and reach the center of the focus inside the vertebral body. In addition, the cervical spine is located in the upper vertebrae, and the 11G puncture needle is more likely to damage important blood vessels and nerves. This is the technical difficulty of vessel-plasty in cervical fractures. This study adopts improved exchange technology. Firstly, 17G puncture needle is selected to puncture into the vertebral body. When puncture around important blood vessels before entering the bone, the additional blunt needle shall be used alternately to protect important blood vessels. After the 17G puncture needle was safely inserted into the center of the vertebral body, replace it with 11G trocar through the expansion tube to establish a working path with the same diameter of expander. The expander was rotated in multiple directions to repair the collapsed endplate, and thus raising the height of the vertebral body. Rotation in multiple directions will form as much space as possible and most importantly to prevent the BFMC from being pierced by bone fragments. It can also reduce the pressure of bone cement injection. In the process of injection, the bone cement is firstly wrapped by the BFMC to avoid multi-directional flow. As the injection continues, the cement slowly exudes through the mesh and forms interdigitations with the surrounding bone trabeculae, which avoids the loosening of the bone cement block.
Limitations
Obvious limitations of this study are the small sample size and the lack of a control group. Another limitation is that new metastasis at other vertebral levels with the progress of the primary tumor or taking antigenic tumor medicine may affect the clinical follow-up results as confounding factors in the long-term follow-up after discharge. Finally, it should be noted that all procedures in this study were performed by doctors with rich experience in osteoarticular interventions; similar results may not be achieved by doctors unfamiliar with this technique.
Conclusion
Vessel-plasty using BFMC appears to be safe and effective for cervical malignant severe compression fractures. It can relieve pain, stabilize the vertebral body. It may be a new treatment option for these high-risk patients.
Ethical Statement and Informed Consent
This study was approved by the institutional review board of Shanghai Sixth People’s Hospital, and written informed consent was obtained from each patient. All study procedures were in accordance with the Declaration of Helsinki of the World Medical Association.
Funding
This study was funded by Natural Fund from Shanghai Science and Technology Commission (grant No. 19411971800).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Dodwad SN, Savage J, Scharschmidt TJ, Patel A. Evaluation and treatment of spinal metastatic disease. Cancer Treat Res. 2014;162:131–150.
2. Cole JS, Patchell RA. Metastatic epidural spinal cord compression. The Lancet Neurology. 2008;7:459–466.
3. Yang Z, Yang Y, Zhang Y, et al. Minimal access versus open spinal surgery in treating painful spine metastasis: a systematic review. World J Surg Oncol. 2015;13:68.
4. Boriani S, Gasbarrini A, Bandiera S, Ghermandi R, Lador R. En Bloc Resections in the Spine: the Experience of 220 Patients During 25 Years. World Neurosurg. 2017;98:217–229.
5. Weitao Y, Qiqing C, Songtao G, Jiaqiang W. Open vertebroplasty in the treatment of spinal metastatic disease. Clinical Neurology and Neurosurgery. 2012;114:307–312.
6. Mattie R, Brar N, Tram JT, et al. Vertebral Augmentation of Cancer-Related Spinal Compression Fractures: a Systematic Review and Meta-Analysis. Spine (Phila Pa. 1976;2021(46):1729–1737.
7. Nieuwenhuijse MJ, van Erkel AR, Dijkstra PD. Percutaneous vertebroplasty in very severe osteoporotic vertebral compression fractures: feasible and beneficial. J Vasc Interv Radiol. 2011;22:1017–1023.
8. Gu YF, Tian QH, Li YD, et al. Percutaneous vertebroplasty and interventional tumor removal for malignant vertebral compression fractures and/or spinal metastatic tumor with epidural involvement: a prospective pilot study. J Pain Res. 2017;10:211–218.
9. Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa. 1976;2000(25):923–928.
10. Peh WC, Gilula LA, Peck DD. Percutaneous vertebroplasty for severe osteoporotic vertebral body compression fractures. Radiology. 2002;223:121–126.
11. O’Brien JP, Sims JT, Evans AJ. Vertebroplasty in patients with severe vertebral compression fractures: a technical report. AJNR Am J Neuroradiol. 2000;21:1555–1558.
12. Distefano D, Scarone P, Isalberti M, et al. The ‘armed concrete’ approach: stent-screw-assisted internal fixation (SAIF) reconstructs and internally fixates the most severe osteoporotic vertebral fractures. J Neurointerv Surg. 2021;13:63–68.
13. Cianfoni A, Distefano D, Scarone P, et al. Stent screw-assisted internal fixation (SAIF): clinical report of a novel approach to stabilizing and internally fixating vertebrae destroyed by malignancy. J Neurosurg Spine. 2019;1:1–12.
14. Cianfoni A, Distefano D, Pravatà E, et al. Vertebral body stent augmentation to reconstruct the anterior column in neoplastic extreme osteolysis. J Neurointerv Surg. 2019;11:313–318.
15. Elsaman A, Shaaban MH. Vertebral Augmentation Involving Vertebroplasty or Kyphoplasty for Cancer-Related Vertebral Compression Fractures: a Systematic Review. Ont Health Technol Assess Ser. 2016;16:1–202.
16. Reyad RM, Ghobrial HZ, Hakim SM, Hashem RH, Elsaman A, Shaaban MH. Thick cement usage in percutaneous vertebroplasty for malignant vertebral fractures at high risk for cement leakage. Diagnostic and Interventional Imaging. 2017;98:721–728.
17. Chen L, Su IC, Ni CF, Wang ZT. Percutaneous vertebroplasty performed with an 18-gauge needle for treatment of metastatic severe compression fracture of the cervical vertebral body. J Vasc Interv Radiol. 2014;25:1413–1417.
18. Clarençon F, Fahed R, Cormier E, et al. Safety and effectiveness of cervical vertebroplasty: report of a large cohort and systematic review. Eur Radiol. 2020;30:1571–1583.
19. De la Garza-ramos R, Benvenutti-Regato M, Caro-Osorio E. Vertebroplasty and kyphoplasty for cervical spine metastases: a systematic review and meta-analysis. Int J Spine Surg. 2016;10:7.
20. Chen L, Su IC, Ni C-F, Wang Z-T. Percutaneous Vertebroplasty Performed with an 18-Gauge Needle for Treatment of Metastatic Severe Compression Fracture of the Cervical Vertebral Body. J Vascular Interventional Radiol. 2014;25:1413–1417.
21. Chen C, Li D, Wang Z, Li T, Liu X, Zhong J. Safety and Efficacy Studies of Vertebroplasty, Kyphoplasty, and Mesh-Container-Plasty for the Treatment of Vertebral Compression Fractures: preliminary Report. PLoS One. 2016;11:e0151492–e.
22. Yang XG, Wu G, Sun YY, Pang HR, Huang XQ, Xu GH. Vesselplasty using the Mesh-Hold™ bone-filling container for the treatment of pathological vertebral fractures due to osteolytic metastases: a retrospective study. Eur J Radiol. 2020;126:108962.
23. Zheng Z, Luk KDK, Kuang G, et al. Vertebral Augmentation With a Novel Vessel-X Bone Void Filling Container System and Bioactive Bone Cement. Spine. 2007;1:32.
24. Fisher CG, DiPaola CP, Ryken TC, et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine. 2010;35:E1221–9.
25. Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine. 2001;26:298–306.
26. Bonnard E, Foti P, Kastler A, Amoretti N. Percutaneous vertebroplasty under local anaesthesia: feasibility regarding patients’ experience. Eur Radiol. 2017;27:1512–1516.
27. Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. Cirse Quality Assurance Document and Standards for Classification of Complications: the Cirse Classification System. Cardiovasc Intervent Radiol. 2017;40:1141–1146.
28. Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415.
29. Cazzato RL, de Marini P, Auloge P, et al. Percutaneous vertebroplasty of the cervical spine performed via a posterior trans-pedicular approach. Eur Radiol. 2021;31:591–598.
30. Bao L, Jia P, Li J, et al. Percutaneous Vertebroplasty Relieves Pain in Cervical Spine Metastases. Pain Res Manag. 2017;2017:3926318.
31. Sun H, Yang Z, Xu Y, et al. Safety of percutaneous vertebroplasty for the treatment of metastatic spinal tumors in patients with posterior wall defects. European Spine Journal. 2015;24:1768–1777.
32. Boszczyk B. Volume matters: a review of procedural details of two randomised controlled vertebroplasty trials of 2009. Eur Spine J. 2010;19:1837–1840.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.