Back to Journals » Clinical Interventions in Aging » Volume 18
Vascular Aging: Assessment and Intervention
Authors Li A, Yan J, Zhao Y, Yu Z, Tian S , Khan AH, Zhu Y, Wu A, Zhang C, Tian XL
Received 29 May 2023
Accepted for publication 6 August 2023
Published 17 August 2023 Volume 2023:18 Pages 1373—1395
DOI https://doi.org/10.2147/CIA.S423373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Ao Li,1,2 Jinhua Yan,3 Ya Zhao,2 Zhenping Yu,4 Shane Tian,5 Abdul Haseeb Khan,2 Yuanzheng Zhu,2 Andong Wu,2 Cuntai Zhang,3 Xiao-Li Tian2
1Queen Mary School, Nanchang University, Nanchang, Jiangxi, 330031, People’s Republic of China; 2Aging and Vascular Diseases, Human Aging Research Institute (HARI) and School of Life Science, Nanchang University, and Jiangxi Key Laboratory of Human Aging, Nanchang, Jiangxi, 330031, People’s Republic of China; 3Department of Geriatrics, Institute of Gerontology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 4Institute of Translational Medicine, School of Life Science, Nanchang University, and Jiangxi Key Laboratory of Human Aging, Nanchang, Jiangxi, 330031, People’s Republic of China; 5Department of Biochemistry/Chemistry, Ohio State University, Columbus, OH, USA
Correspondence: Xiao-Li Tian; Cuntai Zhang, Email [email protected]; [email protected]
Abstract: Vascular aging represents a collection of structural and functional changes in a blood vessel with advancing age, including increased stiffness, vascular wall remodeling, loss of angiogenic ability, and endothelium-dependent vasodilation dysfunction. These age-related alterations may occur earlier in those who are at risk for or have cardiovascular diseases, therefore, are defined as early or premature vascular aging. Vascular aging contributes independently to cardio-cerebral vascular diseases (CCVDs). Thus, early diagnosis and interventions targeting vascular aging are of paramount importance in the delay or prevention of CCVDs. Here, we review the direct assessment of vascular aging by examining parameters that reflect changes in structure, function, or their compliance with age including arterial wall thickness and lumen diameter, endothelium-dependent vasodilation, arterial stiffness as well as indirect assessment through pathological studies of biomarkers including endothelial progenitor cell, lymphocytic telomeres, advanced glycation end-products, and C-reactive protein. Further, we evaluate how different types of interventions including lifestyle mediation, such as caloric restriction and salt intake, and treatments for hypertension, diabetes, and hyperlipidemia affect age-related vascular changes. As a single parameter or intervention targets only a certain vascular physiological change, it is recommended to use multiple parameters to evaluate and design intervention approaches accordingly to prevent systemic vascular aging in clinical practices or population-based studies.
Keywords: vascular aging, arterial stiffness, endothelial function, arterial wall thickness, therapeutic intervention
Introduction
Our world is turning into an aging society with medical advances and improved social care. The growing elderly population is susceptible to diversified stresses, particularly diseases, and death. With advanced age, vessels degenerate morphologically and physiologically, referred to as vascular aging, contributing to the common cardio-cerebral vascular diseases (CCVDs) in the elderly, such as hypertension, coronary artery disease, aneurysm, and stroke.1,2 Compared to individuals aged 65–74, the mortality rate of CCVDs increase from 40% to 60% of all deaths in individuals aged ≥80 years.3 These facts demonstrate the significance of vascular aging to the health of an aging society.
Vascular aging is a developing process characterized by age-related deterioration in both structure and function of blood vessels. It may occur early in individuals with cardiovascular risk factors or diseases, such as abnormal blood chemicals, unhealthy life habits, or hypertension, resulting in early or premature vascular aging that promotes or exacerbates CCVDs.4 Therefore, early diagnosis and timely therapeutic interventions are key to success in delaying or preventing CCVDs.5 For this purpose, a few methods have been developed or proposed to detect or intervene in the deleterious changes in vascular structures and functions with age, but their availabilities, including sensitivity and specificity in detection as well as efficacies in the prevention of age-related vascular changes, remain to be evaluated.4
In this review, we examine how to assess vascular aging in clinical practices as well as possible interventions available in the literature, providing general guidelines on how to improve the evaluation and prevention of vascular aging in the future.
Clinical Assessment of Vascular Aging
Vascular Structural Changes with Age
Arterial Wall Thickness
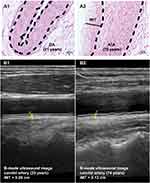
Vascular autopsies show that the arterial wall thickness increases significantly with age due to an increased intima layer, while the load-bearing medial layer has a minor contribution.6 Other morphological changes have been summarized previously.7 Despite that many vascular phenotypes are associated with age, the intimal-medial thickness (IMT) is often used to assess vascular aging in clinical practice, as it can be performed non-invasively through ultrasonography.8–10
For convenience in practice, the most used artery is the carotid artery. It has been shown that the carotid arterial wall thickness (carotid intimal-medial thickness) in the segment free of plagues increases linearly about 2.5-fold from 30 to 90 years in man.11,12 The carotid IMT increased about 0.04 mm per decade from ages 40 to 80,13,14 similar to that in young subjects aged 20–40.15,16 Other arteries, such as common femoral arteries, superficial femoral arteries, popliteal arteries, and brachial arteries, are also used to measure age-dependent wall thickening.17,18
Unlike other parameters used to evaluate vascular aging, the wall thickening of vessel segments free of atherosclerotic plaques, represented as IMT (or carotid IMT), appears very consistent with the chronological ages,19 thus, it potentially represents the physiological vascular aging.11,18
Histomorphological and ultrasonographic observations of IMT in arteries are presented in Figure 1.
Arterial Lumen Diameter
An increase in arterial lumen diameter is reported in aged vessels, likely due to the repeated stretch of the elastic arteries through an entire lifetime, causing elastin fatigue, and thus leading to elastin fracture and fragmentation.20,21 Loss of elastin contributes to the enlarged lumen diameter mostly in the proximal aorta.21 Transthoracic echocardiography is commonly used to measure the diameter of the aortic root at multiple locations, for instance, the aortic annulus, sinuses of Valsalva, sinotubular junction, and ascending aorta, to obtain reliable results.22
Previously, the Framingham Heart Study established a reference value for aortic root diameter by using M-mode echocardiography, based on a large healthy population. They observed that aortic root diameter constantly increased (from 28–33 mm to 33–37 mm) with age from 25 to 75 years, although the age-dependent dilation varies in the subjects with different body surface areas (BSA).23 In a B-mode echocardiography-based study, however, BSA is the most important determinant of aortic root diameter at the aortic annulus and sinuses of Valsalva, while age is mainly associated with the aortic root diameter at ascending aorta.24 Other B-mode echocardiography-based studies showed that the association between diameter at the aortic annulus and age was weak,25 while the diameter of the ascending aorta is more closely related to age.26 Methodologically, the aortic root diameter measured by B-mode echocardiography is larger than that measured by M-mode echocardiography.24 Therefore, when comparing the results from different studies, it is critical to consider the influence of different imaging techniques and measurement positions on the value of aortic root diameter.
A study reported that aortic root diameter steadily increases with age from 15 to 64 years after the adjustment for BSA.27 This finding is consistent with another Caucasian population-based study, which reported an increase of 1.1mm and 0.9mm in the diameter of the sinuses of Valsalva and ascending aorta per decade in people 15 years and older.28 The correlation between age and aortic root diameter is non-linear. The age-dependent dilation of aortic root was faster in children under age 15 than adults, with a rate of nearly 10 mm per decade (age, 1–15) versus 1.1 mm per decade (age>15). Importantly, BSA, rather than age, was the most decisive factor in aortic root dilation in those under 15 years.24,28 These studies pointed out that the diameter of ascending aorta assessed by echocardiography is more closely related to aging.26 Age-related aortic root dilation usually occurs in the adult population, as the aortic dimensions in those younger than 15 are mainly affected by body size.24,28
In addition to the aorta, the positive association between arterial lumen diameter and age was also shown in peripheral arteries such as brachial arteries,29 popliteal arteries,18,30 femoral arteries18,31 as well as carotid arteries.32,33 These suggest that an increased arterial lumen diameter is a good indicator for vascular aging. Vascular structural changes with ages are summarized in Table 1.
 |
Table 1 Aging-Related Vascular Structural Changes on Clinical Assessment |
Vascular Functional Changes with Age
Endothelium-Dependent Vasodilation
Endothelium-dependent vasodilation loses sensitivity with advancing age in response to dilators, such as acetylcholine; therefore, it can be considered as a functional parameter for the degree of vascular aging. Age-related endothelial dysfunction has been documented in coronary arteries,34 peripheral arteries,35 and microcirculation.36 The underlying mechanisms are multifactorial, including increased oxidative stress, inflammation response, and unbalanced release of vasoconstrictors and vasodilators.35,37,38
Acetylcholine-Induced Vasodilation
Intracoronary infusion of acetylcholine induces dilation of healthy coronary arteries by stimulating endothelial cells to release nitric oxide (NO).39 Therefore, quantitative angiographic analysis of acetylcholine-induced coronary atrial dilation is used as the “gold standard” to detect coronary endothelial function. Existing studies demonstrate a positive correlation between aging and declined coronary blood flows upon acetylcholine infusion into the left main coronary artery. The ratio of acetylcholine-induced and papaverine-induced percentage change of blood flow declined by nearly 80% from ages 20 to 80.34,40–42 The acetylcholine-induced vasodilation is dependent on dosages, particularly low dosages. It has been reported that vasodilation was decreased by 2.5, 2.6, and 4.1mm per decade at the dosages of 1, 3, and 10 μg/min, respectively.34 A general illustration of acetylcholine induces dilation is shown in Figure 2A.
The disadvantages of using the intracoronary infusion of acetylcholine to assess vascular dilation are its invasive nature and high cost, therefore, it is only used in patients undergoing angiography. Under this consideration, peripheral arteries, such as the brachial arteries and cutaneous microcirculation are examined instead of the coronary artery.35,43 For example, studies show that the endothelium function of the brachial artery in response to acetylcholine, represented as forearm blood flow, similar to the coronary artery.35,44 The infusion of acetylcholine into the brachial artery led to a decrease of nearly 40% in maximum dilation of healthy subjects aged 20–80.35
Reactive Hyperemia-Induced Vasodilation
During reactive hyperemia, a dramatic increase in arterial blood flow results in vasodilation.45 This process also depends on the increased release of NO from endothelial cells.46 Flow-mediated dilation (FMD), an endothelial-dependent (primarily NO-mediated) dilation of conduit arteries in response to an abrupt rise in blood flow and shear stress, is the widely used method to measure conduit artery endothelial function. Reactive hyperemia-induced vasodilation can also be presented as reactive hyperemia index measured by reactive hyperemia-peripheral arterial tonometry. Both FMD and reactive hyperemia index are noninvasive assessments of endothelial functions and vascular aging.47
FMD is largely attenuated in the elderly.48–51 Studies have shown that the FMD of the elderly (age>70) is nearly 50% lower than that of the young (age<30).48–51 Different from IMT or diameter discussed above, the age-related decrease of FMD is not linear. The decline in FMD is not much changed in the group with ages 24 to 39 years15 but becomes pronounced in men above 40 years and women above 45 years of age.52,53 The decrease rate is reported to be 0.21% and 0.49% per year in men and women, respectively.53 The sexual dimorphism in FMD is likely due to the vasoprotective effects of estrogen. Therefore, it is better to use FMD as a predictor of vascular aging in men over 40 and women over 50.
Arterial Stiffness
Arteries become rigid with aging, attributed to both structural and functional alterations.54 Arterial elasticity is strongly affected by the intimal layer and medial layer of the vascular wall.55 First, the stress-bearing elastin fibers fracture and collagen fibers deposit in the medial layer, and the ratio of elastin to collagen decreases.56 Then, the increase in vascular wall pressure leads to a phenotypic switch of smooth muscle cells, resulting in excessive proliferation and migration of smooth muscle cells, as well as the production of more extracellular matrix.57 Finally, endothelial dysfunction causes a diminished synthesis and release of vasodilators, and endothelial cells become super sensitive to vasoconstrictors while insensitive to vasodilators.37,38 Together, arterial elasticity decreases with ages that impairs the cushion function of arteries. The stiffened arteries is not able to buffer cardiac output, which causes quicker propagation and reflection of pulse waves.21
Pulse Wave Velocity
Pulse wave velocity (PWV) measures the velocity of a pulse wave traveling between two arterial sites, based on the principles of applanation tonometry. When arterial elasticity decreases, the pulse wave propagates rapidly along the vasculature, which is manifested as increased PWV.58 It can be performed in various arterial segments, such as carotid-brachial arteries, brachial-ankle arteries, and carotid-femoral arteries. Among these, the carotid-femoral PWV (cfPWV) is often used as it is easy to measure, closely correlated with central arterial stiffness, and has the most clinical relevance.59–61
The cfPWV was reported to increase by about 2.5-folds from the age of 21 to 96 years in subjects from the Baltimore Longitudinal Study of Aging.62 Another study in a Chinese-population showed that the cfPWV was increased by 83% from 2-month-old infants to 90-year-old elderly.63 The association between increased cfPWV and age is not linear, as cfPWV increases more rapidly after the fifth decade.54,64,65 Similar findings were reported in the different cohorts.66
It is noteworthy that age-related arterial stiffness is regionally heterogeneous and is influenced by blood pressure.63,64,67 It has also been reported that the carotid-brachial PWV is weakly correlated with age,64 but another arterial stiffness index, brachial-ankle PWV (baPWV) shows a strong correlation with ages, similar to the cfPWV.66,68,69
To better understand the age-related changes of PWV, a baPWV in Chinese men and women from ages 60–90 is presented (Figure 2B).70
Augmentation Index
The augmentation index (AIx) is determined by pulse wave reflection and represented as a surrogate parameter for arterial stiffness.71 As the conduit artery loses its elasticity, the reflected wave from the impedance mismatch returns earlier, which reaches to the central aorta in the late systolic period and merges with the systolic blood pressure. This process leads to an increment in aortic systolic blood pressure.72 Accordingly, aortic AIx, which measures the contribution of the early reflected waveform to the late increased systolic blood pressure in ascending aorta, is considered to be another indicator of systemic arterial stiffness.
Although PWV and AIx are both used to measure arterial stiffness, they provide different information about arterial properties and are not interchangeable.73,74 AIx is primarily affected by two factors, the wave propagation velocity (PWV) and the distance between the aortic root and major impedance mismatch sites. Thus, AIx is not only an indicator that reflects arterial stiffness.73 For this reason, the age-related changes of AIx are not fully consistent with changes in PWV.
As we mentioned earlier, cfPWV persistently increases with ages (from 2 months to 90 years old).63 However, studies have shown that AIx is markedly higher in young children, and gradually declines with ages until adolescence (15–18 years) while the cfPWV increases slowly.75,76 Therefore, the higher AIx in children is likely attributed to the short length of the aorta rather than the accelerated wave reflection. After puberty, AIx is reported to be positively associated with age. In a large, healthy cohort, it was shown that AIx increases nearly 5-fold from adolescents aged 20 to the elderly aged 96.73 Like the cfPWV, the increase of AIx with age is non-linear.
Previous studies demonstrated that cfPWV increases more significantly in subjects aged 50 and above, while AIx increases more prominently in subjects under 50 years old.54,64 The increase of AIx in the elderly is likely attenuated due to the reversal of the central-to-peripheral arterial stiffness gradient.64 Central arterial stiffness consistently increases with age, and ultimately exceeds peripheral arterial stiffness in the elderly.64 This change shifts the reflecting point distally, thereby reducing AIx increments. There are two turning points for age-related changes in AIx. It first occurs in adolescence when the age-related decrease in AIx stops and turns into an age-related increase, then during middle age when the increment rate of AIx diminishes.66,77
Thus, AIx is more likely to be an indicator of age-related arterial stiffness in people aged over 18, whereas cfPWV reflects the course of age-related arterial stiffness across lifespan.
Other Methods to Detect Arterial Stiffness
Recent studies have proposed a novel A-mode ultrasonic technique named ARTerial Stiffness Evaluation for Non-invasive Screening (ARTSENS). It is an image-free device for measuring arterial wall thickness based on Gaussian-mixture modeling.78,79 More than a dozen of published articles have claimed the advantages of ARTSENS in clinical applications, including its portability, high-throughput, and efficiency in evaluating vascular wall and stiffness, but a large cohort-based clinical trial has yet to yield any conclusive results.80–83 Vascular functional changes are listed in Table 2.
 |
Table 2 Aging-Related Vascular Functional Changes on Clinical Assessment |
Laboratory Tests and Indirect Measurements of Vascular Aging
In addition to an assessment of age-associated vascular structural and functional alterations by using complex and expensive devices, the measurement of blood biomarkers is another way to indirectly evaluate vascular aging. In the following section, we will highlight several frequently used biomarkers.
Endothelial Progenitor Cells
Endothelial progenitor cell (EPC), the precursor of endothelial cell, is thought to be originated from bone marrow.86 EPCs are recruited to the site of injury where they differentiate into endothelial cells, thereby regenerating damaged endothelium.87–90
The number of EPC colonies has been identified as an independent predictor of FMD. The EPC colonies were approximately 3-fold higher in subjects with a higher FMD than those with a low FMD.91 Moreover, EPCs are correlated with arterial stiffness and arterial elasticity.92 In addition to counting the number of EPCs, the changes of EPCs in their functions are associated with vascular aging as well. The proliferative and migratory capacity of EPCs is linearly reduced with the increases of baPWV.93 The age-dependent increase in carotid IMT is negatively associated with EPC function, and the survival rate of EPCs from the elderly who have high carotid IMT is decreased significantly in a healthy population-based study.94 Accordingly, EPCs appear to be a surrogate biomarker for predicting vascular aging.95
Telomere Length
Telomere is located at the end of each eukaryotic chromosome to maintain genetic integrity. When the telomere length shortens to critical region, the cell permanently loses its ability to divide and enters senescence.96 Thus, telomere attrition is considered a hallmark of replicative senescence or chronological aging, and it is also involved in some premature or accelerated aging.97,98
Blood leukocyte telomere lengths (LTL) are closely related to carotid IMT and cfPWV, therefore, LTL serves as an indirect marker of vascular aging. The correlation of LTL with carotid IMT has been tested in healthy elderly populations free of cardiovascular diseases99 as well as in hypertensive and diabetic patients.100–102 LTL is negatively correlated with cfPWV after excluding known confounding factors such as gender, menopausal status, blood pressure, blood glucose, and lipid level,103,104 therefore, considered as a better indicator of biological aging in vasculature than chronologic age in patients with cardiovascular damage.105
Advanced Glucagon End Products
Advanced glycation end-products (AGEs) are heterogeneous compounds formed by the non-enzymatic glycosylation reactions between reducing sugars and the amino group of proteins.106 They are accumulated in the vascular wall during aging and contribute to arterial stiffness by cross-linking with collagen fibers.107 Endothelial cells, on the other hand, express receptors for AGEs (RAGEs). Activation of AGEs-RAGEs signaling elicits vascular inflammation and oxidative stress, which ultimately leads to endothelial dysfunction.108 RAGEs can be cleaved into a soluble form, soluble RAGEs, which lack a membrane-anchoring domain; therefore, it circulates in the blood.109 Binding of soluble RAGEs to AGEs acts dominant-negatively, neutralizing the deleterious effects of AGEs on vessels.110,111 Elevated circulating levels of AGEs were independently associated with increased arterial stiffness in healthy individuals as well as in diabetic and hypertensive patients.112–114
An accelerated age-dependent increase in cfPWV has been reported in subjects with low circulating soluble RAGEs.115 Based on these findings, the combination of the AGE-soluble RAGE should provide more enriched information than a single parameter in evaluating vascular aging.110,116 Supportively, the ratio of skin-deposited AGEs to soluble RAGEs is a better predictor of arterial stiffness than that in blood.116–118
C-Reactive Protein
C-reactive protein (CRP) is an inflammation biomarker that is linearly correlated with cfPWV, forearm blood flow, and AIx.119–121 Serum CRP levels of elderly populations without overt CCVDs are increased with cfPWV,122,123 and AIx is increased by about 35% from 0.6 mg/L to 3.6 mg/L of CRP.124–126
It is important to know that a single assessment of CRP concentration is unlikely to provide credible information as CRP levels are susceptible to other factors such as age, gender, and infection.127 However, the cumulative CRP is shown to be a reliable marker in the prediction of arterial stiffness.127,128 To sum up, those cellular and molecular markers that associated with age-related vascular structural and functional changes are presented in Table 3.
 |
Table 3 Cellular and Molecular Markers of Vascular Aging |
Interventions for Vascular Aging
Pharmacologic Interventions
Anti-Hypertensive Agents
Hypertension is prevalent in the elderly and bring a huge burden for the society. It is a predominant risk factor that facilitates age-related changes in vascular function and structure.129 The cfPWV is accelerated in hypertensive patients with 0.93m/s per decade, compared with 0.44 m/s per decade in normotensive patients.130 The maximum forearm blood flow in response to acetylcholine decreased by nearly 42% in hypertensive patients.131 Furthermore, elevated blood pressure increases tension on the vascular wall, exacerbating vascular wall remodeling.20
Anti-hypertensive drugs are effective treatments to halt the progression of vascular aging. Here, we discuss five of the most commonly used antihypertensive drugs, including renin–angiotensin II-aldosterone system inhibitors (ACEI: angiotensin-converting enzyme inhibitors; ARB: angiotensin receptor blockers, AdRB: aldosterone receptor blockers), calcium channel blockers (CCB), β-receptor blockers, and diuretics on vascular aging.
It has been reported that all anti-hypertensive treatments, including ACEI, CCBs, β-blockers, and diuretics effectively reduced blood pressure; however, only ACEI improved endothelium-dependent vasodilation in randomly selected hypertensive patients treated with monotherapy.132 Supportively, in a meta-analysis, ACEI was reported to be more effective in enhancing endothelial function against all other drugs administered as a monotherapy in hypertensive patients.133 Another study reported that both ACEI and ARB improved FMD in hypertensive patients compared to β-blockers and CCB.134 These suggest that inhibition of the renin–angiotensin system is better for the improvement of endothelium-dependent vasodilation.
On the other hand, it was found that ACEI, CCB, β-blocker, and diuretics alleviate arterial stiffness in the long-term treatment compared with the control group. There was no significant difference in therapeutic effects among these groups.135 Other studies also reported that ACEI and ARB do not exhibit superiority in alleviating arterial stiffness compared with other types of antihypertensive treatments.136–140 ACEI effectively improved arterial elasticity in hypertensive patients compared with control groups but found no significant extra benefit compared with other types of anti-hypertensive drugs.141
Unlike ARB, AdRB, or CCB, diuretics exhibit only a moderate effect on vascular stiffness.142,143 Studies showed that ARB (losartan) and diuretics (chlorothiazide) both reduced blood pressure to normal levels in hypertensive patients after 4 weeks of treatment, while only losartan led to a significant decrease in cfPWV and AIx,144 while both AdRB (eplerenone) and CCB (amlodipine) were effective in reducing cfPWV with no significant difference between the two groups.139 It was further reported in the EXPLOR trial that ARB (valsartan), not β-blocker (atenolol), significantly decreased cfPWV after adjusting for blood pressure and heart rate.145 Atenolol improves arterial elasticity, however, it increases AIx attributed to the lower heart rate.146 The third generation of β-blockers, such as nebivolol and carvedilol, showed better results in the prevention of vascular aging. For example, nebivolol significantly reduces AIx.147 Further, with the infusion of nebivolol into the iliac artery, arterial elasticity was reported to improve only using nebivolol.148 Even if β-blockers reduce cfPWV to the same extent as ACEI or ARB does, the latter causes a long-lasting reduction in cfPWV.149,150
Treatment for Diabetes
Hyperglycemia, a feature of patients with diabetes or impaired glucose metabolism, contributes to vascular aging.151 Hyperglycemia can facilitate AGEs formation and endothelial cell senescence,152,153 which in turn aggravate arterial stiffness and endothelial dysfunction.112 Compared with age-matched healthy subjects, FMD is reduced by approximately 14% in newly diagnosed diabetic patients.154 The endothelium function is inversely correlated with the severity of diabetes and blood glucose levels.155 In addition, the age-dependent increase of arterial stiffness and arterial wall thickness is accelerated in diabetic patients.156–158 Therefore, anti-hyperglycemic treatment should be considered in the prevention of vascular aging.
Metformin is the first line of treatment for diabetes, as it re-sensitizes patients to insulin. It also improves age-related vascular function significantly.159 It has been reported in the REMOVAL trial that the age-related carotid IMT increase was reversed with a 0.012mm/year reduction in diabetic patients treated with metformin for 5 years.160 The percentage changes in coronary blood flow in response to acetylcholine were increased by 75% after 6 weeks of metformin treatment in pre-diabetic patients compared with the non-treated group.161 Moreover, FMD and reactive hyperemia index were increased by 1.8-fold and 1.3-fold, respectively, after 12-week treatment with metformin in type-2 diabetic patients.162 Metformin also improves AIx, cfPWV, and endothelial-dependent vasodilation in polycystic ovary syndrome patients treated with metformin for 8 weeks.163
Other anti-diabetic agents, such as peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist (Thiazolidinediones), such as pioglitazone, troglitazone, and rosiglitazone, and sodium-glucose co-transporter-2 inhibitor, such as dapagliflozin, empagliflozin, and pioglitazone, similar to Metformin, can improve age-related vascular phenotypes. A study reported that carotid IMT was decreased by 0.08 mm in diabetic patients treated with troglitazone for 3 months, whereas increased by 0.016 mm in placebo-treated subjects.164 Notably, the carotid IMT increased by 0.031 mm after 48 weeks of placebo treatment, and decreased by 0.012 mm after 48 weeks of rosiglitazone treatment in those with normoglycemia, although there was no significant change in fasting blood glucose level after treatment.165,166 Thus, the effect of thiazolidinediones in delaying vascular aging is at least in part independent of its hypoglycemic ability.167
Sodium-glucose co-transporter-2 inhibitor block glucose re-absorption in the proximal renal tubes and accelerate glucose excretion.168 Empagliflozin improves endothelial function to the same extent as metformin does, however, it showed superior benefits in reducing cfPWV in type 1 diabetic patients.162 Dapagliflozin improves FDM even when the treatment lasts as short as two days in type-2 diabetic patients169 and is more efficient than hydrochlorothiazide.170
Similar to thiazolidinediones, sodium-glucose co-transporter-2 inhibitor mediated restoration of vascular function is not fully dependent on their abilities to reduce glycemia and blood pressure.
Sulfonylureas appear to have little effect on improving vascular function in diabetic patients.171–178
Glucagon-like peptide-1 seems to ameliorate endothelial function through its indirect mechanism in improving glucose and lipid metabolisms.179 Exenatide, a glucagon-like peptide-1 analog, has been proven to improve brachial FMD in diabetic patients after long-term administration.180,181 Besides, even single dose of exenatide had favorable effects on endothelial function over two sequential meals in diabetic patients.182,183 Moreover, chronic administration of exenatide can also improve cfPWV,184,185 AIx179 and carotid IMT in people with type 2 diabetes mellitus.186
Intensive insulin therapy has been reported to improve acetylcholine-induced vasodilation in patients with type 1 diabetes.187 In addition, it has been shown that endothelial function is better in type 2 diabetes patients with insulin therapy combined with metformin, compared with metformin monotherapy.188,189 However, there are debated results which demonstrate that FMD in diabetic patients is not increased after 14 weeks of insulin glargine therapy.179
Lipid-Lowering Agents
Hyperlipidemia directly damages endothelial cells and EPCs impeding their abilities to repair the injured endothelium.190 Both carotid IMT and cfPWV are increased in patients with familial hypercholesterolemia than in normal people,191–193 suggesting that patients with hyperlipidemia suffer from premature vascular aging.
3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) inhibitors, also known as statins are widely used in the treatment of hyperlipidemia. Statins improve blood profile and restore vascular structure and function.194–196 However, it is still under heat discussion that whether the improvement of endothelial function is due to its lipid lowering effects or through its arterial pleiotropic effect. Some studies raise that statin therapy has pleiotropic effects because it can improve endothelial function in such a short time when the lipid-lowering effect of statins is incomplete,194 while recent studies that combine simvastatin treatment with ezetimibe to reach a lower LDL-C level suggest that the improvement of endothelial function is more correlated with the reduction of lipid level.197,198 Besides, researches focusing on the effects of statin therapy in ameliorating arterial stiffness have produced mixed results.199–202 It is also discovered that the improvement of arterial stiffness index may be transient under statin therapy.203 Patients with chronic kidney diseases possess lots of cardiovascular risk factors, and manifested as premature vascular aging.204 One study investigating the effects of statin in restoring arterial stiffness in patients with chronic kidney diseases has found that statin may retard the progression of arterial stiffness.205
It was found in a longitudinal study that the aortic root diameter was decreased in hypercholesterolemia patients treated with atorvastatin for two years.206 Other studies revealed that a low dose of simvastatin only moderately reduced the LDL-cholesterol level (25%) but did not reduce carotid IMT values, while high doses of atorvastatin largely reduced the LDL-cholesterol (45%) concentration, and reversed the age-related progression of carotid IMT.195,196 These indicate that structural improvements of arteries require more intensive and longer statin treatments.
Non-Pharmacologic Interventions
Physical Activity
It has been reported in a Spanish cross-sectional study that vascular aging was positively correlated with sedentary time but inversely related to physical activity.207 The arterial stiffness index cfPWV in sedentary people was 26% higher than the age-matched senior athletes.62 Additionally, sedentary men exhibited a more prominent age-related decline in endothelial function than their peers who exercised regularly.208
Physical exercise that lasts 12 weeks can effectively restore impaired endothelial-dependent vasodilation in both hypertensive patients and the normotensive elderly,209 possibly attributed to the enhancement of NO release as well as the reduction of oxidative stress and inflammatory responses.210,211 A longitudinal study showed that the age-related increase in baPWV was delayed in exercise-training people. After 10 years of follow-up, there was a 5.0% increase in baPWV among men who regularly exercised and a 13% increase in their inactive peers.212 With aerobic exercise for 3 months, central artery elasticity was effectively restored in sedentary subjects in a cross-sectional study.213 Notably, endurance exercise seems to have different effects on the structure of the carotid and peripheral arteries, therefore, producing debated results on whether endurance exercise can reduce the age-related increase in carotid IMT.214–218 More likely, endurance training changes the local arterial shear stress of the femoral artery and has little effect on the carotid artery.
Nevertheless, in healthy older adults, a decrease of nearly 11% in IMT and 9% in femoral arterial lumen diameter were detected 3 months after aerobic exercise intervention.218 Interestingly, it was found that racquet players had lower carotid IMT values than inactive peers. Their study suggested that more vigorous and intensive exercise is required to reduce carotid IMT.219 In contrast to endurance training, chronic resistance training has negative effects on arterial elasticity.220 The resistance-trained subjects showed a prominent age-dependent reduction in arterial elasticity compared with the sedentary-control subjects.220
Dietary Control
Diet habits influence vascular function profoundly. For example, the Mediterranean diet improves vascular function, while high-fat diets can acutely impair endothelial-dependent vasodilation.221,222 Thus, dietary control provides a cost-effective way to prevent vascular aging.
Several studies reported that Mediterranean diet consumption significantly improved FMD in healthy older adults, obese individuals, and patients with metabolic syndrome.223–225 It has been shown that the six-week low-fat diets (30% of total calories from fat) restored endothelial function in obese individuals, and increased FMD by 34%.226,227 An eight-week of low-fat diet reduced cfPWV in hypercholesterolemic patients, and the decreased cfPWV was significantly correlated with the reduction of plasma CRP levels.228
A high-fiber diet was associated with a low risk of CCVDs,229 decreased carotid IMT,230 restored endothelial dysfunction in patients with metabolic syndrome,231 and prevented endothelial dysfunction caused by high-fat meals.232,233
Excessive salt intake is another life habit that impairs vascular function.234–237 The arterial elasticity in untreated hypertensive patients was significantly improved after 2 weeks of salt restriction,238 and the age-dependent growth of cfPWV was effectively delayed during 2 years of salt restriction in normotensive people although the salt restriction did not decrease blood pressure, compared with the normal diet group.239 These findings suggest that the beneficial effects of a low-salt diet do not depend solely on blood pressure reduction.
Caloric restriction is considered to be an effective nutritional intervention to delay vascular aging.240,241 In hypertensive obese individuals, baseline forearm blood flow did not change after 2 weeks of a low-calorie diet (800 kcal/day), but endothelium-dependent vasodilation improved.242 The study in overweight young adults also revealed a 10% weight loss and a 3.3% increase in FMD after 6 weeks of a very-low-calorie diet (580 kcal/day).243 In a longitudinal study, the FMD of obese diabetic patients increased from 5.64% to 10.16% after 8 weeks of caloric restriction (1000 kcal/day) and continued to increase to 12.46% over the next 44 weeks.226 Another trial showed no difference in FMD changes between the caloric restriction group and the control group, however, PWV was decreased by 1.2m/s in the caloric restriction group after 3 months of a low-calorie diet (<1400 kcal/day).244
Intermittent fasting, time-restricted food intake, is another way to control energy intake.245 Current knowledge about its effect on our blood vessels is very limited. In a comparative study, circulating markers of endothelial function (total nitrate, asymmetrical dimethylarginine, vascular cell adhesion molecule-1) were improved in patients with metabolic syndrome after 8 weeks of a 2-day fasting dietary schedule.246 Furthermore, a recent animal study reported an increase in FMD in obese rats undergoing 6 weeks of intermittent fasting.247 More studies are required to delineate the effects of intermittent fasting on vascular aging. It is important to understand that both, the strength and timing, present a large effect on the outcome, and may explain the conflicts which are not discussed here.248–251 The interventions of vascular aging and their outcomes are outlined in Table 4.
 |
Table 4 Interventions for Vascular Aging and the Beneficial Effects |
Anti-Aging Therapy
The above strategies mainly focus on controlling risk factors of vascular aging to delay this process and have far-reaching significance. However, these measures alone seem to be insufficient in solving the problem. Currently, the most promising therapeutic approach in delaying vascular aging is anti-aging therapy, which relies on compounds that straightforwardly target molecular mechanisms underlying cellular senescence.
Senolytic is a kind of chemotherapeutics, which refers to the group of drugs that can selectively eliminate senescent cells. The common combination is dasatinib and quercetin. Recent studies have confirmed that senolytics can improve many age-related diseases and extend life span in aged mice.252–254 Besides, senolytics can significantly reduce senescent cells in the medial layer of the aorta in the atherosclerotic model mice (ApoE−/−) and improve the vasomotor response to NO.255 Moreover, eliminating senescent cells can increase atherosclerotic plaque stability and prevent the development of atherosclerotic lesions.256
Metformin is not only a commonly used drug for diabetes, it also has direct cardiovascular protective effects.257 Age-related vascular indicators such as IMT, FMD, AIx and PWV were improved in diabetic patients with metformin.160,162,163 Furthermore, studies demonstrated that metformin can delay cellular senescence.252 The underlying mechanisms are complex and not fully understood.258 There was a mass of papers investigated the anti-aging actions of metformin with data obtained from various species include C. elegans, Drosophila melanogaster, rodents and humans. Nonetheless, their conclusions are controversial, with some reviews being supportive of the anti-aging effect of metformin,259–261 whereas others hold different opinions.257,262–264
Rapamycin is mTOR inhibitor. It has been reported to increase life span in many species,265–267 and also it can inhibit the senescence-associated secretory phenotype of senescent cells.268 Besides, plenty of evidences demonstrate that rapamycin has cardiovascular protective benefits. In old mice, after treatment of rapamycin, the endothelial-dependent dilation and NO bioavailability were largely improved, potentially due to its action in mediating redox balance or inflammation in advanced age.269 In addition, structural changes of aged vasculature may be ameliorated, contributing to attenuated age-related vascular stiffening.269 More importantly, these cardiovascular protective benefits were also confirmed in human. Studies have reported that those who received rapamycin therapy after kidney transplantation have significantly greater FMD, PWV and AIx.270,271
Nicotinamide adenine dinucleotide (NAD+) is a coenzyme that catalyzes cell metabolism and can enhance Sirtuins 1 activation.272 Its concentration is decreased in senescent cells. Many studies have demonstrated that supplementation of NAD+ precursor can retard cellular senescence and prolong lifespan.273,274 Moreover, supplying NAD+ to old mice can reverse their age-related endothelial dysfunction and arterial stiffness.275 Nevertheless, when translated to humans, only vascular elasticity, no endothelial function, was found to improve after providing people with NAD+ precursor for 6 weeks.276 These evidences proved that Sirtuins 1 activation may be effective in ameliorating vascular aging.
Anti-aging therapy has now become a promising weapon in age-related diseases. However, there is still a long way to go “from bench to bedside”.
Summary and Discussion
Blood vessels change their structures, functions, and compliance along the aging process. These changes can be detected and visualized to assess the degree of vascular aging directly; 1) Structure: both arterial wall thickness and lumen diameter are increased. 2) Function: the endothelium-dependent vasodilation induced by acetylcholine or reactive hyperemia becomes desensitized. 3) The vascular compliance: stiffness, which is measured by PWV or AIx, is increased. In addition to these direct measurements, the laboratory tests can provide many other indirect biomarkers or indicators for vascular aging, for example, endothelial progenitor cells, lymphocytic telomere, advanced glycation end-products, and C-reactive protein, as these biomarkers are well correlated with direct measurements.
However, it should be noted that these parameters reflect different information about arterial properties and may not be interchangeable even in the same category. For example, PWV and AIx are not fully consistent at all ages. Importantly, most of these parameters are influenced by not only age but also other factors, such as health status, gender, and lifestyle, and not all parameters are linearly correlated with age, while, some of them are only altered in the elderly population. Therefore, it is essential to measure multiple parameters to get a broader assessment of vascular aging.
Many diseases, particularly cardiovascular and metabolic diseases including their risk factors, significantly affect vascular aging. Thus, treating these diseases and avoiding the relevant risk factors prove to be effective in the prevention of vascular aging. Notably, only a few approaches can improve all parameters for vascular aging, since the outcome of the intervention is affected by many factors, such as strength, frequency, and age. This again points to the importance of measuring multiple parameters to evaluate the results of applied approaches to evaluate their abilities in preventing vascular aging.
It is worthy of being mentioned that there also exist controversies in both vascular phenotypic alterations and the outcomes of interventions. For example, whether aortic root dilation is a reasonable marker for vascular aging, as it is influenced by many factors, including body surface area, and how the listed indirect laboratory tests reflect vascular aging specifically. We also leave some questions that need more investigations to clarify. For instance, which clinical assessment(s) cover(s) more vascular changes with ages and can be used as a representative indicator(s) to quantify vascular aging and which intervention gives best outcome, and so on. Answers to these questions not only provide an update in knowledge but also make a step forward to clinical translation in vascular aging.
In summary, vascular aging is a multi-dimensional process that requires a comprehensive set of parameters to be measured in clinical practices and it can be prevented to maintain healthy vasculature by avoiding modifiable risk factors and adhering to a healthy lifestyle.
Abbreviations
ACERI, angiotensin-converting enzyme inhibitors; AdRB, aldosterone receptor blockers; AGEs, advanced glycation end-products; ARB, angiotensin receptor blockers; AIx, augmentation index; baPWV, brachial-ankle PWV; BSA, body surface areas; CCB, calcium channel blockers; CCVDs, cardio-cerebral vascular diseases; cfPWV, carotid-femoral PWV; CRP, C-reactive protein; EPC, endothelial progenitor cell; FMD, flow-mediated dilation; IMT, intimal-medial thickness; LTL, leukocyte telomere lengths; NAD+, nicotinamide adenine dinucleotide; NO, nitric oxide; PWV, pulse wave velocity; RAGE, receptors for AGE.
Ethic Statements
The processes that we achieved vascular tissues from patients conformed to the guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of The Second Affiliated Hospital of Nanchang University.
Funding
This study was funded by the National Key Research and Development Program of the Ministry of Science and Technology, China (2020YFC2002900 to XLT), the Key Program of the National Natural Science Foundation of China (81630034 to XLT), and the Key Programs of the Jiangxi Province, China (20192ACB70002 and 20181BCD40001 to XLT).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi:10.1161/CIRCRESAHA.111.246876
2. Cortes-Canteli M, Iadecola C. Alzheimer’s disease and vascular aging: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):942–951. doi:10.1016/j.jacc.2019.10.062
3. Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol a Biol Sci Med Sci. 2010;65(10):1028–1041. doi:10.1093/gerona/glq113
4. Zhang C, Tao J; Cardiovascular Group SoGCMA. Expert consensus on clinical assessment and intervention of vascular aging in China (2018). Aging Med. 2018;1(3):228–237. doi:10.1002/agm2.12049
5. Tian XL, Li Y. Endothelial cell senescence and age-related vascular diseases. J Genet Genomics. 2014;41(9):485–495. doi:10.1016/j.jgg.2014.08.001
6. Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139(5):1119–1129.
7. Cai Y, Song W, Li J, et al. The landscape of aging. Sci China Life Sci. 2022;65(12):2354–2454. doi:10.1007/s11427-022-2161-3
8. Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74(6):1399–1406. doi:10.1161/01.CIR.74.6.1399
9. O’Leary DH, Polak JF, Kronmal RA, et al. Distribution and correlates of sonographically detected carotid artery disease in the Cardiovascular Health Study. The CHS Collaborative Research Group. Stroke. 1992;23(12):1752–1760. doi:10.1161/01.STR.23.12.1752
10. Salonen R, Salonen JT. Progression of carotid atherosclerosis and its determinants: a population-based ultrasonography study. Atherosclerosis. 1990;81(1):33–40. doi:10.1016/0021-9150(90)90056-O
11. Homma S, Hirose N, Ishida H, Ishii T, Araki G. Carotid plaque and intima-media thickness assessed by b-mode ultrasonography in subjects ranging from young adults to centenarians. Stroke. 2001;32(4):830–835. doi:10.1161/01.STR.32.4.830
12. Nagai Y, Metter EJ, Earley CJ, et al. Increased carotid artery intimal-medial thickness in asymptomatic older subjects with exercise-induced myocardial ischemia. Circulation. 1998;98(15):1504–1509. doi:10.1161/01.CIR.98.15.1504
13. Ando F, Takekuma K, Niino N, Shimokata H. Ultrasonic evaluation of common carotid intima-media thickness (IMT)--influence of local plaque on the relationship between IMT and age. J Epidemiol. 2000;10(1 Suppl):S10–S17. doi:10.2188/jea.10.1sup_10
14. Koç AS, Sümbül HE. Age should be considered in cut-off values for increased carotid intima-media thickness. Turk Kardiyol Dern Ars. 2019;47(4):301–311. doi:10.5543/tkda.2018.94770
15. Juonala M, Kähönen M, Laitinen T, et al. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in Young Finns Study. Eur Heart J. 2008;29(9):1198–1206. doi:10.1093/eurheartj/ehm556
16. Stein JH, Douglas PS, Srinivasan SR, et al. Distribution and cross-sectional age-related increases of carotid artery intima-media thickness in young adults: the Bogalusa Heart Study. Stroke. 2004;35(12):2782–2787. doi:10.1161/01.STR.0000147719.27237.14
17. Dinenno FA, Jones PP, Seals DR, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. Am J Physiol Heart Circ Physiol. 2000;278(4):H1205–H1210. doi:10.1152/ajpheart.2000.278.4.H1205
18. van den Munckhof I, Scholten R, Cable NT, Hopman MT, Green DJ, Thijssen DH. Impact of age and sex on carotid and peripheral arterial wall thickness in humans. Acta Physiol. 2012;206(4):220–228. doi:10.1111/j.1748-1716.2012.02457.x
19. Watanabe D, Gando Y, Murakami H, et al. Longitudinal trajectory of vascular age indices and cardiovascular risk factors: a repeated-measures analysis. Sci Rep. 2023;13(1):5401. doi:10.1038/s41598-023-32443-5
20. O’Rourke MF, Nichols WW. Aortic diameter, aortic stiffness, and wave reflection increase with age and isolated systolic hypertension. Hypertension. 2005;45(4):652–658. doi:10.1161/01.HYP.0000153793.84859.b8
21. O’Rourke MF. Arterial aging: pathophysiological principles. Vasc Med. 2007;12(4):329–341. doi:10.1177/1358863X07083392
22. Vriz O, Driussi C, Bettio M, Ferrara F, D’Andrea A, Bossone E. Aortic root dimensions and stiffness in healthy subjects. Am J Cardiol. 2013;112(8):1224–1229. doi:10.1016/j.amjcard.2013.05.068
23. Vasan RS, Larson MG, Benjamin EJ, Levy D. Echocardiographic reference values for aortic root size: the Framingham Heart Study. J Am Soc Echocardiogr. 1995;8(6):793–800. doi:10.1016/S0894-7317(05)80003-3
24. Roman MJ, Devereux RB, Kramer-Fox R, O’Loughlin J. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64(8):507–512. doi:10.1016/0002-9149(89)90430-X
25. Daimon M, Watanabe H, Abe Y, et al. Normal values of echocardiographic parameters in relation to age in a healthy Japanese population: the JAMP study. Circ J. 2008;72(11):1859–1866. doi:10.1253/circj.CJ-08-0171
26. Wang X, Ren XS, An YQ, et al. A specific assessment of the normal anatomy of the aortic root in relation to age and gender. Int J Gen Med. 2021;14:2827–2837. doi:10.2147/IJGM.S312439
27. Devereux RB, de Simone G, Arnett DK, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons ≥15 years of age. Am J Cardiol. 2012;110(8):1189–1194. doi:10.1016/j.amjcard.2012.05.063
28. Campens L, Demulier L, De Groote K, et al. Reference values for echocardiographic assessment of the diameter of the aortic root and ascending aorta spanning all age categories. Am J Cardiol. 2014;114(6):914–920. doi:10.1016/j.amjcard.2014.06.024
29. Bjarnegård N, Länne T. Arterial properties along the upper arm in humans: age-related effects and the consequence of anatomical location. J Appl Physiol. 2010;108(1):34–38. doi:10.1152/japplphysiol.00479.2009
30. Sandgren T, Sonesson B, Ahlgren AR, Länne T. Factors predicting the diameter of the popliteal artery in healthy humans. J Vasc Surg. 1998;28(2):284–289. doi:10.1016/S0741-5214(98)70164-8
31. Sandgren T, Sonesson B, Ahlgren R, Länne T. The diameter of the common femoral artery in healthy human: influence of sex, age, and body size. J Vasc Surg. 1999;29(3):503–510. doi:10.1016/S0741-5214(99)70279-X
32. Bia D, Zócalo Y, Farro I, et al. Integrated evaluation of age-related changes in structural and functional vascular parameters used to assess arterial aging, subclinical atherosclerosis, and cardiovascular risk in Uruguayan adults: cUiiDARTE project. Int J Hypertens. 2011;2011:587303. doi:10.4061/2011/587303
33. Astrand H, Rydén-Ahlgren A, Sandgren T, Länne T. Age-related increase in wall stress of the human abdominal aorta: an in vivo study. J Vasc Surg. 2005;42(5):926–931. doi:10.1016/j.jvs.2005.07.010
34. Egashira K, Inou T, Hirooka Y, et al. Effects of age on endothelium-dependent vasodilation of resistance coronary artery by acetylcholine in humans. Circulation. 1993;88(1):77–81. doi:10.1161/01.CIR.88.1.77
35. Taddei S, Virdis A, Ghiadoni L, et al. Age-related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38(2):274–279. doi:10.1161/01.HYP.38.2.274
36. James MA, Tullett J, Hemsley AG, Shore AC. Effects of aging and hypertension on the microcirculation. Hypertension. 2006;47(5):968–974. doi:10.1161/01.HYP.0000209939.05482.61
37. Soltis EE. Effect of age on blood pressure and membrane-dependent vascular responses in the rat. Circ Res. 1987;61(6):889–897. doi:10.1161/01.RES.61.6.889
38. Vanhoutte PM. Aging and vascular responsiveness. J Cardiovasc Pharmacol. 1988;12(8):S11–S19. doi:10.1097/00005344-198812081-00004
39. Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315(17):1046–1051. doi:10.1056/NEJM198610233151702
40. Egashira K, Inou T, Hirooka Y, et al. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91(1):29–37. doi:10.1172/JCI116183
41. Ishida S, Hamasaki S, Kamekou M, et al. Advancing age is associated with diminished vascular remodeling and impaired vasodilation in resistance coronary arteries. Coron Artery Dis. 2003;14(6):443–449. doi:10.1097/00019501-200309000-00005
42. Yasue H, Matsuyama K, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81(2):482–490. doi:10.1161/01.CIR.81.2.482
43. Tew GA, Klonizakis M, Saxton JM. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. Eur J Appl Physiol. 2010;109(2):173–181. doi:10.1007/s00421-009-1342-9
44. Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. doi:10.1016/0735-1097(95)00327-4
45. Anderson EA, Mark AL. Flow-mediated and reflex changes in large peripheral artery tone in humans. Circulation. 1989;79(1):93–100. doi:10.1161/01.CIR.79.1.93
46. Cho JM, Park SK, Kwon OS, et al. Activating P2Y1 receptors improves function in arteries with repressed autophagy. Cardiovasc Res. 2023;119(1):252–267. doi:10.1093/cvr/cvac061
47. Tajima E, Sakuma M, Tokoi S, et al. The comparison of endothelial function between conduit artery and microvasculature in patients with coronary artery disease. Cardiol J. 2020;27(1):38–46. doi:10.5603/CJ.a2018.0077
48. Babcock MC, DuBose LE, Witten TL, et al. Assessment of macrovascular and microvascular function in aging males. J Appl Physiol. 2021;130(1):96–103. doi:10.1152/japplphysiol.00616.2020
49. Donato AJ, Eskurza I, Silver AE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi:10.1161/01.RES.0000269183.13937.e8
50. Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. J Physiol. 2006;571(Pt 3):661–668. doi:10.1113/jphysiol.2005.102566
51. Aizawa K, Ramalli A, Sbragi S, et al. Arterial wall shear rate response to reactive hyperaemia is markedly different between young and older humans. J Physiol. 2019;597(16):4151–4163. doi:10.1113/JP278310
52. Skaug EA, Aspenes ST, Oldervoll L, et al. Age and gender differences of endothelial function in 4739 healthy adults: the HUNT3 Fitness Study. Eur J Prev Cardiol. 2013;20(4):531–540. doi:10.1177/2047487312444234
53. Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi:10.1016/0735-1097(94)90305-0
54. McEniery CM, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR, Cockcroft JR. Normal vascular aging: differential effects on wave reflection and aortic pulse wave velocity: the Anglo-Cardiff Collaborative Trial (ACCT). J Am Coll Cardiol. 2005;46(9):1753–1760. doi:10.1016/j.jacc.2005.07.037
55. Aguilar VM, Paul A, Lazarko D, Levitan I. Paradigms of endothelial stiffening in cardiovascular disease and vascular aging. Front Physiol. 2022;13:1081119. doi:10.3389/fphys.2022.1081119
56. Bulpitt CJ, Rajkumar C, Cameron JD. Vascular compliance as a measure of biological age. J Am Geriatr Soc. 1999;47(6):657–663. doi:10.1111/j.1532-5415.1999.tb01586.x
57. González-Clemente JM, Cano A, Albert L, et al. Arterial stiffness in type 1 diabetes: the case for the arterial wall itself as a target organ. J Clin Med. 2021;10(16):3616. doi:10.3390/jcm10163616
58. Heffernan KS, Stoner L, London AS, Augustine JA, Lefferts WK. Estimated pulse wave velocity as a measure of vascular aging. PLoS One. 2023;18(1):e0280896. doi:10.1371/journal.pone.0280896
59. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi:10.1093/eurheartj/ehl254
60. Lehmann ED, Hopkins KD, Gosling RG. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension. 1996;27(5):1188–1191.
61. Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54(6):1328–1336. doi:10.1161/HYPERTENSIONAHA.109.137653
62. Vaitkevicius PV, Fleg JL, Engel JH, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Pt 1):1456–1462. doi:10.1161/01.CIR.88.4.1456
63. Avolio AP, Deng FQ, Li WQ, et al. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: comparison between urban and rural communities in China. Circulation. 1985;71(2):202–210. doi:10.1161/01.CIR.71.2.202
64. Mitchell GF, Parise H, Benjamin EJ, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–1245. doi:10.1161/01.HYP.0000128420.01881.aa
65. AlGhatrif M, Strait JB, Morrell CH, et al. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62(5):934–941. doi:10.1161/HYPERTENSIONAHA.113.01445
66. Avolio AP, Kuznetsova T, Heyndrickx GR, Kerkhof PLM, Li JK. Arterial flow, pulse pressure and pulse wave velocity in men and women at various ages. Adv Exp Med Biol. 2018;1065:153–168.
67. Reference Values for Arterial Stiffness’ Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31(19):2338–2350. doi:10.1093/eurheartj/ehq165
68. Kim OY, Paik JK, Lee JY, Lee SH, Lee JH. Follow-ups of metabolic, inflammatory and oxidative stress markers, and brachial-ankle pulse wave velocity in middle-aged subjects without metabolic syndrome. Clin Exp Hypertens. 2013;35(5):382–388. doi:10.3109/10641963.2012.739232
69. Ishida A, Fujisawa M, Del Saz EG, et al. Arterial stiffness, not systolic blood pressure, increases with age in native Papuan populations. Hypertens Res. 2018;41(7):539–546. doi:10.1038/s41440-018-0047-z
70. Sang Y, Wu X, Miao J, Cao M, Ruan L, Zhang C. Determinants of Brachial-Ankle pulse wave velocity and vascular aging in healthy older subjects. Med Sci Monit. 2020;26:e923112. doi:10.12659/MSM.923112
71. Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17(5):543–551. doi:10.1097/00001573-200209000-00016
72. Yao Y, Hao L, Xu L, et al. Diastolic augmentation index improves radial augmentation index in assessing arterial stiffness. Sci Rep. 2017;7(1):5864. doi:10.1038/s41598-017-06094-2
73. Sakurai M, Yamakado T, Kurachi H, et al. The relationship between aortic augmentation index and pulse wave velocity: an invasive study. J Hypertens. 2007;25(2):391–397. doi:10.1097/HJH.0b013e3280115b7c
74. Filipovský J, Tichá M, Cífková R, Lánská V, Stastná V, Roucka P. Large artery stiffness and pulse wave reflection: results of a population-based study. Blood Press. 2005;14(1):45–52. doi:10.1080/08037050510008814
75. Hidvégi EV, Illyés M, Molnár FT, Cziráki A. Influence of body height on aortic systolic pressure augmentation and wave reflection in childhood. J Hum Hypertens. 2015;29(8):495–501. doi:10.1038/jhh.2014.118
76. Diaz A, Zócalo Y, Bia D, Cabrera Fischer E. Reference Intervals of central aortic blood pressure and augmentation index assessed with an oscillometric device in healthy children, adolescents, and young adults from Argentina. Int J Hypertens. 2018;2018:1469651. doi:10.1155/2018/1469651
77. Kelly R, Hayward C, Avolio A, O’Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–1659. doi:10.1161/01.CIR.80.6.1652
78. Kiran VR, Nabeel PM, Shah MI, Sivaprakasam M, Joseph J. Gaussian-Mixture modelling of a-mode radiofrequency scans for the measurement of arterial wall thickness.
79. Raj KV, Joseph J, Nabeel PM, Sivaprakasam M. Automated measurement of compression-decompression in arterial diameter and wall thickness by image-free ultrasound. Comput Methods Programs Biomed. 2020;194:105557. doi:10.1016/j.cmpb.2020.105557
80. Nabeel PM, Manoj R, Abhidev VV, Joseph J, Kiran VR, Sivaprakasam M. High-throughput vascular screening by ARTSENS pen during a medical camp for early-stage detection of chronic kidney disease.
81. Nabeel PM, Raj KV, Joseph J. Image-free ultrasound for local and regional vascular stiffness assessment: the ARTSENS Plus. J Hypertens. 2022;40(8):1537–1544. doi:10.1097/HJH.0000000000003181
82. Joseph J, Nabeel PM, Rao SR, Venkatachalam R, Shah MI, Kaur P. Assessment of carotid arterial stiffness in community settings with ARTSENS(R). IEEE J Transl Eng Health Med. 2021;9:1900111. doi:10.1109/JTEHM.2020.3042386
83. Joseph J, Kiran R, Nabeel PM, et al. ARTSENS((R)) Pen-portable easy-to-use device for carotid stiffness measurement: technology validation and clinical-utility assessment. Biomed Phys Eng Express. 2020;6(2):025013. doi:10.1088/2057-1976/ab74ff
84. Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291(6):H3043–H3049. doi:10.1152/ajpheart.00190.2006
85. Thijssen DH, de Groot P, Kooijman M, Smits P, Hopman MT. Sympathetic nervous system contributes to the age-related impairment of flow-mediated dilation of the superficial femoral artery. Am J Physiol Heart Circ Physiol. 2006;291(6):H3122–H3129. doi:10.1152/ajpheart.00240.2006
86. Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi:10.1161/01.RES.85.3.221
87. Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi:10.1161/01.CIR.0000018166.84319.55
88. Kawamoto A, Gwon HC, Iwaguro H, et al. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi:10.1161/01.CIR.103.5.634
89. Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108(21):2710–2715. doi:10.1161/01.CIR.0000096490.16596.A6
90. Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93(10):980–989. doi:10.1161/01.RES.0000099245.08637.CE
91. Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi:10.1056/NEJMoa022287
92. Tao J, Wang Y, Yang Z, Tu C, Xu MG, Wang JM. Circulating endothelial progenitor cell deficiency contributes to impaired arterial elasticity in persons of advancing age. J Hum Hypertens. 2006;20(7):490–495. doi:10.1038/sj.jhh.1001996
93. Yang Z, Chen L, Su C, et al. Impaired endothelial progenitor cell activity is associated with reduced arterial elasticity in patients with essential hypertension. Clin Exp Hypertens. 2010;32(7):444–452. doi:10.3109/10641961003686435
94. Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol. 2008;103(6):582–586. doi:10.1007/s00395-008-0742-z
95. Buffa S, Borzì D, Chiarelli R, et al. Biomarkers for vascular ageing in aorta tissues and blood samples. Exp Gerontol. 2019;128:110741. doi:10.1016/j.exger.2019.110741
96. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi:10.1016/0014-4827(61)90192-6
97. Strazhesko I, Tkacheva O, Boytsov S, et al. Association of insulin resistance, arterial stiffness and telomere length in adults free of cardiovascular diseases. PLoS One. 2015;10(8):e0136676. doi:10.1371/journal.pone.0136676
98. Mehdizadeh M, Aguilar M, Thorin E, Ferbeyre G, Nattel S. The role of cellular senescence in cardiac disease: basic biology and clinical relevance. Nat Rev Cardiol. 2022;19(4):250–264. doi:10.1038/s41569-021-00624-2
99. Strazhesko ID, Tkacheva ON, Akasheva DU, et al. Growth hormone, insulin-like growth factor-1, insulin resistance, and leukocyte telomere length as determinants of arterial aging in subjects free of cardiovascular diseases. Front Genet. 2017;8:198. doi:10.3389/fgene.2017.00198
100. Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165(1):14–21. doi:10.1093/aje/kwj346
101. Spigoni V, Aldigeri R, Picconi A, et al. Telomere length is independently associated with subclinical atherosclerosis in subjects with type 2 diabetes: a cross-sectional study. Acta Diabetol. 2016;53(4):661–667. doi:10.1007/s00592-016-0857-x
102. Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–185. doi:10.1161/01.HYP.0000113081.42868.f4
103. Raymond AR, Norton GR, Woodiwiss AJ, Brooksbank RL. Impact of gender and menopausal status on relationships between biological aging, as indexed by telomere length, and aortic stiffness. Am J Hypertens. 2015;28(5):623–630. doi:10.1093/ajh/hpu212
104. Peng H, Zhu Y, Yeh F, et al. Impact of biological aging on arterial aging in American Indians: findings from the Strong Heart Family Study. Aging. 2016;8(8):1583–1592. doi:10.18632/aging.101013
105. Nakashima H, Ozono R, Suyama C, Sueda T, Kambe M, Oshima T. Telomere attrition in white blood cell correlating with cardiovascular damage. Hypertens Res. 2004;27(5):319–325. doi:10.1291/hypres.27.319
106. Bucala R, Cerami A. Advanced glycosylation: chemistry, biology, and implications for diabetes and aging. Adv Pharmacol. 1992;23:1–34.
107. Sims TJ, Rasmussen LM, Oxlund H, Bailey AJ. The role of glycation cross-links in diabetic vascular stiffening. Diabetologia. 1996;39(8):946–951. doi:10.1007/BF00403914
108. Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196(1):9–21. doi:10.1016/j.atherosclerosis.2007.07.025
109. Yonekura H, Yamamoto Y, Sakurai S, et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem J. 2003;370(Pt 3):1097–1109. doi:10.1042/bj20021371
110. Prasad K. Is there any evidence that AGE/sRAGE is a universal biomarker/risk marker for diseases? Mol Cell Biochem. 2019;451(1–2):139–144. doi:10.1007/s11010-018-3400-2
111. Geroldi D, Falcone C, Emanuele E, et al. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J Hypertens. 2005;23(9):1725–1729. doi:10.1097/01.hjh.0000177535.45785.64
112. Schram MT, Schalkwijk CG, Bootsma AH, Fuller JH, Chaturvedi N, Stehouwer CD. Advanced glycation end products are associated with pulse pressure in type 1 diabetes: the EURODIAB Prospective Complications Study. Hypertension. 2005;46(1):232–237. doi:10.1161/01.HYP.0000164574.60279.ba
113. McNulty M, Mahmud A, Feely J. Advanced glycation end-products and arterial stiffness in hypertension. Am J Hypertens. 2007;20(3):242–247. doi:10.1016/j.amjhyper.2006.08.009
114. Semba RD, Najjar SS, Sun K, Lakatta EG, Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am J Hypertens. 2009;22(1):74–79. doi:10.1038/ajh.2008.320
115. Gelžinský J, Mayer O Jr, Seidlerová J, et al. Soluble receptor for advanced glycation end-products independently influences individual age-dependent increase of arterial stiffness. Hypertens Res. 2020;43(2):111–120. doi:10.1038/s41440-019-0347-y
116. Mayer O, Gelžinský J, Seidlerová J, et al. The role of advanced glycation end products in vascular aging: which parameter is the most suitable as a biomarker? J Hum Hypertens. 2021;35(3):240–249. doi:10.1038/s41371-020-0327-3
117. Gelžinský J, Mayer O Jr, Seidlerová J, et al. Serum biomarkers, skin autofluorescence and other methods. Which parameter better illustrates the relationship between advanced glycation end products and arterial stiffness in the general population? Hypertens Res. 2021;44(5):518–527. doi:10.1038/s41440-020-00601-1
118. Birukov A, Cuadrat R, Polemiti E, Eichelmann F, Schulze MB. Advanced glycation end-products, measured as skin autofluorescence, associate with vascular stiffness in diabetic, pre-diabetic and normoglycemic individuals: a cross-sectional study. Cardiovasc Diabetol. 2021;20(1):110. doi:10.1186/s12933-021-01296-5
119. Du Clos TW. Function of C-reactive protein. Ann Med. 2000;32(4):274–278. doi:10.3109/07853890009011772
120. Vlachopoulos C, Dima I, Aznaouridis K, et al. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–2200. doi:10.1161/CIRCULATIONAHA.105.535435
121. Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102(9):1000–1006. doi:10.1161/01.CIR.102.9.1000
122. Mattace-Raso FU, van der Cammen TJ, van der Meer IM, et al. C-reactive protein and arterial stiffness in older adults: the Rotterdam Study. Atherosclerosis. 2004;176(1):111–116. doi:10.1016/j.atherosclerosis.2004.04.014
123. Yasmin MCM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler Thromb Vasc Biol. 2004;24(5):969–974. doi:10.1161/01.ATV.zhq0504.0173
124. Kampus P, Kals J, Ristimäe T, Fischer K, Zilmer M, Teesalu R. High-sensitivity C-reactive protein affects central haemodynamics and augmentation index in apparently healthy persons. J Hypertens. 2004;22(6):1133–1139. doi:10.1097/00004872-200406000-00014
125. Kullo IJ, Seward JB, Bailey KR, et al. C-reactive protein is related to arterial wave reflection and stiffness in asymptomatic subjects from the community. Am J Hypertens. 2005;18(8):1123–1129. doi:10.1016/j.amjhyper.2005.03.730
126. Nakhai-Pour HR, Grobbee DE, Bots ML, Muller M, van der Schouw YT. C-reactive protein and aortic stiffness and wave reflection in middle-aged and elderly men from the community. J Hum Hypertens. 2007;21(12):949–955. doi:10.1038/sj.jhh.1002255
127. Sun L, Ning C, Liu J, et al. The association between cumulative C-reactive protein and brachial-ankle pulse wave velocity. Aging Clin Exp Res. 2020;32(5):789–796. doi:10.1007/s40520-019-01274-8
128. McEniery CM, Spratt M, Munnery M, et al. An analysis of prospective risk factors for aortic stiffness in men: 20-year follow-up from the Caerphilly prospective study. Hypertension. 2010;56(1):36–43. doi:10.1161/HYPERTENSIONAHA.110.150896
129. Khutan H, Aggarwal S, Kajal KS, Garg R, Kaur R, Kaur A. Study of carotid intimal medial thickness in essential hypertension with or without left ventricular hypertrophy. Ann Afr Med. 2017;16(4):192–195. doi:10.4103/aam.aam_9_17
130. Diaz A, Tringler M, Wray S, Ramirez AJ, Cabrera Fischer EI. The effects of age on pulse wave velocity in untreated hypertension. J Clin Hypertens. 2018;20(2):258–265. doi:10.1111/jch.13167
131. Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. Effect of the angiotensin-converting enzyme inhibitor imidapril on reactive hyperemia in patients with essential hypertension: relationship between treatment periods and resistance artery endothelial function. J Am Coll Cardiol. 2001;37(3):863–870. doi:10.1016/S0735-1097(00)01177-3
132. Higashi Y, Sasaki S, Nakagawa K, et al. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta-blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35(2):284–291. doi:10.1016/S0735-1097(99)00561-6
133. Ding H, Liu S, Zhao KX, Pu J, Xie YF, Zhang XW. Comparative Efficacy of Antihypertensive Agents in Flow-Mediated Vasodilation of Patients with Hypertension: network Meta-Analysis of Randomized Controlled Trial. Int J Hypertens. 2022;2022:2432567. doi:10.1155/2022/2432567
134. Shahin Y, Khan JA, Samuel N, Chetter I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: a meta-analysis of randomised controlled trials. Atherosclerosis. 2011;216(1):7–16. doi:10.1016/j.atherosclerosis.2011.02.044
135. Ong KT, Delerme S, Pannier B, et al. Aortic stiffness is reduced beyond blood pressure lowering by short-term and long-term antihypertensive treatment: a meta-analysis of individual data in 294 patients. J Hypertens. 2011;29(6):1034–1042. doi:10.1097/HJH.0b013e328346a583
136. Boutouyrie P, Lacolley P, Briet M, et al. Pharmacological modulation of arterial stiffness. Drugs. 2011;71(13):1689–1701. doi:10.2165/11593790-000000000-00000
137. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113(9):1213–1225. doi:10.1161/CIRCULATIONAHA.105.595496
138. Wu CF, Liu PY, Wu TJ, Hung Y, Yang SP, Lin GM. Therapeutic modification of arterial stiffness: an update and comprehensive review. World J Cardiol. 2015;7(11):742–753. doi:10.4330/wjc.v7.i11.742
139. White WB, Duprez D, St Hillaire R, et al. Effects of the selective aldosterone blocker eplerenone versus the calcium antagonist amlodipine in systolic hypertension. Hypertension. 2003;41(5):1021–1026. doi:10.1161/01.HYP.0000067463.13172.EA
140. Chen X, Huang B, Liu M, Li X. Effects of different types of antihypertensive agents on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. J Thorac Dis. 2015;7(12):2339–2347. doi:10.3978/j.issn.2072-1439.2015.12.58
141. Li X, Chang P, Wang Q, et al. Effects of angiotensin-converting enzyme inhibitors on arterial stiffness: a systematic review and meta-analysis of randomized controlled trials. Cardiovasc Ther. 2020;2020:7056184. doi:10.1155/2020/7056184
142. Alem M, Milia P, Muir S, Lees K, Walters M. Comparison of the effects of diuretics on blood pressure and arterial stiffness in patients with stroke. J Stroke Cerebrovasc Dis. 2008;17(6):373–377. doi:10.1016/j.jstrokecerebrovasdis.2008.04.011
143. Kithas PA, Supiano MA. Spironolactone and hydrochlorothiazide decrease vascular stiffness and blood pressure in geriatric hypertension. J Am Geriatr Soc. 2010;58(7):1327–1332. doi:10.1111/j.1532-5415.2010.02905.x
144. Mahmud A, Feely J. Effect of angiotensin ii receptor blockade on arterial stiffness: beyond blood pressure reduction. Am J Hypertens. 2002;15(12):1092–1095. doi:10.1016/S0895-7061(02)02982-5
145. Boutouyrie P, Beaussier H, Achouba A, Laurent S. Destiffening effect of valsartan and atenolol: influence of heart rate and blood pressure. J Hypertens. 2014;32(1):108–114. doi:10.1097/HJH.0000000000000014
146. Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–270. doi:10.1111/j.1469-7793.2000.t01-1-00263.x
147. Mahmud A, Feely J. Beta-blockers reduce aortic stiffness in hypertension but nebivolol, not atenolol, reduces wave reflection. Am J Hypertens. 2008;21(6):663–667. doi:10.1038/ajh.2008.156
148. McEniery CM, Schmitt M, Qasem A, et al. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44(3):305–310. doi:10.1161/01.HYP.0000137983.45556.6e
149. Koumaras C, Tziomalos K, Stavrinou E, et al. Effects of renin-angiotensin-aldosterone system inhibitors and beta-blockers on markers of arterial stiffness. Am Soc Hypertens. 2014;8(2):74–82. doi:10.1016/j.jash.2013.09.001
150. Theodorakopoulou M, Loutradis C, Bikos A, et al. The effects of nebivolol and irbesartan on ambulatory aortic blood pressure and arterial stiffness in hemodialysis patients with intradialytic hypertension. Blood Purif. 2021;50(1):73–83. doi:10.1159/000507913
151. Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43(2):176–181. doi:10.1161/01.HYP.0000111829.46090.92
152. Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. doi:10.1146/annurev.med.46.1.223
153. Wan Y, Liu Z, Wu A, et al. Hyperglycemia Promotes Endothelial Cell Senescence through AQR/PLAU Signaling Axis. Int J Mol Sci. 2022;23(5):2879. doi:10.3390/ijms23052879
154. Tian J, Wen Y, Yan L, et al. Vascular endothelial dysfunction in patients with newly diagnosed type 2 diabetes and effects of 2-year and 5-year multifactorial intervention. Echocardiography. 2011;28(10):1133–1140. doi:10.1111/j.1540-8175.2011.01514.x
155. Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 2003;41(4):661–665. doi:10.1016/S0735-1097(02)02894-2
156. Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care. 2003;26(7):2133–2138. doi:10.2337/diacare.26.7.2133
157. de la Cruz-Ares S, Cardelo MP, Gutiérrez-Mariscal FM, et al. Endothelial dysfunction and advanced glycation end products in patients with newly diagnosed versus established diabetes: from the CORDIOPREV study. Nutrients. 2020;12(1):238. doi:10.3390/nu12010238
158. Dayem SM, Battah AA, El Bohy AE. Assessment of increase in aortic and carotid intimal medial thickness in type 1 diabetic patients. Open Access Maced J Med Sci. 2016;4(4):630–635. doi:10.3889/oamjms.2016.118
159. Abdelgawad IY, Agostinucci K, Sadaf B, Grant MKO, Zordoky BN. Metformin mitigates SASP secretion and LPS-triggered hyper-inflammation in Doxorubicin-induced senescent endothelial cells. Front Aging. 2023;4:1170434. doi:10.3389/fragi.2023.1170434
160. Timmons JG, Greenlaw N, Boyle JG, et al. Metformin and carotid intima-media thickness in never-smokers with type 1 diabetes: the REMOVAL trial. Diabet Obes Metab. 2021;23(6):1371–1378. doi:10.1111/dom.14350
161. Sardu C, Paolisso P, Sacra C, et al. Effects of metformin therapy on coronary endothelial dysfunction in patients with prediabetes with stable angina and nonobstructive coronary artery stenosis: the CODYCE multicenter prospective study. Diabet Care. 2019;42(10):1946–1955. doi:10.2337/dc18-2356
162. Lunder M, Janić M, Japelj M, Juretič A, Janež A, Šabovič M. Empagliflozin on top of metformin treatment improves arterial function in patients with type 1 diabetes mellitus. Cardiovasc Diabetol. 2018;17(1):153. doi:10.1186/s12933-018-0797-6
163. Agarwal N, Rice SP, Bolusani H, et al. Metformin reduces arterial stiffness and improves endothelial function in young women with polycystic ovary syndrome: a randomized, placebo-controlled, crossover trial. J Clin Endocrinol Metab. 2010;95(2):722–730. doi:10.1210/jc.2009-1985
164. Minamikawa J, Tanaka S, Yamauchi M, Inoue D, Koshiyama H. Potent inhibitory effect of troglitazone on carotid arterial wall thickness in type 2 diabetes. J Clin Endocrinol Metab. 1998;83(5):1818–1820. doi:10.1210/jcem.83.5.4932
165. Sidhu JS, Kaposzta Z, Markus HS, Kaski JC. Effect of rosiglitazone on common carotid intima-media thickness progression in coronary artery disease patients without diabetes mellitus. Arterioscler Thromb Vasc Biol. 2004;24(5):930–934. doi:10.1161/01.ATV.0000124890.40436.77
166. Hetzel J, Balletshofer B, Rittig K, et al. Rapid effects of rosiglitazone treatment on endothelial function and inflammatory biomarkers. Arterioscler Thromb Vasc Biol. 2005;25(9):1804–1809. doi:10.1161/01.ATV.0000176192.16951.9a
167. Satoh N, Ogawa Y, Usui T, et al. Antiatherogenic effect of pioglitazone in type 2 diabetic patients irrespective of the responsiveness to its antidiabetic effect. Diabet Care. 2003;26(9):2493–2499. doi:10.2337/diacare.26.9.2493
168. Liu L, Ni YQ, Zhan JK, Liu YS. The role of SGLT2 INHIBITORS IN VASCULAR AGING. Aging Dis. 2021;12(5):1323–1336. doi:10.14336/AD.2020.1229
169. Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol. 2017;16(1):138. doi:10.1186/s12933-017-0621-8
170. Solini A, Seghieri M, Giannini L, et al. The effects of dapagliflozin on systemic and renal vascular function display an epigenetic signature. J Clin Endocrinol Metab. 2019;104(10):4253–4263. doi:10.1210/jc.2019-00706
171. Koren S, Shemesh-Bar L, Tirosh A, et al. The effect of sitagliptin versus glibenclamide on arterial stiffness, blood pressure, lipids, and inflammation in type 2 diabetes mellitus patients. Diabet Technol Ther. 2012;14(7):561–567. doi:10.1089/dia.2011.0296
172. Cosenso-Martin LN, Giollo-Júnior LT, Fernandes LAB, et al. Effect of vildagliptin versus glibenclamide on endothelial function and arterial stiffness in patients with type 2 diabetes and hypertension: a randomized controlled trial. Acta Diabetol. 2018;55(12):1237–1245. doi:10.1007/s00592-018-1204-1
173. Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract. 2010;64(13):1796–1801. doi:10.1111/j.1742-1241.2010.02399.x
174. Chen LL, Yu F, Zeng TS, Liao YF, Li YM, Ding HC. Effects of gliclazide on endothelial function in patients with newly diagnosed type 2 diabetes. Eur J Pharmacol. 2011;659(2–3):296–301. doi:10.1016/j.ejphar.2011.02.044
175. Fava D, Cassone-Faldetta M, Laurenti O, De Luca O, Ghiselli A, De Mattia G. Gliclazide improves anti-oxidant status and nitric oxide-mediated vasodilation in Type 2 diabetes. Diabet Med. 2002;19(9):752–757. doi:10.1046/j.1464-5491.2002.00762.x
176. Kraaijenhof J, Muskiet MHA, Tonneijck L, et al. Effects of dipeptidyl peptidase-4 inhibitor linagliptin versus sulphonylurea glimepiride on systemic haemodynamics in overweight patients with type 2 diabetes: a secondary analysis of an 8-week, randomized, controlled, double-blind trial. Diabetes Obes Metab. 2020;22(10):1847–1856. doi:10.1111/dom.14107
177. van Bommel EJM, Smits MM, Ruiter D, et al. Effects of dapagliflozin and gliclazide on the cardiorenal axis in people with type 2 diabetes. J Hypertens. 2020;38(9):1811–1819. doi:10.1097/HJH.0000000000002480
178. Tamura H, Kondo Y, Ito K, Hasebe M, Satoh S, Terauchi Y. Comparison of the effects of empagliflozin and glimepiride on endothelial function in patients with type 2 diabetes: a randomized controlled study. PLoS One. 2022;17(2):e0262831. doi:10.1371/journal.pone.0262831
179. Nomoto H, Miyoshi H, Furumoto T, et al. A comparison of the effects of the GLP-1 analogue liraglutide and insulin glargine on endothelial function and metabolic parameters: a randomized, controlled trial Sapporo Athero-Incretin Study 2 (SAIS2). PLoS One. 2015;10(8):e0135854. doi:10.1371/journal.pone.0135854
180. Gurkan E, Tarkun I, Sahin T, Cetinarslan B, Canturk Z. Evaluation of exenatide versus insulin glargine for the impact on endothelial functions and cardiovascular risk markers. Diabet Res Clin Pract. 2014;106(3):567–575. doi:10.1016/j.diabres.2014.09.046
181. Irace C, De Luca S, Shehaj E, et al. Exenatide improves endothelial function assessed by flow mediated dilation technique in subjects with type 2 diabetes: results from an observational research. Diab Vasc Dis Res. 2013;10(1):72–77. doi:10.1177/1479164112449562
182. Koska J, Sands M, Burciu C, et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015;64(7):2624–2635. doi:10.2337/db14-0976
183. Song X, Jia H, Jiang Y, et al. Anti-atherosclerotic effects of the glucagon-like peptide-1 (GLP-1) based therapies in patients with type 2 Diabetes Mellitus: a meta-analysis. Sci Rep. 2015;5:10202. doi:10.1038/srep10202
184. Scalzo RL, Moreau KL, Ozemek C, et al. Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complications. 2017;31(2):449–455. doi:10.1016/j.jdiacomp.2016.10.003
185. Hong JY, Park KY, Kim BJ, Hwang WM, Kim DH, Lim DM. Effects of short-term exenatide treatment on regional fat distribution, glycated hemoglobin levels, and aortic pulse wave velocity of obese type 2 diabetes mellitus patients. Endocrinol Metab. 2016;31(1):80–85. doi:10.3803/EnM.2016.31.1.80
186. Patti AM, Nikolic D, Magan-Fernandez A, et al. Exenatide once-weekly improves metabolic parameters, endothelial dysfunction and carotid intima-media thickness in patients with type-2 diabetes: an 8-month prospective study. Diabet Res Clin Pract. 2019;149:163–169. doi:10.1016/j.diabres.2019.02.006
187. Franklin VL, Khan F, Kennedy G, Belch JJ, Greene SA. Intensive insulin therapy improves endothelial function and microvascular reactivity in young people with type 1 diabetes. Diabetologia. 2008;51(2):353–360. doi:10.1007/s00125-007-0870-2
188. Vehkavaara S, Yki-Järvinen H. 3.5 years of insulin therapy with insulin glargine improves in vivo endothelial function in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2004;24(2):325–330. doi:10.1161/01.ATV.0000113817.48983.c5
189. Makino H, Tanaka A, Hosoda H, et al. Effect of basal insulin therapy on vascular endothelial function and adipokine profiles in people with Type 2 diabetes. Diabet Med. 2016;33(12):1737–1743. doi:10.1111/dme.13151
190. Pirro M, Schillaci G, Paltriccia R, et al. Increased ratio of CD31+/CD42- microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26(11):2530–2535. doi:10.1161/01.ATV.0000243941.72375.15
191. Reiner Ž, Simental-Mendía LE, Ruscica M, et al. Pulse wave velocity as a measure of arterial stiffness in patients with familial hypercholesterolemia: a systematic review and meta-analysis. Arch Med Sci. 2019;15(6):1365–1374. doi:10.5114/aoms.2019.89450
192. Braamskamp M, Langslet G, McCrindle BW, et al. Effect of rosuvastatin on carotid intima-media thickness in children with heterozygous familial hypercholesterolemia: the CHARON Study (Hypercholesterolemia in children and adolescents taking rosuvastatin open label). Circulation. 2017;136(4):359–366. doi:10.1161/CIRCULATIONAHA.116.025158
193. Masoura C, Pitsavos C, Aznaouridis K, Skoumas I, Vlachopoulos C, Stefanadis C. Arterial endothelial function and wall thickness in familial hypercholesterolemia and familial combined hyperlipidemia and the effect of statins. A systematic review and meta-analysis. Atherosclerosis. 2011;214(1):129–138. doi:10.1016/j.atherosclerosis.2010.10.008
194. John S, Schneider MP, Delles C, Jacobi J, Schmieder RE. Lipid-independent effects of statins on endothelial function and bioavailability of nitric oxide in hypercholesterolemic patients. Am Heart J. 2005;149(3):473. doi:10.1016/j.ahj.2004.06.027
195. Smilde TJ, van Wissen S, Wollersheim H, Trip MD, Kastelein JJ, Stalenhoef AF. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357(9256):577–581. doi:10.1016/S0140-6736(00)04053-8
196. Smilde TJ, van den Berkmortel FW, Wollersheim H, van Langen H, Kastelein JJ, Stalenhoef AF. The effect of cholesterol lowering on carotid and femoral artery wall stiffness and thickness in patients with familial hypercholesterolaemia. Eur J Clin Invest. 2000;30(6):473–480. doi:10.1046/j.1365-2362.2000.00654.x
197. Garcia MM, Varela CG, Silva PF, et al. Endothelial effect of statin therapy at a high dose versus low dose associated with ezetimibe. Arq Bras Cardiol. 2016;106(4):279–288. doi:10.5935/abc.20160048
198. Takase S, Matoba T, Nakashiro S, et al. Ezetimibe in combination with statins ameliorates endothelial dysfunction in coronary arteries after stenting: the CuVIC Trial (Effect of cholesterol absorption inhibitor usage on target vessel dysfunction after coronary stenting), a multicenter randomized controlled trial. Arterioscler Thromb Vasc Biol. 2017;37(2):350–358. doi:10.1161/ATVBAHA.116.308388
199. Upala S, Wirunsawanya K, Jaruvongvanich V, Sanguankeo A. Effects of statin therapy on arterial stiffness: a systematic review and meta-analysis of randomized controlled trial. Int J Cardiol. 2017;227:338–341. doi:10.1016/j.ijcard.2016.11.073
200. Nechyporenko A, Tedla YG, Korcarz C, Tattersall MC, Greenland P, Gepner AD. Association of statin therapy with progression of carotid arterial stiffness: the Multi-Ethnic Study of Atherosclerosis (Mesa). Hypertens Res. 2023;46(3):679–687. doi:10.1038/s41440-022-01095-9
201. Castejon R, Castañeda A, Sollet A, et al. Short-term atorvastatin therapy improves arterial stiffness of middle-aged systemic lupus erythematosus patients with pathological pulse wave velocity. Lupus. 2017;26(4):355–364. doi:10.1177/0961203316662719
202. Cavero-Redondo I, Deeks JJ, Alvarez-Bueno C, et al. Comparative effect of physical exercise versus statins on improving arterial stiffness in patients with high cardiometabolic risk: a network meta-analysis. PLoS Med. 2021;18(2):e1003543. doi:10.1371/journal.pmed.1003543
203. Gepner AD, Lazar K, Hulle CV, Korcarz CE, Asthana S, Carlsson CM. Effects of simvastatin on augmentation index are transient: outcomes from a randomized controlled trial. J Am Heart Assoc. 2019;8(20):e009792. doi:10.1161/JAHA.118.009792
204. Jankowski J, Floege J, Fliser D, Böhm M, Marx N. Cardiovascular disease in chronic kidney disease: pathophysiological insights and therapeutic options. Circulation. 2021;143(11):1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
205. Fassett RG, Robertson IK, Ball MJ, Geraghty DP, Sharman JE, Coombes JS. Effects of atorvastatin on arterial stiffness in chronic kidney disease: a randomised controlled trial. J Atheroscler Thromb. 2010;17(3):235–241. doi:10.5551/jat.2683
206. Kontopoulos AG, Athyros VG, Pehlivanidis AN, Demitriadis DS, Papageorgiou AA, Boudoulas H. Long-term treatment effect of atorvastatin on aortic stiffness in hypercholesterolaemic patients. Curr Med Res Opin. 2003;19(1):22–27. doi:10.1185/030079902125001290
207. Gomez-Sanchez M, Gomez-Sanchez L, Patino-Alonso MC, et al. Vascular aging and its relationship with lifestyles and other risk factors in the general Spanish population: early Vascular Ageing Study. J Hypertens. 2020;38(6):1110–1122. doi:10.1097/HJH.0000000000002373
208. DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi:10.1161/01.CIR.102.12.1351
209. Higashi Y, Sasaki S, Kurisu S, et al. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100(11):1194–1202. doi:10.1161/01.CIR.100.11.1194
210. Golbidi S, Laher I. Exercise and the aging endothelium. J Diabetes Res. 2013;2013:789607. doi:10.1155/2013/789607
211. Higashi Y, Sasaki S, Sasaki N, et al. Daily aerobic exercise improves reactive hyperemia in patients with essential hypertension. Hypertension. 1999;33(1 Pt 2):591–597. doi:10.1161/01.HYP.33.1.591
212. Sugawara J, Tomoto T, Noda N, et al. Effects of endothelin-related gene polymorphisms and aerobic exercise habit on age-related arterial stiffening: a 10-yr longitudinal study. J Appl Physiol. 2018;124(2):312–320. doi:10.1152/japplphysiol.00697.2017
213. Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102(11):1270–1275. doi:10.1161/01.CIR.102.11.1270
214. Tanaka H, Seals DR, Monahan KD, Clevenger CM, DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase in carotid artery intima-media thickness in healthy men. J Appl Physiol. 2002;92(4):1458–1464. doi:10.1152/japplphysiol.00824.2001
215. Popovic M, Puchner S, Endler G, Foraschik C, Minar E, Bucek RA. The effects of endurance and recreational exercise on subclinical evidence of atherosclerosis in young adults. Am J Med Sci. 2010;339(4):332–336. doi:10.1097/MAJ.0b013e3181cefbb9
216. Moreau KL, Donato AJ, Seals DR, et al. Arterial intima-media thickness: site-specific associations with HRT and habitual exercise. Am J Physiol Heart Circ Physiol. 2002;283(4):H1409–H1417. doi:10.1152/ajpheart.00035.2002
217. Moreau KL, Silver AE, Dinenno FA, Seals DR. Habitual aerobic exercise is associated with smaller femoral artery intima-media thickness with age in healthy men and women. Eur J Cardiovasc Prev Rehabil. 2006;13(5):805–811. doi:10.1097/01.hjr.0000230103.55653.42
218. Dinenno FA, Tanaka H, Monahan KD, et al. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534(Pt 1):287–295. doi:10.1111/j.1469-7793.2001.00287.x
219. Thijssen DH, Cable NT, Green DJ. Impact of exercise training on arterial wall thickness in humans. Clin Sci. 2012;122(7):311–322. doi:10.1042/CS20110469
220. Miyachi M, Donato AJ, Yamamoto K, et al. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension. 2003;41(1):130–135. doi:10.1161/01.HYP.0000047649.62181.88
221. Jennings A, Berendsen AM, de Groot L, et al. Mediterranean-style diet improves systolic blood pressure and arterial stiffness in older adults. Hypertension. 2019;73(3):578–586. doi:10.1161/HYPERTENSIONAHA.118.12259
222. Plotnick GD, Corretti MC, Vogel RA. Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997;278(20):1682–1686. doi:10.1001/jama.1997.03550200058032
223. Davis CR, Hodgson JM, Woodman R, Bryan J, Wilson C, Murphy KJ. A Mediterranean diet lowers blood pressure and improves endothelial function: results from the MedLey randomized intervention trial. Am J Clin Nutr. 2017;105(6):1305–1313. doi:10.3945/ajcn.116.146803
224. Rallidis LS, Lekakis J, Kolomvotsou A, et al. Close adherence to a Mediterranean diet improves endothelial function in subjects with abdominal obesity. Am J Clin Nutr. 2009;90(2):263–268. doi:10.3945/ajcn.2008.27290
225. Esposito K, Marfella R, Ciotola M, et al. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–1446. doi:10.1001/jama.292.12.1440
226. Khoo J, Piantadosi C, Duncan R, et al. Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med. 2011;8(10):2868–2875. doi:10.1111/j.1743-6109.2011.02417.x
227. Phillips SA, Jurva JW, Syed AQ, et al. Benefit of low-fat over low-carbohydrate diet on endothelial health in obesity. Hypertension. 2008;51(2):376–382. doi:10.1161/HYPERTENSIONAHA.107.101824
228. Pirro M, Schillaci G, Savarese G, et al. Attenuation of inflammation with short-term dietary intervention is associated with a reduction of arterial stiffness in subjects with hypercholesterolaemia. Eur J Cardiovasc Prev Rehabil. 2004;11(6):497–502. doi:10.1097/01.hjr.0000152243.51327.2a
229. Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78(3):383–390. doi:10.1093/ajcn/78.3.383
230. Mellen PB, Liese AD, Tooze JA, Vitolins MZ, Wagenknecht LE, Herrington DM. Whole-grain intake and carotid artery atherosclerosis in a multiethnic cohort: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2007;85(6):1495–1502. doi:10.1093/ajcn/85.6.1495
231. Brock DW, Davis CK, Irving BA, et al. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care. 2006;29(10):2313–2315. doi:10.2337/dc06-0917
232. Whisner CM, Angadi SS, Weltman NY, et al. Effects of low-fat and high-fat meals, with and without dietary fiber, on postprandial endothelial function, triglyceridemia, and glycemia in adolescents. Nutrients. 2019;11(11):11. doi:10.3390/nu11112626
233. Katz DL, Nawaz H, Boukhalil J, et al. Effects of oat and wheat cereals on endothelial responses. Prev Med. 2001;33(5):476–484. doi:10.1006/pmed.2001.0918
234. Edwards DG, Farquhar WB. Vascular effects of dietary salt. Curr Opin Nephrol Hypertens. 2015;24(1):8–13. doi:10.1097/MNH.0000000000000089
235. Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension. 2008;51(6):1525–1530. doi:10.1161/HYPERTENSIONAHA.108.109868
236. DuPont JJ, Greaney JL, Wenner MM, et al. High dietary sodium intake impairs endothelium-dependent dilation in healthy salt-resistant humans. J Hypertens. 2013;31(3):530–536. doi:10.1097/HJH.0b013e32835c6ca8
237. Todd AS, Macginley RJ, Schollum JB, et al. Dietary salt loading impairs arterial vascular reactivity. Am J Clin Nutr. 2010;91(3):557–564. doi:10.3945/ajcn.2009.28645
238. Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;44(1):35–41. doi:10.1161/01.HYP.0000132767.74476.64
239. Avolio AP, Clyde KM, Beard TC, Cooke HM, Ho KK, O’Rourke MF. Improved arterial distensibility in normotensive subjects on a low salt diet. Arteriosclerosis. 1986;6(2):166–169. doi:10.1161/01.ATV.6.2.166
240. Nicoll R, Henein MY. Caloric restriction and its effect on blood pressure, heart rate variability and arterial stiffness and dilatation: a review of the evidence. Int J Mol Sci. 2018;19(3):751. doi:10.3390/ijms19030751
241. Weiss EP, Fontana L. Caloric restriction: powerful protection for the aging heart and vasculature. Am J Physiol Heart Circ Physiol. 2011;301(4):H1205–H1219. doi:10.1152/ajpheart.00685.2011
242. Sasaki S, Higashi Y, Nakagawa K, et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am J Hypertens. 2002;15(4 Pt 1):302–309. doi:10.1016/S0895-7061(01)02322-6
243. Raitakari M, Ilvonen T, Ahotupa M, et al. Weight reduction with very-low-caloric diet and endothelial function in overweight adults: role of plasma glucose. Arterioscler Thromb Vasc Biol. 2004;24(1):124–128. doi:10.1161/01.ATV.0000109749.11042.7c
244. Clifton PM, Keogh JB, Foster PR, Noakes M. Effect of weight loss on inflammatory and endothelial markers and FMD using two low-fat diets. Int J Obes. 2005;29(12):1445–1451. doi:10.1038/sj.ijo.0803039
245. Di Daniele N, Marrone G, Di Lauro M, et al. Effects of Caloric restriction diet on arterial hypertension and endothelial dysfunction. Nutrients. 2021;13(1):274. doi:10.3390/nu13010274
246. Guo Y, Luo S, Ye Y, Yin S, Fan J, Xia M. Intermittent fasting improves cardiometabolic risk factors and alters gut microbiota in metabolic syndrome patients. J Clin Endocrinol Metab. 2021;106(1):64–79. doi:10.1210/clinem/dgaa644
247. Azemi AK, Siti-Sarah AR, Mokhtar SS, Rasool AHG. Time-Restricted feeding improved vascular endothelial function in a high-fat diet-induced obesity rat model. Vet Sci. 2022;9(5). doi:10.3390/vetsci9050217
248. Petersen KS, Clifton PM, Lister N, Keogh JB. Effect of weight loss induced by energy restriction on measures of arterial compliance: a systematic review and meta-analysis. Atherosclerosis. 2016;247:7–20. doi:10.1016/j.atherosclerosis.2016.01.042
249. Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2015;35(1):243–252. doi:10.1161/ATVBAHA.114.304798
250. Nordstrand N, Gjevestad E, Hertel JK, et al. Arterial stiffness, lifestyle intervention and a low-calorie diet in morbidly obese patients-a nonrandomized clinical trial. Obesity. 2013;21(4):690–697. doi:10.1002/oby.20099
251. Van Schinkel LD, Bakker LE, Jonker JT, et al. Cardiovascular flexibility in middle-aged overweight South Asians vs white Caucasians: response to short-term caloric restriction. Nutr Metab Cardiovasc Dis. 2015;25(4):403–410. doi:10.1016/j.numecd.2014.12.007
252. Campisi J, Kapahi P, Lithgow GJ, Melov S, Newman JC, Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571(7764):183–192. doi:10.1038/s41586-019-1365-2
253. Pignolo RJ, Passos JF, Khosla S, Tchkonia T, Kirkland JL. Reducing Senescent Cell Burden in Aging and Disease. Trends Mol Med. 2020;26(7):630–638. doi:10.1016/j.molmed.2020.03.005
254. Khosla S, Farr JN, Tchkonia T, Kirkland JL. The role of cellular senescence in ageing and endocrine disease. Nat Rev Endocrinol. 2020;16(5):263–275. doi:10.1038/s41574-020-0335-y
255. Roos CM, Zhang B, Palmer AK, et al. Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice. Aging Cell. 2016;15(5):973–977. doi:10.1111/acel.12458
256. Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735. doi:10.1038/nrd.2017.116
257. Triggle CR, Mohammed I, Bshesh K, et al. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133:155223. doi:10.1016/j.metabol.2022.155223
258. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60(9):1577–1585. doi:10.1007/s00125-017-4342-z
259. Barzilai N, Crandall JP, Kritchevsky SB, Espeland MA. Metformin as a Tool to Target Aging. Cell Metab. 2016;23(6):1060–1065. doi:10.1016/j.cmet.2016.05.011
260. Campbell JM, Bellman SM, Stephenson MD, Lisy K. Metformin reduces all-cause mortality and diseases of ageing independent of its effect on diabetes control: a systematic review and meta-analysis. Ageing Res Rev. 2017;40:31–44. doi:10.1016/j.arr.2017.08.003
261. Podhorecka M, Ibanez B, Dmoszyńska A. Metformin - its potential anti-cancer and anti-aging effects. Postepy Hig Med Dosw. 2017;71:170–175. doi:10.5604/01.3001.0010.3801
262. Glossmann HH, Lutz OMD. Metformin and aging: a review. Gerontology. 2019;65(6):581–590. doi:10.1159/000502257
263. Soukas AA, Hao H, Wu L. Metformin as anti-aging therapy: is it for everyone? Trends Endocrinol Metab. 2019;30(10):745–755. doi:10.1016/j.tem.2019.07.015
264. Wang C, Chen B, Feng Q, Nie C, Li T. Clinical perspectives and concerns of metformin as an anti-aging drug. Aging Med. 2020;3(4):266–275. doi:10.1002/agm2.12135
265. Kaeberlein M, Powers RW, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310(5751):1193–1196. doi:10.1126/science.1115535
266. Bjedov I, Toivonen JM, Kerr F, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi:10.1016/j.cmet.2009.11.010
267. Robida-Stubbs S, Glover-Cutter K, Lamming DW, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15(5):713–724. doi:10.1016/j.cmet.2012.04.007
268. Wang R, Yu Z, Sunchu B, et al. Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism. Aging Cell. 2017;16(3):564–574. doi:10.1111/acel.12587
269. Lesniewski LA, Seals DR, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16(1):17–26. doi:10.1111/acel.12524
270. Joannides R, Etienne I, Iacob M, et al. Comparative effects of sirolimus and cyclosporin on conduit arteries endothelial function in kidney recipients. Transpl Int. 2010;23(11):1135–1143. doi:10.1111/j.1432-2277.2010.01122.x
271. Joannidès R, Monteil C, de Ligny BH, et al. Immunosuppressant regimen based on sirolimus decreases aortic stiffness in renal transplant recipients in comparison to cyclosporine. Am J Transplant. 2011;11(11):2414–2422. doi:10.1111/j.1600-6143.2011.03697.x
272. Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi:10.1016/j.cmet.2011.08.014
273. Yoshida M, Satoh A, Lin JB, et al. Extracellular vesicle-contained eNAMPT delays aging and extends lifespan in mice. Cell Metab. 2019;30(2):329–342.e325. doi:10.1016/j.cmet.2019.05.015
274. Das A, Huang GX, Bonkowski MS, et al. Impairment of an endothelial NAD(+)-H(2)S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74–89.e20. doi:10.1016/j.cell.2018.02.008
275. de Picciotto NE, Gano LB, Johnson LC, et al. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15(3):522–530. doi:10.1111/acel.12461
276. Martens CR, Denman BA, Mazzo MR, et al. Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD(+) in healthy middle-aged and older adults. Nat Commun. 2018;9(1):1286. doi:10.1038/s41467-018-03421-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.