Back to Journals » Medical Devices: Evidence and Research » Volume 8
Validation of pressure gradient and peripheral fractional flow reserve measured by a pressure wire for diagnosis of iliofemoral artery disease with intermediate stenosis
Authors Murata N, Aihara H, Soga Y, Tomoi Y, Hiramori S, Kobayashi Y, Ichihashi K, Tanaka N, Yokoi H
Received 1 March 2015
Accepted for publication 9 June 2015
Published 9 November 2015 Volume 2015:8 Pages 467—472
DOI https://doi.org/10.2147/MDER.S83768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Naotaka Murata,1 Hideaki Aihara,2 Yoshimitsu Soga,1 Yusuke Tomoi,1 Seiichi Hiramori,1 Yohei Kobayashi,1 Kei Ichihashi,1 Nobuhiro Tanaka3
1Department of Cardiology, Kokura Memorial Hospital, Kitakyushu, 2Department of Cardiology, Tsukuba Medical Center Hospital, Ibaraki, 3Department of Cardiology, Tokyo Medical University, Tokyo, Japan
Objective: To examine the pressure gradient and peripheral fractional flow reserve (pFFR) measured by a pressure wire as indicators of hemodynamic significance in iliofemoral angiographic intermediate stenosis.
Background: The utility of pressure measurements using a pressure wire with vasodilators is unclear in cases with intermediate iliofemoral stenosis.
Methods: The mean pressure gradient (MPG) and mean pressure ratio (MPR) were measured at baseline and after injection of isosorbide dinitrate in 23 lesions with angiographically intermediate iliofemoral stenosis. Patients with complex lesions, infrapopliteal artery lesions, chronic total occlusion, and surgical bypass grafts were excluded. Hyperemic MPR was considered equivalent to pFFR. Changes in parameters in response to vasodilators were assessed and correlations of peak systolic velocity ratio (PSVR) with hyperemic MPG and pFFR were examined using duplex ultrasound.
Results: After injection of isosorbide dinitrate, hyperemic MPG increased significantly (from 9.0±5.7 to 16.3±6.2 mmHg; P<0.05) and hyperemic MPR (pFFR) decreased significantly (from 0.92±0.06 to 0.81±0.07; P<0.05). PSVR was significantly correlated with hyperemic MPG (R=0.52; P<0.05) and pFFR (R=–0.50; P<0.05). The optimal cut-off value of pFFR as an indicator of significant hemodynamic stenosis (PSVR >2.5) was 0.85 (area under the curve 0.72; sensitivity 94%; specificity 50%, P<0.05).
Conclusion: pFFR measured using a pressure wire is reliable for prediction of hemodynamic significance in iliofemoral intermediate stenosis.
Keywords: endovascular therapy, peripheral artery disease, pressure measurements, hyperemia, vasodilators, hemodynamics
Introduction
Endovascular treatment (EVT) for iliofemoral artery lesions has become common in the last decade due to technical developments related to self-expandable nitinol stents, and EVT is now an alternative first-line treatment with long-term outcomes that are not inferior to those after surgery.1,2 The high procedural success rate and reduced invasiveness has encouraged extension of indications for endovascular revascularization, but with little focus on hemodynamic significance. The current guidelines for peripheral artery disease indicate that EVT should be used with optimal drug treatment and supervised exercise therapy when clinical findings suggest a reasonable likelihood of symptomatic improvement. These guidelines also recommend pressure measurements across lesions at rest and under induced hyperemia in cases in which the hemodynamic significance of the target lesion is unclear.3,4 Various parameters have been proposed to determine hemodynamic significance, including mean pressure gradient (MPG), peak-to-peak PG, and ratio of mean pressure (also referred to as the peripheral fractional flow reserve [pFFR]) with or without vasodilators.5–9 However, the validity of these parameters for treatment planning is unclear. Therefore, the purpose of this study was to verify whether trans-stenotic absolute PG and pFFR measured using a 0.014-inch pressure wire under hyperemia can be used to establish hemodynamic significance in iliofemoral angiographically intermediate stenosis.
Methods
Study design and patient population
Pressure measurements were performed for 37 consecutive patients (40 lesions) with claudication and critical limb ischemia who had iliofemoral atherosclerotic artery disease for which EVT was planned according to comprehensive consideration of symptoms, quantitative vessel angiography results or duplex ultrasound (DUS) study in advance between April and August 2012 at Kokura Memorial Hospital. Patients who were planning to undergo EVT for TransAtlantic Inter-Society Consensus (TASC) II class C or D lesions, infrapopliteal artery lesions, chronic total occlusion, and surgical bypass grafts were excluded. Based on these criteria, the subjects were 22 patients (23 lesions) with intermediate stenosis, defined as <75% stenosis on quantitative vessel angiography, identified retrospectively from our database. We did not defer the EVT from the results of pressure measurements. Kokura Memorial Hospital review board/ ethics committee approved the study protocol. Written informed consent was obtained from each patient.
Pressure measurements
Pressure measurements were performed using a 0.014-inch pressure wire (300 cm PrimeWire Prestage®; Volcano Corporation, San Diego, CA, USA) following angiography and just before starting EVT. The pressure wire was calibrated before the procedure, equalized at the end of the guiding system, advanced over the lesion, and positioned in a distal healthy area. The mean trans-stenotic absolute PG (baseline MPG) and mean pressure ratio (baseline MPR) were then measured. Next, 250 μg of isosorbide dinitrate (ISDN) was injected via the guiding system to induce a hyperemic state. Hyperemic MPG and MPR were then measured, usually approximately 1 minute after flushing the guiding system with saline. MPG was defined as the difference between the mean pressure in the distal healthy area across the lesion from the pressure wire (mean distal pressure) and the mean central aorta pressure from the guiding system (mean aorta pressure). MPR was calculated as mean distal pressure/mean aorta pressure. Hyperemic MPR was considered equivalent to pFFR.
DUS study
The peak systolic velocity ratio (PSVR) on duplex (DUS) was used to define the hemodynamic severity of lesions. Tests were performed by certified sonographers within 30 days before pressure measurements. PSVR was calculated by dividing the velocity measured at the point of maximum stenosis by the velocity in the closest adjacent healthy vessel segment.
Outcomes
Comparisons of baseline MPG to hyperemic MPG, and baseline MPR to hyperemic MPR (pFFR) were performed to assess responses of these parameters to vasodilators. Correlations of PSVR on DUS with hyperemic MPG and pFFR were examined. Optimal cut-off values were determined for hyperemic MPG and pFFR as indicators of hemodynamically significant stenosis (PSVR >2.5 on DUS).
Statistical analysis
Continuous variables are presented as means with standard deviation and categorical variables as numbers with percentages. Continuous variables were compared by Student’s t-test and correlations between continuous variables were examined using simple linear regression. A receiver operating characteristic analysis was used to determine the optimal cut-off values for hyperemic MPG and pFFR. P<0.05 was regarded as significant. All analyses were performed using JMP® version 10.0 (SAS Institute Inc., Cary, NC, USA).
Results
The characteristics of the patients are shown in Table 1 and the characteristics of lesions are shown in Table 2. The patients had a mean age of 72.2±6.8 years (range: 61–86 years) and 19 were male. Almost half of the patients had coronary artery disease (CAD) and three were receiving hemodialysis. All patients had intermittent claudication, except for four with critical limb ischemia. The mean % stenosis diameter was 57.3%±13.2%, and 91% of the lesions were classified as TASC II A.
  | Table 1 Patient characteristics for pressure measurements (N=22) |
  | Table 2 Lesion characteristics (N=23) |
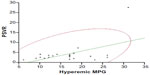
MPG increased significantly after injection of ISDN (from 9.0±5.7 to 16.3±6.2 mmHg; P<0.05, Figure 1). Baseline MPG was not significantly correlated with PSVR (R=0.33; P=0.12); however, hyperemic MPG was significantly correlated with PSVR (R=0.52; P<0.05, Figure 2).
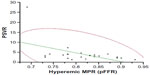
MPR decreased significantly after injection of ISDN (from 0.92±0.06 to 0.81±0.07; P<0.05, Figure 3). Baseline MPR was not significantly correlated with PSVR (R=−0.32; P=0.16); however, hyperemic MPR (pFFR) was significantly correlated with PSVR (R=−0.50; P<0.05, Figure 4).
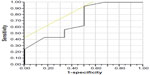
The optimal cut-off value for pFFR for hemodynamically significant stenosis (defined as PSVR >2.5) was 0.85 (area under the curve [AUC] 0.72; sensitivity 94%; specificity 50%, P<0.05, Figure 5). Hyperemic MPG was not a significant predictor of hemodynamically significant stenosis (AUC 0.48; sensitivity 71%; specificity: 87%, P=0.95).
Discussion
The major findings in this study of iliofemoral angiographically intermediate stenosis were that hemodynamic significance can be established more accurately under hyperemia induced by ISDN, compared to a resting state; that hyperemic MPG and hyperemic MPR (pFFR) are both significantly correlated with PSVR on DUS; and that the optimal cut-off value for pFFR as an indicator of hemodynamic significance (PSVR >2.5) was 0.85.
In CAD, common physiological findings are included in planning a revascularization strategy for non-severe stenosis. We routinely employ stress tests for coronary blood flow through exercise or pharmacologic stimulation, and coronary pressure measurements are commonly used for assessment of the ischemic potential of a stenosis in our daily practice. Recent large clinical trials have established fractional flow reserve (FFR) measured using a pressure wire as a standard diagnostic tool in patients with non-severe stenosis.10,11 In peripheral artery disease, hyperemic translesional MPG and renal FFR are important as physiological parameters in endovascular revascularization for renal artery stenosis (RAS). Current American College of Cardiology/American Heart Association guidelines recommend pressure measurements for understanding the functional significance in RAS.12,13
Unlike in CAD or RAS, pressure measurements are not commonly used for iliofemoral disease with intermediate stenosis although there might be high frequencies of visual-functional mismatch between moderate morphologic stenosis (>50% in angiography) and pressure ratio.14 A residual MPG <10 mmHg after intervention has been suggested as an indicator of hemodynamic significance in iliac arteries.4 However, this criterion is based on findings obtained using 5 Fr catheters, and large diameter catheters always overestimate the PG compared with a pressure wire.5 In addition, there are no data on the clinical value of using vasodilators and pFFR measurements for iliofemoral disease with intermediate stenosis. In contrast, DUS is frequently used for planning of iliofemoral artery revascularization (both endovascular and surgical) in daily practice. The quality of this examination depends on the experience of sonographers and data interpretation can be time-consuming; but the method is noninvasive, and provides information on lesion locations and stenosis in two dimensions, and on hemodynamic severity based on PSVR.15,16
The results of the current study revealed the importance of inducing a hyperemic state in evaluation of iliofemoral artery circulation. The findings support our hypothesis that pressure measurements for iliofemoral angiographic mild to moderate stenosis can be used in place of DUS to evaluate hemodynamic significance. Thus, measurement of hyperemic MPG and pFFR using a pressure wire can be used to assess the hemodynamic severity of a lesion (which was not evaluated in DUS before EVT) in a catheter laboratory and to perform intervention with the pressure wire across the lesion based on pFFR reaching the threshold described above. We note that modern pressure wires have improved durability and torque, and thus EVT using a pressure wire is not stressful for operators, except for treating complex lesions such as those in the TASC II C/D class. Hyperemic MPG also had a significant correlation with PSVR on DUS, but we were unable to establish a threshold MPG as an indicator of hemodynamic significance in the current study. This parameter is an absolute value that depends strongly on blood pressure. Therefore, there is likely to be a wide range of hyperemic MPG values in iliofemoral arteries that may or may not reflect hemodynamic significance (Figure 2). Thus, we conclude that hyperemic MPR (pFFR) is a better measure of hemodynamic significance.
Limitations
There were several limitations in the study. First, the study population was identified retrospectively and the sample size was small. Second, correlations between hyperemic pressure measurements and PSVR on DUS were significant, but only moderate. Third, some cases had diffuse long lesions (especially for superficial femoral lesions); thus, some of the lesions on which pressure measurements were performed in our catheter laboratory did not strictly correspond to the positions of lesions evaluated on DUS by sonography in the vascular laboratory. This inadequate correspondence might be one of the reasons behind the poor specificity in pFFR measurement (only 50%) in the present study. Finally, a dose of 250 μg of ISDN was used to induce hyperemia, but a different dose of ISDN or another vasodilator (nitroglycerine, papaverine, adenosine) may alter the results.17 In the future, a validation study examining the type and dose of vasodilator would be needed.
Conclusion
Induction of a hyperemic state is required for pressure measurements in iliofemoral artery circulation. Hyperemic MPR (pFFR) measured using a 0.014-inch pressure wire is reliable for prediction of hemodynamic significance in iliofemoral angiographically mild to moderate stenosis. The optimal cut-off value of pFFR for hemodynamic significance was 0.85.
Acknowledgments
The authors thank Hiroyoshi Yokoi, MD (Fukuoka Sanno Hospital, Cardiovascular Medicine Center) and Professor Akira Yamashina (Tokyo Medical University, Cardiology) for providing critical review of the manuscript.
Disclosure
The authors have no conflicts of interest to disclose.
References
Kashyap VS, Pavkov ML, Bena JF, et al. The management of severe aortoiliac occlusive disease: endovascular therapy rivals open reconstruction. J Vasc Surg. 2008;48(6):1451–1457. | |
Soga Y, Iida O, Hirano K, Yokoi H, Nanto S, Nobuyoshi M. Mid-term clinical outcome and predictors of vessel patency after femoropopliteal stenting with self-expandable nitinol stent. J Vasc Surg. 2010;52(3):608–615. | |
European Stroke Organisation; Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases. Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J. 2011;32(22):2851–2906. | |
Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial. Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–S67. | |
Garcia LA, Carrozza JP Jr. Physiologic evaluation of translesion pressure gradients in peripheral arteries: Comparison of pressure wire and catheter-derived measurements. J Interv Cardiol. 2007;20(1):63–65. | |
Tetteroo E, Van der Graaf Y, Bosch JL, et al. Randomised comparison of primary stent placement versus primary angioplasty followed by selective stent placement in patients with iliac-artery occlusive disease. Dutch Iliac Stent Trial Study Group. Lancet. 1998;351(9110):1153–1159. | |
Kinney TB, Rose SC. Intraarterial pressure measurements during angiographic evaluation of peripheral vascular disease: techniques, Interpretation, applications, and limitations. AJR Am J Roentgenol. 1996;166(2):277–284. | |
Bonn J. Percutaneous vascular intervention: value of hemodynamic measurements. Radiology. 1996;201(1):18–20. | |
Lotfi AS, Sivalingam SK, Giugliano GR, Ashraf J, Visintainer P. Use of fraction flow reserve to predict changes over time in management of superficial femoral artery. J Interv Cardiol. 2012;25(1):71–77. | |
Bech GJ, DeBruyne B, Pijls NH, et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: a randomized trial. Circulation. 2001;103(24):2928–2934. | |
Bech GJ, De Bruyne B, Bonnier HJ, et al. Long-term follow-up after deferral of percutaneous transluminal coronary angioplasty of intermediate stenosis on the basis of coronary pressure measurement. J Am Coll Cardiol. 1998;31(4):841–847. | |
Drieghe B, Madaric J, Sarno G, et al. Assessment of renal artery stenosis severity by pressure gradient measurements. Eur Heart J. 2008; 29(4):517–524. | |
Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for VascularSurgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. | |
Chantadansuwan T, Kehasukcharoen W, Kanoksilp A, et al. Visual-functional mismatch and results of fractional flow reserve guided percutaneous coronary revascularization. J Med Assoc Thai. 2014; 97(10):1064–1076. | |
Koelemay MJ, den Hartog D, Prins MH, Kromhout JG, Legemate DA, Jacobes MJ. Diagnosis of arterial disease of the lower extremities with duplex ultrasonography. Br J Surg. 1996;83(3):404–409. | |
Ranke C, Creutzig A, Alexander K. Duplex scanning of the peripheral arteries: Correlation of the peak velocity ratio with angiographic diameter reduction. Ultrasound Med Biol. 1992;18(5):433–440. | |
Bragadeesh T, Sari I, Pascotto M, Micari A, Kaul S, Lindner JR. Detection of peripheral vascular stenosis by assessing skeletal muscle flow reserve. J Am Coll Cardiol. 2005;45(5):780–785. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.