Back to Journals » Eye and Brain » Volume 14
Value of Optic Nerve Sheath Diameter in Diagnosis and Follow Up of Patients with Disturbed Conscious Level
Authors Momtaz OM, Said OM , Mohamed AM, Abdel Mawla TS
Received 13 April 2022
Accepted for publication 2 September 2022
Published 27 September 2022 Volume 2022:14 Pages 115—126
DOI https://doi.org/10.2147/EB.S369813
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Margaret Wong-Riley
Osama Mahmoud Momtaz,1 Omar M Said,2 Amany Mahmoud Mohamed,1 Tamer Sayed Abdel Mawla1
1Critical Care Department, Fayoum University, Fayoum, Egypt; 2Ophthalmology Department, Fayoum University, Fayoum, Egypt
Correspondence: Omar M Said, Ophthalmology Department, Fayoum University, Fayoum, Egypt, Email [email protected]
Background: Ultrasonographic measurement of optic nerve sheath diameter is a simple, non-invasive, and reliable method of detecting elevated intracranial pressure (ICP) in critical patients. Optic nerve sheath communicates with the dura mater covering the brain and contains cerebrospinal fluid, allowing pressure transmission from the cranium. Therefore, changes in cerebrospinal fluid (CSF) pressure have been shown to produce changes in ONSD.
Objective: This study aimed to assess the accuracy of optic nerve sheath diameter (ONSD) in diagnosis and follow-up patients with disturbed conscious levels compared with CT brain and fundus examination.
Patients and Methods: One hundred forty-one participants were included in the study, classified into 76 cases admitted with disturbed conscious levels due to elevated ICP and 65 controls. All patients were subjected to CT brain and optic nerve US and fundus examination at the time of admission and follow-up after 48 h after proper management.
Results: The current study showed that ONSD is significant in predicting elevated ICP at the cut-off point of average ONSD of 5.19 mm with 97% sensitivity and 98% specificity, and the area under the curve (AUC) was 0.996. The present study revealed a significant inverse correlation between ONSD and GCS in patients with increased ICP.
Conclusion: Ultrasonic measurement of ONSD is a promising technique in diagnosing and following patients with disturbed conscious levels.
Keywords: ultrasonographic, optic nerve sheath diameter, disturbed conscious level
Introduction
Intracranial hypertension is a widely known severe condition caused by different diseases. Disease management and diagnosis are challenging. The brain could be damaged by compressing effects on the intracranial structures or reducing the blood flow. Brain ischemia or brainstem herniation is a possible complication of intracranial hypertension, which may lead to destructive and fatal deterioration.1 Invasive intracranial devices are still the primary approach to measuring intracranial pressure (ICP).2
Intracranial hypertension occurs when ICP is 20 mmHg. Invasive ICP monitoring is associated with many complications such as hemorrhage or infection with a significantly high risk of bacterial colonization.3 As a result, it is essential to find new non-invasive methods for monitoring ICP, even though several techniques are available.4
In case of the lack of invasive intracranial devices or contraindications in some situations, there are non-invasive methods such as magnetic resonance imaging (MRI) and computed tomography (CT) scan that can be rapidly used to predict increased ICP. However, these techniques are too expensive, need prolonged acquisition, are not available in all health care centers, and usually need transporting the patient, which may be limited or harmful.5
Transcranial Doppler sonography (TCD) may give a picture representative of intracranial hypertension. The Transcranial Doppler sonography pulsatility index reveals the reduction in the cerebral perfusion pressure caused by intracranial hypertension. On the other hand, it needs an expert radiologist, as TCD is not easy to perform, even with an experienced physician.6
Fundus examination is widely used as an assessment tool in cases of cerebral edema because it is noninvasiveness, easy to be repeated, and experienced ophthalmologists can be readily available as this technique has been used for decades. However, it has some disadvantages as follows: it is operator-dependent, and the need to prepare the patient to examine the form of mydriatic drops may interfere with assessing pupil responses and cause blurred vision. Also, it is a qualitative assessment; therefore, it is challenging to monitor the changes accurately.7
Ultrasonographic (USG) measuring of optic nerve sheath diameter (ONSD) shows increased interest in the last years. This is because ultrasonography has become a bedside and simple tool commonly used in emergencies.8
The equipment needed is usually cheap and available. The ICP can be indirectly evaluated through the measurement of the optic nerve, anatomically occurring in the subarachnoid space and wrapped with a sheath derived from the meninges, extending towards the orbit.9 This communication allows CSF to transfer, transmitting the changes between ICP and infraorbital subarachnoid spaces pressure.10
Objectives
This research aimed to evaluate the accuracy of optic nerve sheath diameter (ONSD) in diagnosing and follow-up patients with disturbed conscious levels compared with CT brain and fundus examination.
Patients and Methods
The current study was conducted in the ICU of Fayoum University Hospital from January 2020 to September 2021. One hundred forty-one participants were included, divided into 76 cases admitted with disturbed conscious levels due to elevated ICP, and 65 controls.
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of the Faculty of Medicine, Fayoum University (No 403) in January 2020 before the study began. Informed written consents for participation were taken and signed by the eligible relatives before recruitment and randomization.
Inclusion criteria: age older than 18 years, traumatic brain injuries, intracranial hemorrhages, stroke, metabolic (hepatic, uremic, electrolyte disturbance), brain inflammation (meningitis, encephalitis), and intracranial tumors.
Exclusion criteria: age <18 years, eye diseases (such as glaucoma, lens opacity), orbit diseases, and fracture.
All Patients Were Subjected to the Following
All patients were subjected to CT brain and optic nerve US and fundoscopy at the admission before starting management and follow-up after 48 h after proper surveillance.
- Full medical history was taken from all patients, including personal history, chronic disease, history of present illness (including the cause of admission and presentation), and history including drug intake.
- Clinical examination: general and local, especially the common Glasgow Coma Scale (GCS). The scale comprised three tests: eye, verbal, and motor responses. The three values separately, as well as their sum, were considered. The lowest possible GCS (graded 1 in each element) was three (deep coma or death), while the highest was 15 (fully awake person).
- Laboratory investigations: full labs.
- Ocular ultrasound was performed on all patients on admission and followed up after 48 h after proper management. A 7.5-MHz linear probe measuring was used with a Philips ultrasound machine (ModelHS 2000). The patient was placed in a supine position with closed eyes, and the probe was placed on the upper border of the upper eyelid 3 mm away from the port of entry of the nerve into the eye socket. It was considered abnormal if the mean binocular optic nerve sheath diameter exceeded 5.00 mm.10
- Brain CT or MRI was carried out on all patients on admission and followed up after 48 h after proper management. Radiological findings of edema, effacement, or shift suggestive of elevated ICP were used to evaluate the diagnosis of brain edema and optic nerve sheath diameter accuracy in diagnosis and follow-up.
- Fundus examination was performed for all patients on admission and follow-up after 48 h following proper management for comparison with ocular ultrasound and imaging studies.
Statistical Methods
The collected data were tabulated and statistically analyzed using SPSS version 22 (SPSS Inc, USA). The mean, standard deviation (SD), and range were calculated for quantitative data. Independent t-test and dependent t-test were used as a test of significance. Pearson correlation was run to test the relation between GCS and ONSD at baseline and after 48 hours. ROC curve was performed. The cut-off points with the optimum sensitivity and specificity of ONSD in differentiating between the study groups were identified using the Youden index. Qualitative data were presented as numbers and percentages, and chi-square (χ2) was used as a significance test. Statistical significance was considered at P< 0.05.
Results
Demographic Characteristics of Our Study
No statistically significant difference was determined between both groups regarding age (51 ± 15.3 vs 47.6 ± 16.2, P: 0.19) and sex (males: 37 (48.7%) vs 36 (55.4%), P: 0.4, respectively)).
Risk Factors
The risk factors in this study were 27 (35.5%) patients having diabetes, 28 (36.8%) were hypertensive, 5 (6.6%) were with a positive history of ischemic heart disease, 17 (22.4%) were renal, and 11 (14.5%) were hepatic.
Causes of Elevated ICP
Causes of increased ICP in the present study were intracranial tumor in 17 (22.4%) patients, intracranial hemorrhage in 11 (14.5%) patients, head trauma in 11 (14.5%) patients, uremic encephalopathy in 11 (14.5%) patients, hypernatremia in 8 (10.5%) patients, DKA in 5 (6.6%) patients, hepatic encephalopathy in 4 (5.3%) patients, CO2 narcosis in 3 (3.9%) patients, brain abscess in 3 (3.9%) patients, ischemic Stroke in 2 (2.6%) patients, and post-arrest in a patient (1.3%).
Glasgow Coma Scale (GCS)
There was a statistically significant difference between the GCS of patients on admission and after 48 h (after proper management) (11.0 ± 2.8 vs 12.8 ± 2.9, p< 0.0001), respectively.
Management of Patients
In our study, 43 (56.6%) patients were managed conservatively according to the cause (Lasix, dialysis, mannitol,) and 33 (43.4%) patients were managed by surgical intervention.
CT Signs of Elevated ICP
CT brain was performed for all patients and showed 39 (51.3%) patients with signs of elevated ICP as follows: 9 (11.8%) patients showed signs of hydrocephalus, 9 (11.1%) showed signs of midline shift, 21 (27.6%) showed signs of mass effect and 39 (51%) showed signs of brain edema.
After 48 h, 5 (6.6%) patients showed signs of hydrocephalus, 1 (1.3%) showed signs of midline shift, 7 (9.2%) showed signs of mass effect, and 39 (51.3%) showed signs of brain edema.
Fundoscopic Examination
The fundoscopic examination was carried out on all patients in the current study upon admission and after 48h. It was found that 21 patients had papilledema on admission and no significant difference after 48 h. An insignificant difference was determined regarding the grades of papilledema between both eyes on admission and after 48 h (Table 1).
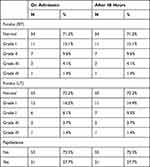 |
Table 1 The Fundus Examinations |
ONSD of the Study Population
The ultrasound on the optic nerve of the patients showed that there was a statistically significant difference between cases and control as follows: RT ONSD (6.24 ± 0.57 VS 4.66 ± 0.40, p< 0.0001), LT ONSD (6.28 ± 0.60 vs 4.62 ± 0.52, p< 0.0001), and average ONSD (6.26 ± 0.53 vs 4.64 ± 0.37, p< 0.0001). There was a statistically significant difference between ONSD on admission and after 48 h in RT ONSD (6.24 ± 0.57 vs 5.81 ± 0.58, p< 0.0001), LT ONSD (6.28 ± 0.60 vs 5.78 ± 0.68, p< 0.0001), and average ONSD (6.26 ± 0.53 vs 5.79 ± 0.59, p <0.0001) (Figures 1 and Figure 2).
 |
Figure 1 The ONSD of the right eye on admission (before surgical intervention) (5.89 mm). |
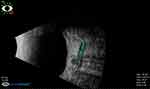 |
Figure 2 The follow-up of ONSD after 48hs of surgical interventions of the previous patient is shown in Figure 1 (4.86 mm). |
ONSD According to Causes of Coma
The present study found that there was a significantly lower ONSD in patients with increased ICP due to metabolic causes than in other patients regarding average ONSD on admission (6.01 ± 0.26 vs 6.42 ± 0.6) and after 48 h (5.41 ± 0.25 vs 6.04 ± 0.61) with p< 0.0001.
Among patients with increased ICP, there was a significant reduction in average ONSD after 48 h mainly due to metabolic causes than other causes (5.41± 0.25 vs 6.04 ± 0.61, p< 0.0001) from the baseline (6.01 ± 026 vs 6.46± 0.6, p< 0.0001).
Correlation Between GCS and ONSD
There was a negative significant correlation between ONSD and GCS (r= −0.237, p-value 0.040) on admission and (r=−0.404, p <0.0001) after 48 h (Figures 3 and Figure 4).
 |
Figure 3 The correlation between Glasgow coma scale and ONSD on admission. Scattered dot diagram (Blue circles refer to cases). |
 |
Figure 4 The correlation between Glasgow coma scale and ONSD after 48hs. Scattered dot diagram (Blue circles refer to cases). |
Relation Between ONSD and CT on Admission
ONSD increased in all studied patients, with a significantly higher value in a patient with CT, confirming increased ICP compared to those without (6.45± 0.59 vs 6.06 ± 0.38, p: 0.001) (Table 2).
 |
Table 2 The Relation Between ONSD and CT on Admission |
Relation Between ONSD and CT Findings After 48 Hours
Significantly higher values of ONSD in patients with CT showed increased ICP after 48 h (6.14 ± 0.59 vs 5.43 ± 0.32, p-value< 0.0001) (Table 3).
 |
Table 3 The Relation Between ONSD and CT Findings After 48 Hours |
Fundoscopic Examination in Correlation to ONSD
There was a positive correlation between fundoscopic examination and ONSD on admission. After 48h, all patients with papilledema had a significantly higher ONSD on admission. After 48 h, it increased significantly with increasing the grades of fundus examination with a p-value of 0.001 on the admission for RT eye and 0.033 for LT eye, and after 48h, 0.002 for RT eye and 0.019 for LT eye. Significantly higher values were observed in patients with papilledema with an average ONSD P-value of 0.006, but many patients with no papilledema showed increased ONSD measurements. This supports that papilledema by fundus examination is not a sensitive marker (Table 4).
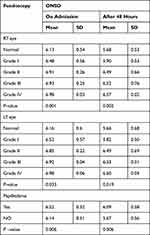 |
Table 4 The Fundoscopic Examination in Correlation to ONSD |
Receiver-Operating Characteristic (ROC) Curves Analysis for ONSD
The receiver-operating characteristic (ROC) curve analysis for prediction of increased ICP using average ONSD showed that ONSD had an excellent discriminative power in differentiating case from control at a cut-off point of average ONSD of 5.19 mm with 97% sensitivity and 98% specificity, and the area under the curve (AUC) was 0.996.
ROC curve was calculated using ONSD as an index for brain edema. It was a good sign in the predicted elevated ICP compared to the CT brain with an AUC of 0.723. The optimal cut-off point of average ONSD for patients with evidence of the radiological finding of increased ICP was 6.63, with a sensitivity of 53.8% and a specificity of 94.6% (Table 5, Figures 5 and Figure 6).
 |
Table 5 The Validity of ONSD |
 |
Figure 5 ROC curve for the validity of ONSD (cases vs controls). |
 |
Figure 6 ROC curve for the validity of ONSD patients with the evidence of the radiological finding of increased ICP. |
Morality Rate in Correlation to ONSD
The current study showed no statistical correlation between ONSD and in and out hospital mortality (Table 6).
 |
Table 6 The Mortality Rate in Correlation to ONSD |
Discussion
The optic nerve emerges in the cerebrospinal fluid and is part of the central nervous system. This communication permits CSF to circulate between the intracranial and intraorbital subarachnoid areas resulting in comparable pressure variations.9 As a result, ultrasonographic measuring of ONSD has become a standard bedside method for detecting increased ICP. This technique is recommended in neurosurgery, trauma, critical care, and emergency department.10 One hundred forty-one participants were included in this study, classified into 76 cases (patients) who were diagnosed with disturbed conscious levels due to elevated ICP and 65 controls.
This study showed that ONSD significantly predicts elevated ICP at a cut-off point of average ONSD of 5.19 mm with 97% sensitivity, 98% specificity, and an AUC of 0.996. ONSD was significantly higher in a patient with CT, showing increased ICP compared to those without (6.45± 0.59 vs 6.06 ± 0.38, p-value 0.001). The best cut-off point of average ONSD for patients with evidence of the radiological finding of increased ICP was 5.9, with an 82% sensitivity and 51% specificity. ONSD was more sensitive in the detection of increased ICP than fundus examination.
The Current results Were Consistent with the Following Studies
Mahmoud et al found that the cut-off value of mean ONSD for detecting elevated ICP was > 5 mm with a sensitivity of 100%, specificity of 96%, and AUC of 0.99.11
Kim et al showed that ONSD of 5.3 mm for identifying high ICP had a sensitivity of 75.4% and a specificity of 90.8%.12
Juxiang Wang et al also reported that the mean value of ONSD in raised ICP (> 13 mm Hg) was 5.48 mm, while the mean value for ICP (>22 mm Hg) was > 5.83 mm.13
Altayar et al agreed with the current study and found that the mean ONSD in the positive CT findings group compared with the negative group was 6.3 ± 0.06 vs 5.5 ± 0.07 mm p-value < 0.001, respectively. Also, the mean value of ONSD in patients with increased ICP group compared with normal ICP group (measured by intraventricular device) was 6.6 ± 0.05 vs 5.8 ± 0.08, p = 0.004, respectively, with optimal cut-off value for predicting elevated ICP > 6.1 cm with 84.62% sensitivity, 66.67% specificity, and AUC of 0.85.14
Hanafi et al, the mean ONSD critical values were 5.3 mm, with 96.4% sensitivity and 71.4% specificity. It showed that ultrasonography to identify elevated ICP caused by trauma was a safe, non-invasive, and effective method.15
Raghunandan et al reported a statistically significant increase in ONSD associated with increasing papilledema severity as graded by Frisén’s grading. Papilledema can be absent in patients with optic atrophy, despite coexisting elevated ICPs in those patients.16
Jeon et al reported that an optimal cut-off value of ONSD for elevated ICP was 5.6 mm with 93.8% sensitivity and 86.7% specificity in Korean populations.17
Ohle et al reported that ultrasonic ONSD ≥ 5 mm revealed higher accuracy for detecting increased ICP than CT features (such as hydrocephalus and the collapse of ventricles).18
The Following Studies Were Inconsistent with the Current Results
Jenjitranant et al reported a lower ONSD cut-off value of 3.15 mm, which had 97.4% and 13.8% specificity.19
Wang et al documented significantly higher ONSD of increased opening pressure in the lumbar puncture group than that of the normal opening pressure in the LP group (4.58±0.46 mm vs 3.55±0.38 mm) with a cut-off value> 7.2 mm for ONSD at opening pressure of 22 mm Hg, with 82% sensitivity, 79% specificity, and 0.81 AUC.9
Conclusion
- Ultrasonography of the ONSD is a non-invasive, reliable, fast, and accessible approach for assessing patients with disturbed conscious levels (traumatic or non-traumatic).
- Compared to classic CT signs of brain edema such as ventricular compression, cisternal effacement, or sulcal effacement, ONSD had a better predictive value for brain edema.
Data Sharing Statement
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent
Our study complies with the declaration of Helsinki and was approved by the ethics committee of Faculty of Medicine, Fayoum University (No 403) on January 2020 prior to the study beginning. Informed written consents for participation were taken and signed by the eligible relatives before recruitment and randomization.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Antonelli M, Azoulay E, Bonten M, et al. Year in review in intensive care medicine, 2008: i. Brain injury and neurology, renal failure and endocrinology, metabolism and nutrition, sepsis, infections and pneumonia. Intensive Care Med. 2009;35(1):30–44. doi:10.1007/s00134-008-1371-6
2. Hwan Kim Y, Ho Lee J, Kun Hong C, et al. Feasibility of optic nerve sheath diameter measured on initial brain computed tomography as an early neurologic outcome predictor after cardiac arrest. Acad Emerg Med. 2014;21(10):1121–1128. doi:10.1111/acem.12477
3. Hagel S, Bruns T, Pletz MW, Engel C, Kalff R, Ewald C. External ventricular drain infections: risk factors and outcome. Interdiscip Perspect Infect Dis. 2014;2014:1–6. doi:10.1155/2014/708531
4. Hamarat Y, Deimantavicius M, Kalvaitis E, et al. Location of the internal carotid artery and ophthalmic artery segments for non-invasive intracranial pressure measurement by multi-depth TCD. Libyan J Med. 2017;12(1):1384290. doi:10.1080/19932820.2017.1384290
5. Sekhon MS, Griesdale DE, Robba C, et al. Erratum to: optic nerve sheath diameter on computed tomography is correlated with simultaneously measured intracranial pressure in patients with severe traumatic brain injury. Intensive Care Med. 2014;41(1):177. doi:10.1007/s00134-014-3560-9
6. Raboel PH, Bartek J, Andresen M, Bellander BM, Romner B. Intracranial pressure monitoring: invasive versus non-invasive methods—a review. Crit Care Res Pract. 2012;2012:1–14. doi:10.1155/2012/950393
7. Driessen C, Bannink N, Lequin M, et al. Are ultrasonography measurements of optic nerve sheath diameter an alternative to funduscopy in children with syndromic craniosynostosis? J Neurosurg Pediatr. 2011;8(3):329–334. doi:10.3171/2011.6.PEDS10547
8. Liu H, Yang D, Ma T, Shi W, Zhu Q, Kang J. Measurement and associations of the optic nerve subarachnoid space in normal tension and primary open-angle glaucoma. Am J Ophthalmol. 2018;186:128–137. doi:10.1016/j.ajo.2017.11.024
9. Wang L, Feng L, Yao Y, et al. Optimal optic nerve sheath diameter threshold for the identification of elevated opening pressure on lumbar puncture in a Chinese population. PLoS One. 2015;10(2):e0117939. doi:10.1371/journal.pone.0117939
10. Amin D, McCormick T, Mailhot T. Elevated intracranial pressure diagnosis with emergency department bedside ocular ultrasound. Case Rep Emerg Med. 2015;2015:1–3. doi:10.1155/2015/385970
11. Mahmoud SMM, Attia SM, El Adalany MA, Ahmed MES. Accuracy of optic nerve sheath diameter measurement as a predictor of intracranial pressure in traumatic brain injury. Egypt J Hosp Med. 2021;85(1):3092–3097. doi:10.21608/ejhm.2021.194063
12. Kim DY, Kim SY, Hong DY, et al. Comparison of ultrasonography and computed tomography for measuring optic nerve sheath diameter for the detection of elevated intracranial pressure. Clin Neurol Neurosurg. 2021;204:106609. doi:10.1016/j.clineuro.2021.106609
13. Juxiang Wang MD, Li K, Li H, et al. Ultrasonographic optic nerve sheath diameter correlation with ICP and accuracy as a tool for non-invasive surrogate ICP measurement in patients with decompressive craniotomy. J Neurosurg. 2020;133:514–520. doi:10.3171/2019.4.JNS183297
14. Altayar AS, Abouelela AZ, Abdelshafey EE, et al. Optic nerve sheath diameter by ultrasound is a good screening tool for high intracranial pressure in traumatic brain injury. Ir J Med Sci. 2020;190(1):387–393. doi:10.1007/s11845-020-02242-2
15. Hanafi M, Verki M, Parei S. Ultrasonic assessment of optic nerve sheath to detect increased intracranial pressure. J Med Ultrasound. 2019;27(2):69. doi:10.4103/JMU.JMU_54_18
16. Raghunandan N, Joseph M, Nithyanandam S, Karat S. Role of ultrasonographic optic nerve sheath diameter in the diagnosis and follow-up of papilledema and its correlation with Frisén’s severity grading. Indian J Ophthalmol. 2019;67(8):1310. doi:10.4103/ijo.IJO_1827_18
17. Jeon JP, Lee SU, Kim S-E, et al. Correlation of optic nerve sheath diameter with directly measured intracranial pressure in Korean adults using bedside ultrasonography. PLoS One. 2017;12(9):e0183170. doi:10.1371/journal.pone.0183170
18. Ohle R, McIsaac SM, Woo MY, Perry JJ. Sonography of the optic nerve sheath diameter for detection of raised intracranial pressure compared to computed tomography. J Ultrasound Med. 2015;34(7):1285–1294. doi:10.7863/ultra.34.7.1285
19. Jenjitranant P, Tunlayadechanont P, Prachanukool T, Kaewlai R. Correlation between optic nerve sheath diameter measured on imaging with acute pathologies found on computed tomography of trauma patients. Eur J Radiol. 2020;125:108875. doi:10.1016/j.ejrad.2020.108875
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
