Back to Journals » OncoTargets and Therapy » Volume 9
Values of 99mTc-methoxyisobutylisonitrile imaging after first-time large-dose 131I therapy in treating differentiated thyroid cancer
Authors Pan X, Duan D, Zhu Y, Pang H, Guan L, Lv Z
Received 8 August 2015
Accepted for publication 11 November 2015
Published 10 February 2016 Volume 2016:9 Pages 723—730
DOI https://doi.org/10.2147/OTT.S94036
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Daniele Santini
Xiaomei Pan, Dong Duan, Yuquan Zhu, Hua Pang, Lili Guan, Zhixiang Lv
Department of Nuclear Medicine, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Objective: The aim of this study is to investigate the use of 99mTc-methoxyisobutylisonitrile (MIBI) imaging for evaluating the treatment response of differentiated thyroid cancer (DTC) after the first administration of a high dose of 131I.
Methods: Patients with DTC who received 131I therapy underwent 99mTc-MIBI imaging after successive increases in the therapeutic dose of 131I, and the serum levels of thyroglobulin (Tg) were measured.
Results: A total of 191 patients were enrolled in the final analysis, including 65 metastases and/or thyroid remnant-positive patients (22 patients with metastases and 43 patients with thyroid remnants). The sensitivity of 99mTc-MIBI imaging for detecting positive cases and thyroid remnants was 56.9% and 39.5%, respectively, which was significantly lower than that of 131I imaging (92.3% and 100%, respectively, P<0.01 for both). The sensitivity of 99mTc-MIBI imaging for detecting metastases was 90.9%, which was slightly higher than that of 131I imaging (77.3%, P>0.05). The Tg levels in the positive group were significantly higher than that in the negative group (P<0.01). In addition, the Tg levels in the 99mTc-MIBI+/131I- group were significantly higher than that in the 131I+/99mTc-MIBI group (P<0.05).
Conclusion: After the first 131I therapy, although 99mTc-MIBI imaging was able to detect the existence of metastatic lesions in patients with DTC better, its assessment for the removal efficiency of thyroid remnants was unsatisfactory. The results of 99mTc-MIBI imaging showed good correlations with the Tg level.
Keywords: differentiated thyroid cancer, 99mTc-MIBI imaging, therapeutic dose 131I imaging, thyroglobulin
Introduction
Thyroid cancer (TC), the most common malignant tumor among endocrine and head–neck tumors, with an increasing incidence in recent years, accounts for ~3% of all malignancies.1 Differentiated thyroid cancer (DTC) accounts for >90% of all TC cases. Currently, the routine treatment for DTC is surgery plus 131I therapy plus thyroid-stimulating hormone (TSH) suppression therapy.2,3 Total or near-total thyroidectomy is the most commonly used surgical procedure. 131I therapy includes the ablation of thyroid remnants and local/distant metastases with the postoperative use of different activities of 131I. The suppression of TSH using supraphysiologic doses of levothyroxine to supplement thyroid hormone and to inhibit the growth of DTC cells is an effort to decrease the risk of recurrence.
After the first (postoperative) application of 131I therapy, the patient should be evaluated for the presence of remnants or metastases, which is important for determining the necessity of repeated 131I therapy.4 Serum thyroglobulin (Tg) level is the most sensitive and specific indicator for monitoring DTC recurrence or metastases, but it cannot be used to locate the lesions or distinguish remnants from metastases; therefore, combining with other imaging methods is needed for evaluation. Ultrasonography and computed tomography are commonly used, but they have certain deficiencies for detecting functional TC recurrent or metastatic lesions, especially for the diagnosis of some minor metastatic lesions. 131I imaging with diagnostic dose might not be able to detect small metastases and might induce a stunning effect (a reduction in the uptake of 131I therapy dose induced by a pretreatment diagnostic activity) of remnants/metastases, which might influence the efficacy of subsequent 131I therapy.5–8 99mTcO4-imaging9 can provide information about the clearance of thyroid remnants, but it is not a tumor-specific imaging; therefore, it cannot be used to evaluate the clearance of TC metastases. Although 18F-fluoro-2-deoxy-D-glucose positron emission tomography (18FDG-PET) imaging can provide information about remnants and metastases with a high metabolism, it is too expensive for wider use in clinical application; besides, partial metastatic lesions (especially lymph node metastases) show no high intake of fluorodeoxyglucose.10 99mTc-methoxyisobutylisonitrile (MIBI) is a nonspecific tumor-philic imaging agent; it has been used for diagnosing and evaluating the efficacy of treatments for lung cancer, breast cancer, bone cancer, gliomas, and other malignant tumors.11–16 The present study compared the results of 99mTc-MIBI imaging and therapeutic-dose 131I imaging, aiming to assess the use of 99mTc-MIBI imaging in patients with DTC after surgery and the first (postoperative) application of 131I therapy.
Materials and methods
Clinical data
A total of 192 patients with DTC (38 men and 154 women; aged 7–78 years [mean±SD: 43.2±8.6 years]) who underwent total or near-total thyroidectomy for pathologically diagnosed DTC (including 171 cases of papillary carcinoma and 21 cases of follicular carcinoma) and who were treated in our hospital between February 2010 and March 2014 were enrolled. All patients underwent 99mTc-MIBI imaging and high-dose 131I imaging 6 months after the first (postoperative) application of 131I therapy (the mean dose was 113.5±20.8 mCi) and stopped receiving thyroxin preparation as a medication, 4 weeks before the imaging. This study was conducted in accordance with the Declaration of Helsinki. This study was conducted with the approval from the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University. Written informed consent was obtained from all participants.
Laboratory tests
By using the chemiluminescence method, the serum Tg level (reference value, <35 ng/mL) was measured in all patients, both after they stopped the medication of thyroxin preparation 4 weeks prior to imaging and just before the imaging. The radioimmunoassay method was used to detect the levels of TSH (reference range, 0.3–5.0 mIU/L) and Tg antibody (TgAb, reference value, <30%).
99mTc-MIBI imaging
Patients were intravenously injected with 740–925 MBq 99mTc-MIBI (Beijing Xinke Co., Beijing, People’s Republic of China), and then, neck and chest as well as systemic planar images were obtained after 90 minutes. A single-photon emission computed tomography (SPECT)/computed tomography (CT) instrument (Symbia T2; Siemens, Munich, Germany) was used, which was equipped with a parallel low-energy high-resolution collimator with an energy peak of 140 keV and a window width of 20%. The magnification fold when performing neck and chest scanning was 1.5, with a matrix of 256×256 pixels and an acquisition count of 500 K. The magnification fold when performing systemic scanning was 1.0, with a matrix of 256×1,024 pixels and scanning speed of 15 cm/min.
131I imaging
Patients were orally administered a mean dose of 5,550–8,140 MBq (150–220 mCi) 131I (provided by Chengdu Gaotong Isotope Co., Ltd.) on the second day after 99mTc-MIBI imaging; they underwent neck–chest scanning and systemic scanning on the fourth day after 131I administration (namely, high-dose 131I imaging). A SPECT/CT instrument (Symbia T2) was used, which was equipped with a parallel low-energy high-resolution collimator with a matrix of 256×1,024 pixels, an energy peak of 364 keV, and a window width of 20%; the magnification fold was 1, with a scanning speed of 15 cm/min and acquisition count of 100–150 K.
Image analysis
The images were evaluated independently by two physicians with SPECT/CT diagnostic experience. The images with consistent evaluation results were then included in the final analysis. Positive cases were defined as the presence of an abnormal radioactive uptake outside the thyroid gland bed area or the thyroid gland – as found on either of the above-mentioned imaging methods – and negative cases were defined as no imaging agent uptake outside the thyroid gland bed area and the thyroid gland.
Statistical analysis
SPSS 13.0 software (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. The data were assessed using the chi-square test. The measurement data are expressed as 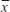 ±s. The intergroup data were compared with an independent samples t-test, with P<0.05 considered statistically significant.
±s. The intergroup data were compared with an independent samples t-test, with P<0.05 considered statistically significant.
Results
Imaging results of all patients
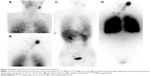
The imaging results of a total of 191 patients with DTC were included in the study (the images of one patient were excluded owing to considerable differences between the evaluation results of the two physicians). Among them, 126 (66.0%) patients were negative on both imaging methods, 32 (16.8%) patients were positive on both imaging methods, 28 (14.7%) patients were high-dose 131I whole body scan (Rx-WBS) positive but 99mTc-MIBI negative, and five (2.6%) patients were 99mTc-MIBI positive but Rx-WBS negative. The imaging results of some patients are shown in Figures 1–4. A total of 65 patients (34.0%) were found to be positive, including 37 patients found by 99mTc-MIBI imaging, with a sensitivity, specificity, and accuracy of 56.9%, 100%, and 85.3%, respectively; while 60 patients were found by Rx-WBS, with a sensitivity, specificity, and accuracy of 92.3%, 100%, and 97.4%, respectively. The sensitivity of 99mTc-MIBI imaging in finding positive cases was significantly lower than that of Rx-WBS (χ2=14.7, P<0.01) (Table 1).
  | Table 1 Imaging results of 191 patients |
Imaging results of 99mTc-MIBI and Rx-WBS toward residual thyroid tissues and metastases
Among the 65 positive patients, 43 patients exhibited residual thyroid tissues; 99mTc-MIBI imaging only detected 17 cases (39.5%) while Rx-WBS detected all 43 cases (100%). Accordingly, the sensitivity of 99mTc-MIBI imaging for detecting residual thyroid tissues was significantly lower than that of Rx-WBS (χ2=24.0, P<0.01). Among the 22 patients with DTC metastases, 99mTc-MIBI imaging detected 20 cases (90.9%), while Rx-WBS detected 17 cases (77.3%). The sensitivity of 99mTc-MIBI imaging for detecting TC metastases was higher than that of Rx-WBS, although the difference was not significant (χ2=0.57, P>0.05). On further analysis, 33 positive patients showed discrepant results between the two imaging methods (only positive on 99mTc-MIBI imaging or on Rx-WBS imaging). A total of 28 cases were positive on Rx-WBS but negative on 99mTc-MIBI, among whom 26 cases exhibited radioactive 131I uptake in the thyroid gland bed area and two cases exhibited an abnormal 131I uptake outside the gland. Five cases were positive on 99mTc-MIBI but negative on Rx-WBS, among whom three cases exhibited 99mTc-MIBI uptake outside the thyroid gland and two cases exhibited 99mTc-MIBI uptake in the thyroid gland bed area (Table 2).
  | Table 2 Imaging results of two imaging methods toward residual thyroid tissues and metastases |
Results of serum Tg in each group
According to imaging results, all patients were classified into four groups: group I (99mTc-MIBI negative and 131I negative), group II (99mTc-MIBI positive and 131I positive), group III (99mTc-MIBI negative and 131I positive), and group IV (99mTc-MIBI positive and 131I negative). The serum Tg levels of the positive groups (namely, groups II, III, and IV) were significantly higher than the negative group (group I), and those of the 99mTc-MIBI-positive group (group IV) was significantly higher than the 131I-positive group (group III). All the groups exhibited TSH levels >30 mIU/L and TgAb levels within the reference range, indicating that the Tg results (Table 3) were not influenced by TgAb.
Discussion
The evaluation of whether postoperative TC metastases exist in patients with DTC should be preferably performed after the first (postoperative) application of 131I therapy (namely, ablation of the thyroid gland) because the existence of residual normal thyroid tissue might influence the 131I uptake of metastases, thus leading to false-negative results. The search for an imaging method that can not only sensitively detect TC metastases but also objectively assess whether residual thyroid tissues in the neck are completely resolved is important for the follow-up treatment of patients with DTC.
99mTc-MIBI is a nonspecific tumor-philic imaging agent with good physicochemical properties and a low radiation absorbance dose; therefore, it can be administered in high doses. In recent years, 99mTc-MIBI imaging has been widely used for the diagnosis of TC, breast cancer, lung cancer, bone tumors, and other malignancies.11–16 99mTc-MIBI is absorbed into the cytoplasm by passive diffusion and then goes into mitochondria by active transportation. Tumor cells have a high number of mitochondria, active metabolism, and an increased local blood supply, and thus, they can absorb and temporarily retain more MIBI. This characteristic of fast uptake and relatively slow excretion is significantly different from the uptake and excretion pattern of benign cells. This is the reason why the tumor tissues can be easily identified.17–19 Accordingly, as the active ratio of the target tumor tissues to nontarget tissues is higher on delayed 99mTc-MIBI imaging, the delayed imaging is more obvious; therefore, this study only analyzed the results in the delayed phase.
The use of 99mTc-MIBI as a radioactive tracer has the following advantages in evaluating the existence of remnants and metastases in patients with DTC after surgery and the first application of 131I therapy: ideal physical properties of 99mTc-MIBI led to less radiation damage in patients and are suitable for SPECT imaging; is easily prepared and has low production cost; does not get affected by the serum TSH level, no need for long-term withdrawal of levothyroxine before imaging and avoids hypothyroid symptoms; its uptake is related to the contents and metabolism of intracellular mitochondria (both functional and nonfunctional metastases can ingest 99mTc-MIBI, which could overcome the limitation of misdiagnosis of nonfunctional or dedifferentiated TC metastases of 131I imaging); and is without the “stunning” effect and does not affect the efficacy of subsequent 131I therapy.
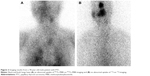
As the most common metastatic areas of TC are the neck and chest, our department normally acquires neck and chest images (enlarged 1.5 times) and systemic images, which are helpful to discover the neck or lung metastases and provide information about iliac or other metastases. 99mTc-MIBI is excreted through the hepatobiliary system, which leads to obvious accumulation in the liver and intestines. Therefore, plain 99mTc-MIBI scanning has substantial limitations in assessing metastases in the spine, gastrointestinal tract, and other organs. Figure 1 shows that systemic 99mTc-MIBI imaging revealed lesions with an abnormal 99mTc-MIBI uptake in the right iliac region of a patient. 131I imaging also exhibited uptake in this site, so it might be considered an iliac metastasis; furthermore, as more 131I uptake was seen in the liver, we could not exclude the possibility that the lesion was accompanied by liver metastasis. While even 99mTc-MIBI imaging exhibits obvious aggregation in the liver and intestinal tract, it cannot be used to assess the possibility of liver metastasis.
Among the 65 positive cases found in this study, 131I imaging could detect 60 cases (92.3%) and 99mTc-MIBI imaging could detect 37 cases (56.9%). The positive rate on 99mTc-MIBI imaging was significantly lower than that on 131I imaging, while the combination of 99mTc-MIBI imaging with 131I imaging can increase the positive rate of diagnosis. A total of 43 patients were found to have residual thyroid tissues; the sensitivity of 131I imaging was 100% and that of 99mTc-MIBI imaging was only 39.5%, which is significantly lower than that of 131I imaging (P<0.01). This difference might be explained owing to the following reasons: after surgery and the first application of 131I therapy, residual normal thyroid tissues are rare, the number of mitochondria in the normal cells is lower, and surgical trauma affects the local blood supply, all of these reasons lead to no or reduced 99mTc-MIBI uptake. Therefore, the use of 99mTc-MIBI imaging in assessing the existence of cervical residual normal thyroid tissues is very limited, and it cannot be used on its own to assess the existence of residual thyroid tissues.
A study has shown that, although 99mTc-MIBI is a nonspecific tracer for TC, it has a high sensitivity and specificity in the detection of functional and nonfunctional metastases.20 Rubello et al21 found that even during thyroid hormone replacement suppression therapy, 99mTc-MIBI imaging shows high sensitivity (93.5%) in detecting cervical lymph node metastases (part of which had no 131I intake), when combined with ultrasonography; the sensitivity was as high as 97.8%. Ronga et al22 found that 99mTc-MIBI had a high sensitivity in detecting mediastinal lymph node metastasis of TC, especially when the metastatic lesions were negative on 131I imaging. In our study, 22 patients had TC metastases; among them, 99mTc-MIBI imaging detected 20 cases (15 of cervical and mediastinal lymph node, three of lung, one of iliac, and one of vertebral) with a sensitivity of 90.9%, consistent with the findings of Campennì et al,23 while 131I imaging only detected 17 cases with a sensitivity of 77.3%. The sensitivity of 131I imaging was lower than that of MIBI imaging, but the difference was not significant (P>0.05).
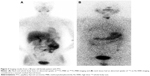
Coelho et al24 reported that ~30% of the primary or metastatic lesions of patients with DTC gradually lost iodine uptake ability during or after 131I treatment, probably owing to dedifferentiation. The study found that the uptake value of 99mTc-MIBI in tumor cells increases with the decrease of differentiation degree of DTC;25,26 poorly differentiated thyroid cancers (pDTCs) and dedifferentiated thyroid cancers (dDTCs) have higher metabolism compared to DTCs, thus enhancing 99mTc-MIBI retention in pDTC and dDTC cells and showing positive results on 99mTc-MIBI imaging, while because of losing iodine uptake ability, the patients show negative results on 131I imaging. In this study, three patients with TC metastases were 99mTc-MIBI positive but 131I negative (the result of one patient is shown in Figure 2). We thought that the possible reasons for this difference might be dedifferentiation or poor differentiation of TC metastases. It is a pity that not one of them had the pathological examination of the metastatic lesions; therefore, the final reason is not clear. For these patients, the efficacy of routine 131I therapy might be poor and other treatment options should be performed. Another two patients’ metastatic lesions show a result of 131I positive but 99mTc-MIBI negative, which might be due to the lower mitochondria content in tumor cells, but the exact reason is still not clear.
Tg is synthesized by thyroid follicular cells. The serum Tg level was a specific marker for monitoring residual tumor cells, recurrence, and metastasis of DTC and for assessing the efficacy of treatment. In patients who underwent total thyroidectomy and who had already been treated with 131I ablation of the thyroid gland, the serum Tg levels should be below the reference line or undetectable; therefore, an increase in Tg might suggest a recurrence or metastasis of TC. In this study, the TgAb levels of all patients were within the reference range, indicating that antibodies did not interfere with the Tg results. The serum Tg level of the positive group was significantly higher than that of the negative group, indicating that the imaging results had good correlations with the Tg levels, consistent with previous reports.23 We noted that the survival rate and the reliability of Tg as a tumor marker during the follow-up period were directly influenced by whether 131I could completely clear patients’ residual thyroid tissues.
In summary, after the first (postoperative) application of 131I therapy, 99mTc-MIBI imaging could sensitively detect metastatic TC lesions in patients with DTC by not only having a high positive rate for cervical lymph node metastases but also finding distant metastases. The use of 99mTc-MIBI for detecting cervical residual thyroid tissues is limited; therefore, it cannot be used instead of 131I imaging, but it can be used as one of the inspection methods for evaluating patients with DTC, thus providing important information about the need for subsequent 131I therapy. 99mTc-MIBI imaging and 131I imaging had good correlations with the serum Tg levels.
Disclosure
The authors report no conflicts of interest in this work.
References
Ward LS. Thyroid tumors: are we unveiling the puzzle? Endocr Relat Cancer. 2014;21(5):E7–E8. | ||
American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. | ||
Tuttle RM, Haddad RI, Ball DW, et al. Thyroid carcinoma, version 2. 2014. J Natl Compr Canc Netw. 2014;12:1671–1680. | ||
Lundh C, Lindencrona U, Postgård P, et al. Radiation-induced thyroid stunning: differential effects of (123)I, (131)I, (99m)Tc, and (211)At on iodide transport and NIS mRNA expression in cultured thyroid cells. J Nucl Med. 2009;50:1161–1167. | ||
Flux GD, Haq M, Chittenden SJ, et al. A dose effect correlation for radioiodine ablation in differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37:270–275. | ||
Mallick U, Harmer C, Yap B, et al. Ablation with low-dose radioiodine and thyrotropin alfa in thyroid cancer. N Engl J Med. 2012;366:1674–1685. | ||
Etchebehere EC, Santos AO, Matos PS, et al. Is thyroid stunning clinically relevant? A retrospective analysis of 208 patients. Arq Bras Endocrinol Metabol. 2014;58:292–300. | ||
McDougall IR, Iagaru A. Thyroid stunning: fact or fiction? Semin Nucl Med. 2011;41:105–112. | ||
Kiratli PO, Kara PP, Ergün EL, et al. Metastatic insular thyroid carcinoma: visualized on Tc-99m pertechnetate, Tc-99m MDP and iodine-131 imaging; a review of the literature for other radionuclide agents. Ann Nucl Med. 2004;18:443–446. | ||
Xu YH, Shen CT, Xue YL, et al. Iodine-131 SPET/CT and 18F-FDG PET/CT for the identification and localization of mediastinal lymph node metastases from differentiated thyroid carcinoma. Hell J Nucl Med. 2013;16:199–203. | ||
Kanaev SV, Novikov SN, Krivorot’ko PV, et al. Combined use of 99mTc-MIBI imaging and ultrasonography (US) in the diagnosis of axillary lymphatic metastasis in patients with breast cancer. Vopr Onkol. 2013;59:52–58. | ||
Nikoletic K, Lucic S, Peter A, et al. Lung 99mTc-MIBI imaging: impact on diagnosis of solitary pulmonary nodule. Bosn J Basic Med Sci. 2011;11:174–179. | ||
Kanaev SV, Novikov SN, Beĭnusov DS, et al. Role of single-photon emission-computed tomography and x-ray computed tomography in diagnosing lymphatic metastases in patients with non-small cell lung cancer. Vopr Onkol. 2014;60:476–481. | ||
Deltuva VP, Jurkienė N, Kulakienė I, et al. Introduction of novel semiquantitative evaluation of (99m)Tc-MIBI SPECT before and after treatment of glioma. Medicina (Kaunas). 2012;48:15–21. | ||
Spyridonidis TJ, Matsouka P, Symeonidis A, et al. (99m)Tc sestamibi as a prognostic factor of response to first-line therapy and outcome in patients with malignant lymphoma. Clin Nucl Med. 2013;38:847–854. | ||
Riazi A, Kalantarhormozi M, Nabipour I, et al. Technetium-99m methoxyisobutylisonitrile imaging in the assessment of cold thyroid nodules: is it time to change the approach to the management of cold thyroid nodules? Nucl Med Commun. 2014;35:51–57. | ||
Carvalho PA, Chiu ML, Kronauge JF, et al. Subcellular distribution and analysis of technetium-99m-MIBI in isolated perfused rat hearts. J Nucl Med. 1992;33:1516–1522. | ||
Mankoff DA, Dunnwald LK, Gralow JR, et al. [Tc-99m]-sestamibi uptake and washout in locally advanced breast cancer are correlated with tumor blood flow. Nucl Med Biol. 2002;29:719–727. | ||
Piwniaca-Worms D, Kronange JF, Chiu ML. Up take and retention of hexak is (2-methoxy isobutyl isonitrile) technetium (I) in cultured chick myocardial dependence. Circulation. 1990;82:1826–1838. | ||
Iwata M, Kasagi K, Misaki T, et al. Comparison of whole-body 18F-FDG PET, 99mTc-MIBI SPET, and post-therapeutic 131I-Na imaging in the detection of metastatic thyroid cancer. Eur J Nucl Med Mol Imaging. 2004;31:491–498. | ||
Rubello D, Mazzarotto R, Casara D. The role of technetium-99m methoxyisobutylisonitrile imaging in the planning of therapy and follow-up of patients with differentiated thyroid carcinoma after surgery. Eur J Nucl Med. 2000;27:431–440. | ||
Ronga G, Ventroni G, Montesano T, et al. Sensitivity of [99mTc]methoxyisobutylisonitrile scan in patients with metastatic differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2007;51:364–371. | ||
Campennì A, Violi MA, Ruggeri RM, et al. Clinical usefulness of 99mTc-MIBI imaging in the postsurgical evaluation of patients with differentiated thyroid cancer. Nucl Med Commun. 2010;31:274–279. | ||
Coelho SM, de Carvalho DP, Vaisman M. New perspectives on the treatment of differentiated thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:612–624. | ||
Ugur O, Kostakglu L, Guler N, et al. Comparison of 99mTc(V)-DMSA, 201Tl and 99mTc-MIBI imaging in the follow-up of patients with medullary carcinoma of the thyroid. Eur J Nucl Med. 1996;23:1367–1371. | ||
Rubello D, Saladini G, Carpi A, et al. Nuclear medicine imaging procedures in differentiated thyroid carcinoma patients with negative iodine scan. Biomed Pharmacother. 2000;54:337–344. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.