Back to Journals » Drug Design, Development and Therapy » Volume 10
Very rapid virologic response and early HCV response kinetics, as quick measures to compare efficacy and guide a personalized response-guided therapy
Authors Yakoot M , Abdo A, Yousry A, Helmy S
Received 27 April 2016
Accepted for publication 11 June 2016
Published 25 August 2016 Volume 2016:10 Pages 2659—2667
DOI https://doi.org/10.2147/DDDT.S111496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Video abstract presented by Professor Mostafa Yakoot and Professor Alaa M Abdo.
Views: 387
Mostafa Yakoot,1,2 Alaa M Abdo,3 Ahmed Yousry,4,5 Sherine Helmy6
1Green Clinical Research Center, 2Abbas Helmy Clinics, 3Tropical Medicine and Hepatology Department, Alexandria Faculty of Medicine, 4Microbiology Department, High Institute of Public Health, Alexandria University, 5Mabarat Asafra Labs, 6Pharco Corporation, Alexandria, Egypt
Background: This is the second and final report for our study designed to compare two generic sofosbuvir products for the degree and speed of virologic response to a dual anti-hepatitis C virus (HCV) treatment protocol. We aimed to test the applicability of the early virus response kinetics and the very rapid virologic response (vRVR) rate as quick outcome measures for accelerated comparative efficacy studies and as a foundation for a personalized response-guided therapy.
Methods: Fifty eligible chronic HCV patients were randomized to either one of two generic sofosbuvir products (Gratisovir or Grateziano) at a daily dose of one 400 mg tablet plus a weight-based ribavirin dose. Data were compared between the groups for early virus response kinetics and vRVR rates in relation to the rates of final sustained virologic response at week 12 posttreatment (SVR12).
Results: The Log10 transformed virus load (Log polymerase chain reaction) curves showed fairly similar rapid decline during the first 2 weeks, with no significant difference between the groups at four analysis points throughout the study by repeated-measures factorial analysis of variance test (P=0.48). The SVR12 rates were 96% (95% confidence interval, 79.6%–99.9%) in Gratisovir group (24/25) and 95.7% (95% confidence interval, 78%–99.9%) in Grateziano group (22/23). There was no statistically significant difference found by exact test (P>0.999). There was a significant association between the vRVR and the SVR12, with 100% positive predictive value (38/38 of those who had vRVR, achieved a final SVR12) and 82.6% sensitivity (among the total 46 with SVR12, 38 were having vRVR).
Conclusion: We can conclude from our study that the early HCV response kinetics and the vRVR rates could be used as sensitive quick markers for efficacy (with a very high positive predictive value for SVR12), based on our accelerated comparative efficacy research model. This might open the way for new models of accelerated equivalence efficacy studies along with the bioequivalence kinetics studies to test a generic drug against a reference. Also, the early response kinetics and the vRVR might be used as qualifiers for a personalized course of treatment. This could shorten unnecessarily long treatment courses in rapid responders and might help to avoid relapses in slow responders.
Keywords: chronic hepatitis C, HCV response kinetics, very rapid virologic response, vRVR, direct-acting antiviral agents, sofosbuvir, ribavirin, dual therapy
Introduction
Hepatitis C virus (HCV) infection is one of the leading causes of chronic liver disease worldwide.1 It is considered a major public health problem in Egypt with an estimated prevalence rate of approximately 15% of the population.2
The introduction of the new directly acting antiviral (DAA) drug class has revolutionized the treatment of chronic HCV with unprecedented high sustained virologic response (SVR) rates with shorter treatment durations coupled with favorable tolerability and safety profiles.3
Now, there is a very fast process of drug discovery, research and developments in that area. This has created a need for new accelerated research methods and faster response predictive models to cope with the speed of new developments.
Frequently information becomes outdated by the time research is completed and the manuscript is published.
It had been observed during interferon-based therapy that early on-treatment virus kinetics was a good predictor of treatment failure. Those who did not achieve an early virologic response (undetectable serum HCV RNA level or ≥logs reduction of virus load after 12 weeks) were at high risk of treatment failure, and this provided the basis for the treatment stopping rule at week 12.4–6
Now, with the faster and >90% SVR rates achieved with the DAA protocols, the early viral kinetics (ie, time to viral negativity) have become better predictors for treatment success rather than failure. The very rapid virologic response (vRVR; undetectable serum HCV RNA level at week 2) was found to be highly correlated with the final SVR achieved with dual sofosbuvir/ribavirin protocol, with very high positive but low negative predictive values.7
It was also observed by many studies that combinations of highly efficacious DAA drugs that had consistently given >90% SVR12 rates in clinical studies for 12 or 24 weeks failed to have similarly high rates when given for shorter durations.8,9
Given these two important observations, we hypothesized that the duration of virus suppression or the time spent on-treatment with undetectable HCV RNA in the serum (or more perfectly with target-not-detected) is a more important determinant factor for cure than the antiviral activity of the molecule itself and there should be sufficient time spent on-treatment after viral negativity in serum for complete eradication of the virus from liver cells and other potential reservoirs such as the platelets and peripheral mononuclear cells.
Thus, the speed of virologic response (rapidity of virus load reduction) could be regarded as an important efficacy marker, as it combines both the molecular activity of the drug and the time factor.
As we have mentioned in our previously published interim report for this study,10 we used two suggested outcome measures as indicators for the speed of response: the vRVR, which was defined as the undetectable HCV RNA level at the end of week 2 of therapy.11 And the ultrarapid virologic response (uRVR), which we defined as an undetectable serum HCV RNA or a 4 log10 or greater drop in HCV RNA at the end of 1 week of therapy.
In this final report of the same study protocol,10 we further evaluated the validity of the speed of virus load reduction during the first 2 weeks of therapy and the vRVR rate as a quick time-saving endpoint for fast-track comparison of the efficacy of the two treatment options. Also, we tested whether the vRVR within 2 weeks could be used as a justification to personalize or shorten the recommended 24-week course of dual sofosbuvir plus ribavirin,12 to only 16 weeks, given our aforementioned suggestion regarding the appropriate time needed for full virus eradication from the whole body cells, which we assumed from our personal unpublished experience and the work of Dahari et al that 3 months spent on-treatment with undetectable serum HCV RNA could be sufficient.13
Objectives
The objectives of our study were: 1) to study a new suggested model for a fast-track comparative-effectiveness research where both the speed and size of effect of the two treatments on HCV RNA virus load within just 2 weeks of therapy are used to compare the efficacy of the two generic sofosbuvir products Gratisovir (Pharco Corporation, Alexandria, Egypt) and Grateziano (European Egyptian Pharmaceutical Industries, Alexandria, Egypt.) in a dual antiviral treatment protocol, each given with a generic ribavirin product (Hepaverin), in patients with chronic hepatitis C genotype 4; 2) to compare the mean Log10 reduction of virus load at weeks 1 and 2 after starting treatment, as well as the proportion of patients achieving uRVR and vRVR in both groups as a suggested fast-track model to compare efficacy in the interim analysis report; 3) to further compare the effect of both drugs (between-factor) on the virus load (within-factor) as repeatedly measured at 4 points (before treatment, week 2, end of treatment and 12 weeks after end of treatment) in this final report to check our fast-track model; 4) to compare the truncated 4 months versus the recommended 6-month duration of therapy in those who achieved vRVR; and 5) to evaluate the positive and negative predictive accuracy, and the utility of vRVR as a marker for comparative efficacy or for the rationalization of a truncated response-guided therapy.
Patients and methods
Study design and setting
The study was conducted in an outpatient setting according to a randomized, open-label, comparative efficacy study design.10
A repeated-measures factorial design with one between-factor (drug) and one within-factor (Log virus load by polymerase chain reaction [PCR]) had two drug groups with 24 subjects each for a total of 48 subjects. The virus load for each subject was measured four times. This design achieves 87% power to test between-factor (B) if a Geisser-Greenhouse corrected F test is used with a 5% significance level and the actual effect standard deviation (SD) is 0.36; it achieves 84% power to test within-factor (W) if a Geisser-Greenhouse corrected F test is used with a 5% significance level and the actual effect SD is 0.18; it achieves 84% power to test the B*W interaction if a Geisser-Greenhouse corrected F test is used with a 5% significance level and the actual effect SD is 0.18.
Fifty eligible patients with documented chronic hepatitis C, genotype 4 had been included in the study according to the following criteria.
Inclusion criteria
Chronic hepatitis C infection genotype 4 with a PCR positive test and a virus load ≥104 ± elevated liver enzymes; males or females between 18 and 70 years old; interferon naïve (not previously treated with interferon-based therapy); and relapsers (patients with a transient virologic response to previous therapy), or nonresponders to interferon or combined therapy were eligible if they stopped the antiviral drugs at least 3 months before inclusion were included in this study.
Exclusion criteria
Pregnant females or a couple intending pregnancy during the course until 6 months after end of therapy; patients with other causes of hepatitis, concurrent HIV virus infection, or active schistosomiasis; and critically ill patients with complications of severe hepatic, cardiac, or kidney dysfunction (creatinine clearance <50 mL/min), malignancy, anemia, or multiorgan failure were excluded from this study.
The study protocol was reviewed and approved by Green Clinic and Research ethical committee according to the Declaration of Helsinki (IRB00008268). All subjects gave written informed consent before any treatment interventions were performed.
Study protocol
Consecutive patients presenting to three outpatient clinics in Alexandria: Green CRC, Alaa Abdo, and Abbas Helmy Clinics starting from June 1, 2015, were assessed for eligibility through full clinical examination and the following laboratory investigations done at Mabarat Asafra Labs. Detection of HCV RNA was by PCR quantitative measurements by COBAS Amplicor 2.0 (Hoffman-La Roche Ltd., Basel, Switzerland), lower limit of detection of 10 IU/mL. Screening test for hepatitis B surface antigen, autoimmune hepatitis, anti-HIV, schistosoma Ag, liver and kidney functions tests, urine and stools analyses, complete blood count, blood glucose, pregnancy test. Upper abdominal and liver ultrasonography was also done at the clinics.
Patients fulfilling the inclusion/exclusion criteria were included and randomly divided into two treatment groups using software-generated balanced 1:1 block randomization technique.
Group 1 started treatment with one Gratisovir 400 mg (Pharco) tablet daily after the main meal plus weight-based dosing of Hepaverin capsules (ribavirin), 1,200 mg if body weight was ≥75 kg or 1,000 mg if body weight was <75 kg, twice daily orally; for a duration of 6 or 4 months according to further randomization for those achieving vRVR.
Group 2 started treatment with one Grateziano 400 mg (EEPI) tablet daily after the main meal plus weight-based dosing of Hepaverin capsules (ribavirin), 1,200 mg if body weight was ≥75 kg or 1,000 mg if body weight was <75 kg, twice daily orally; for a duration of 6 or 4 months according to further randomization for those achieving vRVR.
At the end of week 2 of therapy, the patients were further divided into those who had vRVR (undetectable HCV RNA level at week 2) were randomized with a 1:1.5 block randomization technique into either 16- or 24-week course duration of therapy, respectively; and all those who did not achieve the vRVR completed the 24-week duration.
All included patients were handed their assigned treatment kit and were asked to revisit the clinic every week for follow-up and collection of clinical and laboratory efficacy and safety data. Blood samples were taken weekly for HCV RNA quantitative PCR test, complete blood count, alanine transaminase, aspartate transaminase, serum bilirubin, and creatinine as routine tests. Other nonroutine lab or imaging tests were done, according to each patient’s condition, whenever deemed necessary.
Asking patients about the occurrence of any adverse event and counting the remaining tablets and capsules during each visit as a measure of compliance were also performed weekly. All patients were equally subjected to full psychological support, assurance, and motivation for raising their spirit and optimism during treatment as routinely done in our practice in management of patients with chronic illness.
All patients who had been randomized to the shorter 16-week course were subjected just before the end of the course to a PCR test for HCV RNA in peripheral mononuclear cells in addition to the routine PCR test in the serum done for every patient at the end of treatment. To be eligible to end treatment in the short-course group, we required the result of serum virus load to be target-not-detected in addition to negative result in peripheral mononuclear cells. This was done in order to assure that there was no known potential reservoir for clinical relapse in peripheral cells before the end of the shorter course.
End points
The following end points were evaluated and already reported in our previously published interim report:10 1) the mean reduction of Log10 virus load in both groups after 1 and 2 weeks of starting therapy and reported in our previous interim report, 2) incidence of uRVR following 1 week of treatment, and 3) incidence of vRVR following 2 weeks of treatment.
The following end points were evaluated and reported here in our final report: 1) incidence of end of treatment response at the end of 24 versus 16-week course of treatment in those patients randomized 1.5:1 after achieving vRVR. Those who do not achieve vRVR will have to complete the full 24-week duration of therapy as was recommended in the American Association for the Study of Liver Diseases guidelines;12 2) incidence of SVR measured after 12 weeks following completion of treatment (SVR12) in each drug combination, treatment duration, and a dichotomized Fibrosis-4 (Fib-4) score; 3) percent of patients with vRVR who achieved SVR12 in each drug group and treatment duration; 4) the positive and negative predictive value, sensitivity, specificity, and the utility of the vRVR as a quick end point for rapid comparative efficacy or for the decision of a shortened response-guided course of therapy; 5) different baseline variables as well as on-study measures (vRVR) were tested by a multiple logistic regression model for determinant factors for SVR12, relapse, or null response; and 6) proportion of subjects with an on-treatment serious adverse event.
Statistical analysis
Data were analyzed using the computer software package SPSS Statistics for Windows, Version 21.0 (IBM Corporation, Armonk, NY, USA). Summaries for categorical data were presented as proportions (95% confidence interval [CI]) calculated by the binomial exact method, while summaries for continuous data were presented as mean (±SD). Comparisons of means of reduction in Log10 transformed virus load between the treatment groups were done using Student’s t-test for independent samples and repeated-measures factorial analysis of variance (ANOVA), split-plot. Exact tests and bootstrapping were used for comparisons of proportions of categorical variables as well as for testing our on-treatment predictor (vRVR) for its positive and negative predictive values, sensitivity, and specificity to predict SVR12 at the end of the study.
Multiple logistic regression model, including important baseline and on-study variables and covariates, was applied to test for determinants for SVR12 or relapse.
Results
Fifty eligible patients were included in the study and randomized to either the Grateziano group (n=25) or Gratisovir group (n=25). During the whole period of the study, only two patients dropped out after the fourth week of treatment; one of them reported that he was subjected to emergent surgical operations after a serious road traffic accident, while the other was lost to follow-up as he traveled to work outside the country. Both the patients were from the Grateziano group, and they have been counted in the total denominator and included as missing in the intention-to-treat (ITT) analysis of the overall SVR12 rates, while for other statistical analyses for association of variables, we did a per-protocol analysis (PP), by including in analysis only those who completed the full protocol (Figure 1).
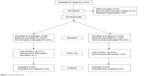  | Figure 1 Patient flowchart. |
The baseline characteristics of both groups were almost comparable (Table 1).
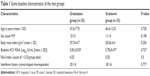  | Table 1 Some baseline characteristics of the two groups |
The overall SVR rates for those remained with undetectable HCV RNA at the end of 12 weeks after end of treatment (SVR12) are presented in Table 2 and Figure 2. The ITT SVR12 rate was 92% (95% CI, 80.77%–97.78%; 46/50 included patients), while the per-protocol (PP) SVR12 rate was 95.8% (95% CI, 85.7%–99.49%; 46/48 subjects who completed the full protocol).
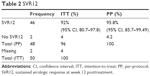  | Table 2 SVR12 |
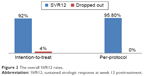  | Figure 2 The overall SVR12 rates. |
The SVR12 rates in those who completed the full protocol in each drug group are presented in Table 3 and Figure 3. SVR12 rates were 96% (95% CI, 79.6%–99.9%) (24/25) in the Gratisovir group and 95.7% (95% CI, 78%–99.9%; 22/23) in the Grateziano group. There was no statistically significant difference found between the two drug groups by the exact test (exact two-sided significance >0.999).
  | Table 3 The per-protocol SVR12 rates for each drug group |
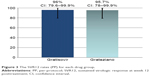  | Figure 3 The SVR12 rates (PP) for each drug group. |
We used the Fib-4 score as a noninvasive marker for cirrhosis in those who scored >3.25. Table 4 presents the SVR12 rates according to dichotomized Fib-4 score.
  | Table 4 SVR12 rates according to dichotomized Fib-4 index score |
SVR rates in those with Fib-4 score ≤3.25 was 100% (95% CI, 90.5%–100%; 37/37) SVR12 rate, while in those with advanced/cirrhotic cases with Fib-4 score >3.25, it was 81.8% (95% CI, 48.2%–97.7%; 9/11). The difference was found to be marginally significant by the exact test (exact two-sided significance, P=0.049).
The relation between having a vRVR and achieving the final SVR12 result is presented in Tables 5 and 6.
  | Table 6 vRVR as a diagnostic predictor for SVR12 |
There was a significant association between the vRVR and the SVR12, with 100% (38/38) of those who had vRVR achieving a final SVR12 (positive predictive value) and 100% (2/2) of those who did not finally achieve SVR12 being those who had not had a vRVR (specificity). Also, 82.6% (38/46) of those who finally achieved SVR12 were those who had had a vRVR (sensitivity), while the negative predictive value for vRVR to predict SVR12 was 20%. This association was found to be statistically significant by the exact test (P=0.04).
All patients who were randomized to the short 16-week course were eligible to end treatment at their planned time. Both eligibility criteria: HCV RNA target-not-detected in serum in addition to negative result in peripheral mononuclear cells were verified in every patient.
No significant difference in SVR12 rates was found in patients who were randomized to either 16- or 24-week duration after having a vRVR. Both groups had 100% (95% CI, 78.2%–100% and 85.2%–100%, respectively) SVR12 rates (Table 7). The SVR12 rates per treatment duration in all patients, as shown in Table 8, also showed no significant difference based on the exact test (P=0.561).
  | Table 7 SVR12 rates per duration of treatment in the vRVR group |
  | Table 8 SVR12 rates per duration of treatment in the whole sample |
The general linear model, repeated-measures factorial ANOVA test, and the split-plot (Tables 9 and 10 and Figure 4) illustrate that, while the within-factor (virus load) showed a very high statistically significant difference in the measurements repeated at four time points with the Geisser-Greenhouse corrected F test (F=1,251.4; P<0.0001), there was no statistically significant difference between the drugs as a between-factor (P=0.48).
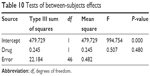  | Table 10 Tests of between-subjects effects |
  | Figure 4 Split-plot showing comparable between-factor (drugs) effects by repeated-measures factorial analysis of variance. |
No serious adverse events were reported during the period of the study in both groups. Similar rates of nonserious treatment-emergent adverse events were reported in both the groups; all were mild in severity. Headache was reported in six patients in the Grateziano group and seven patients in the Gratisovir group; fatigue in seven patients from each group, abdominal pain (six and five), diarrhea (three and two), itching and skin rash (five and five), respectively. Anemia with grade two reduction of hemoglobin (between 8 and <10 g/dL) was reported in three patients from each group at week two, and grade one reduction of hemoglobin (< LLN [lower level of normal] – 10 g/dL) was reported in four and five other patients, respectively. We reduced the dose of ribavirin only for the six cases of grade 2 reduction of hemoglobin; otherwise, no changes were done in the drug doses for all other patients. Mild depression was noticed in three patients from each drug group. This happened late during the last 4 weeks of therapy in the 24 weeks assigned group. Only assurance and psychological support were provided and they improved without antidepressant drug therapy during the 12 weeks posttreatment follow-up period.
Discussion
Our final results have confirmed the previously published interim results that the two drug treatment arms are not statistically significantly different with regard to vRVR rates and virus response kinetics as measured during the first 2 weeks; this was confirmed by the final SVR12 rates and the four points within-subjects “virus load” repeated-measures, factorial ANOVA model.
Also, we found a significant association between the positive vRVR and the positive SVR12, with 38/38 of those who had vRVR achieved a final SVR12 (positive predictive value of 100% [95% CI, 90.75%–100%]). Also, all of those who did not finally achieve SVR12 were those who had not had a vRVR (100% [95% CI, 15.8%–100%], specificity). The negative predictive value for negative vRVR to predict No SVR12 was low as expected (20% [95% CI, 2.5%–55.61%], negative predictive value). Out of the 46 patients who finally achieved SVR12 at the end of the study, 38 were having vRVR at week 2 (82.61% [95% CI, 68.58%–92.18%], sensitivity). This association was found to be statistically significant by the exact test (P=0.04).
Given that there was no difference between the two treatment arms, neither early on (vRVR) nor late (SVR12), we pooled the data of the two arms to calculate the overall ITT SVR12 rate, which was 92% (95% CI, 80.77%–97.78%) and the PP rate, which was 95.8% (95% CI, 85.7%–99.48%).
All our patients who had been randomized to either 16- or 24-week duration after having a vRVR achieved SVR12. Both groups had 100% SVR12 rates (95% CI 78.2%–100% for 16 weeks and 85.2%–100% for 24 weeks). Thus, the group treated with the shorter 16-week course in our study appeared to have a trend toward higher SVR12 rate than other reported rates for the tested 12-week course of treatment by the dual combination of sofosbuvir plus ribavirin in genotype 4.9,14
Doss et al had reported 77% SVR12 rate with 12 weeks and 90% rate with 24 weeks of sofosbuvir and ribavirin therapy,9 while Ruane et al reported 93% (95% CI, 77%–99%) SVR12 rate in the 24-week group and 68% (95% CI, 49%–83%) in the 12-week group.14
We suggest that the reason for this trend to higher SVR12 rate in our truncated 16-week course is that all patients assigned to the shorter 16 weeks were from those who had already responded very early and remained on-treatment for >12 weeks while they had no detected HCV RNA target in their sera. Also, the setting and the design of this study allowed for a very intimate and close patient–physician relationship. So, in addition to the reasonably manageable number of patients per senior treating doctor, the frequent weekly visits and close monitoring of any adverse reaction and appropriate prompt management before it is aggravated or affected patient compliance could have had an impact on patients’ strict compliance and hope for cure. In addition to that, we speculate that the weekly supportive psychological measures that every patient was subjected to, as well as the eye-witnessing of the reports of viral negativity as early as only 2 weeks, could have had an impact on patient motivation, optimism, and hope for cure that may have had a positive impact on his or her immune functions.
We noted that both cases who relapsed were sharing in common two baseline characteristics associated with their schistosomal hepatic fibrosis: fairly large-sized spleen >18 cm and low baseline hemoglobin and platelet count indicating hypersplenism. This observation, in our opinion, is very important for further study in a country like Egypt, where concomitant schistosomal hepatic fibrosis is highly prevalent in chronic HCV cases, especially in the elderly group.
We postulate that the large hyperactive fibrocongestive splenomegaly might harbor a big intracellular reservoir for the virus that needs a longer time to be completely eradicated after serum negativity. We acknowledge the limitations of our small sample size, due to our limited resource setting in the face of significantly costly repeated laboratory tests and other expenses. It was difficult to apply the multivariate logistic regression analysis, as we planned, as a secondary end point because it needed a rather large sample size to give statistically significant odds ratios because of the low treatment failure rate.
Conclusion
We can conclude from our study that the early HCV response kinetics to the dual sofosbuvir and ribavirin therapy and the vRVR rates might be used as sensitive quick markers or end points for efficacy in accelerated comparative efficacy research with a high positive predictive value for SVR12. This might open the way for new models of accelerated equivalent efficacy studies, in addition to the already done pharmacokinetic bioequivalence to test a generic drug against a reference. Also, the early response kinetics might be used as a qualifier for a personalized course of treatment. This could shorten unnecessarily long treatment courses in rapid responders and might help to avoid relapses in slow responders especially for the costly sofosbuvir-based antiviral therapy.
Acknowledgments
The authors would like to thank Abbass Helmy Charity Establishment and Egyptian Cure Bank for their kind help and support to the study.
Disclosure
Sherine Helmy is working for Pharco Corporation. The authors report no other conflicts of interest in this work.
References
Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17(2):107–115. | ||
Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19(8):560–567. | ||
Schinazi R, Halfon P, Marcellin P, Asselah T. HCV direct-acting antiviral agents: the best interferon-free combinations. Liver Int. 2014;34(Suppl 1):69–78. | ||
Ferenci P, Laferl H, Scherzer TM, et al; Austrian Hepatitis Study Group. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology. 2008;135(2):451–458. | ||
Hoofnagle JH, Wahed AS, Brown RS Jr, Howell CD, Belle SH; Virahep-C Study Group. Early changes in hepatitis C virus (HCV) levels in response to peginterferon and ribavirin treatment in patients with chronic HCV genotype 1 infection. J Infect Dis. 2009;199(8):1112–1120. | ||
Esmat GE, Al Akel W, Abdel Aziz RA, et al. Hepatitis C viral kinetic changes in a retrospective cohort study of chronic hepatitis C virus Egyptian patients on pegylated interferon and ribavirin therapy. J Interferon Cytokine Res. 2016;36(3):149–158. | ||
Maasoumy B, Vermehren J, Welker MW, et al. Clinical value of on-treatment HCV RNA levels during different approved sofosbuvir-based antiviral regimens. J Hepatol. Epub 2016 Apr 13. | ||
Kohli A, Kattakuzhy S, Sidharthan S, et al. Four-week direct-acting antiviral regimens in non-cirrhotic patients with hepatitis C virus genotype 1 infection: an open-label, nonrandomized trial. Ann Intern Med. 2015;163(12):899–907. | ||
Doss W, Shiha G, Hassany M, et al. Sofosbuvir plus ribavirin for treating Egyptian patients with hepatitis C genotype 4. J Hepatol. 2015;63(3):581–585. | ||
Yakoot M, Abdo AM, Yousry A, Helmy S. The very-rapid and the ultra-rapid virologic response to two treatment options in patients with chronic hepatitis C: an interim report of a prospective randomized comparative effectiveness study. Drug Des Devel Ther. 2015;9:6027–6033. | ||
Wedemeyer H, Jensen DM, Godofsky E, Mani N, Pawlotsky JM, Miller V; Definitions/Nomenclature Working Group of the HCV DrAG (HCV Drug Development Advisory Group), under the auspices of the Forum for Collaborative HIV Research. Recommendations for standardized nomenclature and definitions of viral response in trials of hepatitis C virus investigational agents. Hepatology. 2012;56(6):2398–2403. | ||
AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932–954. | ||
Dahari H, Canini L, Graw F, et al. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol. 2016;64(6):1232–1239. | ||
Ruane PJ, Ain D, Stryker R, et al. Sofosbuvir plus ribavirin for the treatment of chronic genotype four hepatitis C virus infection in patients of Egyptian ancestry. J Hepatol. 2015;62(5):1040–1046. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


