Back to Journals » International Journal of General Medicine » Volume 14
Validation of Coronary Artery Disease Reporting and Data System (CAD-RADS) and Application of Coronary Artery Calcium Data and Reporting System (CAC-DRS) as New Standardized Tools in the Management of Coronary Artery Disease Patients
Authors Ebaid NY , Khalifa DN, Ragheb AS, Abdelsamie MM, Alsowey AM
Received 7 September 2021
Accepted for publication 19 October 2021
Published 2 November 2021 Volume 2021:14 Pages 7503—7514
DOI https://doi.org/10.2147/IJGM.S336662
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Noha Yahia Ebaid,1 Dalia Nabil Khalifa,1 Ahmad Sabry Ragheb,1 Magdy Mohamad Abdelsamie,2 Ahmed Mohamed Alsowey1
1Department of Radiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt; 2Department of Cardiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Correspondence: Noha Yahia Ebaid
Department of Radiology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
Tel +201064422995
Email [email protected]
Background and Objectives: The coronary artery disease reporting and data system (CAD-RADS) is intended to standardize the reporting of CCTA and the subsequent management guidelines of CAD. The present study was conducted to investigate the validation of CAD-RADS and the application of coronary calcium grading in CAD management.
Patients and Methods: The current study is a single-center prospective study that involved 177 participants with chest pain who were submitted to coronary CT angiography (CCTA). Two reviewers independently assessed CCTA results and gave each patient a CAD-RADS category. The reference standard for determining the clinical utility of CAD-RADS was invasive coronary angiography (ICA). The inter-reviewer agreement (IRA) was tested using the intra-class correlation (ICC).
Results: The study enrolled 111 cases with non-significant CAD and 66 cases with significant CAD based on ICA findings. According to the reviewer, the CAD-RADS had a sensitivity, specificity, and accuracy of 90.9 to 100%, 89.2 to 94.6%, and 93.16 to 93.2%, respectively, for predicting severe CAD. The IRA for CAD-RADS categories was excellent (ICC = 0.960). The best cut-off value for predicting severe CAD was CAD-RADS > 3. Significant relation between Ca and severe CAD (p< 0.001) was detected.
Conclusion: The current study provides a good understanding of CAD-RADS as a standard tool with high diagnostic accuracy.
Keywords: coronary, cardiovascular disease, Agatston, computed tomography, stenosis
Introduction
Globally, Cardiovascular disease (CVD) is the leading cause of death. By 2030, CVD is predicted to overtake cancer as the leading cause of death worldwide.1
Acute myocardial infarction and sudden cardiac death seem to be the most common early symptoms of coronary atherosclerosis (in 50% of males and 64% of females). Before experiencing a coronary event (acute coronary syndromes [ACS] or sudden cardiac death [SCD]), the majority of individuals exhibit no symptoms or warning signs.2
CCTA has been designed as a very efficient non-invasive method for assessing coronary arteries in individuals with a low to moderate risk of developing obstructive CAD.3
The main advantage of CTA is its ability to rule out CAD accurately without invasion. The Prospective Multicenter Imaging study (PROMISE) found that CTA conducts highly functional stress testing to predict unfavorable cardiac events in those with two years of stable chest pain.4
Recently, standardized reporting methods have been created for CCTA and coronary artery calcium scoring (CACS).5,6
The suggested CAD-RADS could consistently provide a wide range of coronary CTA data related to the occurrence, severity, and composition of coronary atherosclerosis. The CAD RADS is a key approach for summarizing specific patient outcomes, which varies from CAD-RADS 0 to CAD-RADS 5, based on the highest coronary luminal stenosis.6
Additionally, it offers guidelines for clinical care for each categorization, involving further testing and therapeutic choices. As a result, CAD-RADS may enable highly efficient coronary CTA, leading to highly precise use of invasive coronary angiograms. Additionally, the broad use of CAD-RADS could facilitate registry-based research on both diagnostic and prognostic characteristics of CTA.6
The extent of maximum coronary stenosis is included in the CAD RADS as follows:
CAD-RADS 0, CAD-RADS 1, CAD-RADS 2, CAD-RADS 3, CAD-RADS 4, CAD-RADS 5 and CAD-RADS N, which indicate 0% absence of CAD, 1–24% minimal non-obstructive CAD, 25–49% mild non-obstructive CAD, 50–69% moderate stenosis, 4A single vessel or two vessels with stenosis 70–99% and 4B: Left main>50% or 3-vessel obstructive disease >70% severe stenosis, 100% total coronary occlusion and obstructive CAD cannot be excluded, so additional or alternative evaluation is needed, respectively.6
Furthermore, patients are classified using the Coronary Artery Calcium – Data and Reporting System (CAC-DRS) based on either a visual or quantitative evaluation of coronary artery calcification as follow: CAC-DRS 0 (0), CAC-DRS 1 (1–99), CAC-DRS 2 (100–299), and CAC-DRS 3 ≥300.7
These scoring systems offer a straightforward way for the referring physician to determine the overall severity of the condition. They also give standardized suggestions for further care and investigations for each category, in addition to the clinical outcomes.5,6
The CCTA shows luminal stenosis and mural atheromatous abnormalities (coronary plaques) and their features. Approximately two-thirds of acute coronary syndrome (ACS) cases, generally described as a lipid-rich and thin fibrous cap, can be attributed to coronary plaque rupture. Signs of plaque vulnerability have been identified as some plaque features in CCTA: low CT density, remodeling index of more than 1.5, spotty calcification, and the sign of napkin ring. Therefore, CCTA plaque evaluation can predict the two-year outcome in patients suspected to have or with proven CAD.8
Recently, CCTA has developed as a potential non-invasive imaging technology typically used before coronary angiography.9
This study aimed to assess the diagnostic performance of CAD-RADS in the evaluation of significant CAD.
Patients and Methods
Study Population
Between June 2019 and December 2020, a sample of 205 patients having normal heart rates was registered for CT coronary angiography, mostly with typical or atypical chest pain. However, 28 patients were excluded from image analysis due to image quality restrictions caused by beam hardening, respiratory and motion artifacts, and a high total calcium score above 1000. To minimize the PCI complexity, our participants were referred by a cardiologist for pre-procedural examination when they complained of typical/atypical chest pain or before coronary revascularization approaches.
All participants had a mean age of 57.05 years, ranging from 28 to 75 years. Overall, there were 123 males and 54 females. Throughout the scan, the average heart rate was 60 beats per minute. A relatively high percentage of our cases (22%) were managed with revascularization procedures as PCI or CABG. The patients’ basic data, clinical characteristics, and investigations are tabulated in Tables 1 and 2. The flow chart of the research process is illustrated in (Figure 1).
 |
Table 1 Patients’ Basic and Clinical Characteristics |
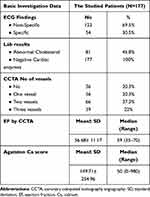 |
Table 2 Patients’ Basic Investigation Data |
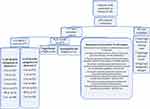 |
Figure 1 Flow chart of the research process shows the enrolled and omitted cases, ICA results, and CAD-RADS categories for each observer. |
The patients were subjected to:
- Clinical evaluation: a complete history of risk factors for CAD, chest pain analysis, ECG abnormalities, and previous revascularization operations such as PCI or CABG.
- Laboratory testing: including cardiac enzymes, renal function tests, as well as a lipid profile.
- CCTA.
- Standard coronary angiography.
The patients were selected based on clinical features suggestive of chest pain of coronary artery origin and negative cardiac enzymes.
Inclusion criteria included any age group, both sexes, symptomatic patients with clinically suspected CAD including angina class I, II, and III, patients who can hold breath for accepted time (20–30 seconds), normal serum creatinine, and sinus heart rhythm.
Exclusion criteria included absolute contraindications such as individuals with impaired renal function (creatinine 1.5 mg/dl) who were not on dialysis, pregnant women, severe obesity, and an Agatston ca score >1000.
Relative contraindications included: patients with renal diseases on dialysis, hemodynamic instability, inability to hold breath for 12 sec, tachycardia (> 70 b/m), arrhythmia, and thyrotoxic patients. Unstable patients (angina class IV and MI) and CADRADS modifier (N) were also excluded from the study.
Methods
Clinical Assessment
Detailed history, clinical assessment of chest pain, and verification of the previous laboratory and other diagnostic investigations as echocardiography were done.
Patient Preparation
Every participant received an in-depth description of each phase of the evaluation. Participants were asked to fast for four to six hours without discontinuing their medicines and stay away from caffeine and atropine for twelve hours before the scanning. Respiratory training, including breath-holding for 15–20 secs with a hand resting on the epigastric area, should be carefully checked to remove respiratory motion irregularities. A beta-blocker (100–200 mg metoprolol) has been administered orally one hour before the scan to maintain a heart rate less than 65 beats per minute unless contraindicated. Verification of the ECG equipment’s secure connection to the gantry and its link to the leads was performed.
CT Coronary Angiography Protocol
A 128-detector scanner has been used to scan patients (Philips Healthcare Ingenuity, Philips Medical System, Best, Netherlands). Initially, scout was done. After that, the score for calcium was assessed, and then CM administration utilizing a bolus tracking method, 70–80 mL of non-ionic CM injected with 5–6 mL /sec injection rate through the dual-head power injector pump with (50mL) saline chaser bolus, has been utilized to wash out the contrast medium from the right side of the heart and start image-acquiring once the threshold of contrast began to reach 180 HU with ROI put on the descending aorta and image acquisition starting from the carina till 1cm below the diaphragm (heart base) for coronary CTA with retrospective ECG gated technique during a breath-hold.
Image Reconstruction and Analysis
All CCTA images were analyzed using PACS (PaxeraUltima — Paxeramed) or a specialized Extended Brilliance Workstation platform (Philips Medical System, Best, The Netherlands). The axial images were checked for image quality as well as the determination of heart and thoracic anatomy. Imaging reconstruction approaches include multiplanar reformation (MPR), curved MPRs, 3D-MIP, and 3D-volume rendered images employing a 0.6mm thickness (3D-VR).
All CCTA examinations were revised and interpreted by two experienced observers with over three years of experience in cardiac imaging for each patient. The following characteristics obtained during CCTA were analyzed individually; (1) coronary calcification (total Agatston calcium score) for all cases excluding patients with stent or CABG; (2) the coronary arteries origin, course, termination, and dominance; (3) coronary artery diameter and extent of luminal stenosis (the percentage of stenosis was quantified using specialized software) classified as minimal (10%), mild (50%), moderate (50–69%), severe (> 70%), subtotal (> 90%), and complete occlusion per coronary segment; (4) plaque characterization utilizing quantitative CTA lesion analysis on all plaques, distinguishing distinct plaque constituents employing different attenuation thresholds and producing a color map of plaque components (−30–60 for lipid plaque, 61–149 for fibrous plaque, and 150–1300 for calcium). Plaque types were classified as: calcified (calcium > 60%), non-calcified (Calcium < 5%), and mixed (calcium 5–60%) according to the volume of the calcium within. Mixed plaque is further classified to mixed plaque dominantly calcified and mixed plaque dominantly non-calcified; (5) signs of plaque vulnerability (spotty calcification less than 3 mm, positive remodeling, napkin ring sign, and low attenuation < 60 or 30 HU) are considered high-risk criteria for V modifier in CAD RADS, involving minimum two of risk criteria; (6) other cardiac and extracardiac findings.
Regarding vessels greater than 1.5 mm in diameter, segmental assessment of the coronary arteries was done only on a patient-by-patient basis employing the society of cardiovascular computed tomography (SCCT) model.
The SCCT established the CAD-RADS classification system, which gave a CAD-RADS category and modifier to each patient. Also, the CAC-DRS category was assigned for each patient.
Before the trial began, referring doctors (cardiologists, cardiothoracic surgeons) were given many clinical sessions to explain the meaning and goal of CAD-RADS. Referring specialists were also given a management strategy based on CAD-RADS recommendations to assess if this reporting method could be used.
Gold Standard Reference
Diagnoses of CAD depending on ICA findings have been verified. ICA was performed at the recommendations of the referring consultants. A computer-assisted, semi-automated edge identification method was used in all ICA investigations with 6-French high-flow Judkins catheters (Cordis, United States) and a quantitative validated coronary angiographic system (Philips Azurion3, Philips Healthcare, The Netherlands). All ICA tests were conducted and evaluated by interventional cardiologists in various projections. Images were collected. Therefore, the extent of coronary stenosis was assessed for the relevance of CAD in two orthogonal perspectives.
Statistical Analysis
Microsoft Excel software was used to collect data and submit it for statistical analysis. After data were imported, they were analyzed using the Statistical Package for the Social Sciences (SPSS version 22.0) software. Qualitative data were represented by number and percentage, while quantitative data by mean ± SD. The intra-class correlation (ICC) statistic was used to examine the overall inter-observer reliability of CCTA findings and CAD-RADS score outcomes for reviewers. K values are assumed as follows: 0.01–0.20 = poor agreement; 0.21–0.40 = reasonable agreement; 0.41–0.60 = fair agreement; 0.61–0.80 = high agreement; and 0.81–1.0 = outstanding agreement. Pearson’s correlation coefficient was used to compute the correlations. The cut-off value and area under the curve were calculated using the receiver operating characteristic (ROC) curve (AUC). Chi-square (X2) test was used to detect the relationship between the calcium score grading and CAC-DRS with ICA results.
Results
The current study enrolled 177 patients with suspected CAD out of 205 who met the inclusion criteria. We successfully conducted all CCTA and ICA examinations without any negative consequences.
We had 111 cases with non-significant stenosis (CADRADS 0, 1, 2, and 3) and 66 cases with significant stenosis (CAD-RADS 4 and 5). The rate of occurrence of severe-obstructive CAD was 37.28%.
CAD-RADS Categories and Modifiers Application
CAD and modifiers categorization based on CADRADS is illustrated in Tables 3 and 4. Regarding observer 1, the highest percentages recorded were CADRADS 5 (27.1%), followed by CADRADS 3 (22%), then CADRADS 0 (20.3%). Regarding observer 2, the highest percentages recorded were CADRADS 3 (27.1%), followed by CADRADS 5 (20.3%), then CADRADS 0 (18.6%).
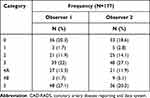 |
Table 3 Frequency Distribution of CAD-RADS Categories by Observer 1 and 2 |
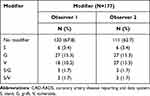 |
Table 4 Frequency Distribution of CAD-RADS Modifiers by Observer 1 and 2 |
PCI (Stent) or a bypass graft are two options for treating obstructive CAD. The modifier G (bypass graft) showed the highest percentage (15.3%).
CAD-RADS Diagnostic Performance in CAD Prediction
On a patient-by-patient basis, the diagnostic performance is excellent. The effectiveness of CAD-RADS in predicting significant obstructive CAD is described in Table 5. According to the observers, the CAD-RADS had a sensitivity, specificity, and accuracy of 90.9% to 100%, 89.2% to 94.6%, and 93.16% to 93.2%, respectively.
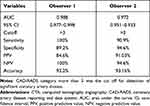 |
Table 5 Validity of CTA CAD-RADS Reported by Observer 1 and 2 |
Inter-Reviewer Agreement on the Conclusions of the CCTA and the results of the CADRADS Categories
Table 6 shows the intra-class correlation for CAD-RADS scoring results. In CADRADS 0, the ICC was 0.947. In CAD-RADS 2, it was 0.902, and in CAD-RADS 5, it was (0.824). The overall CADRADS ICC was (0.960), and regarding modifier, the ICC was (0.891).
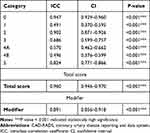 |
Table 6 Interclass Correlation Coefficient of CAD-RADS Reported by Observer 1 and Observer 2 |
Analysis of the Receiver Operating Characteristics
The ROC curve was used to establish the cut-off value for predicting severe obstructive CAD based on the diagnostic performance of CAD-RADS (Figures 2 and 3).
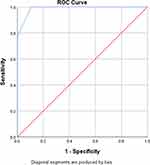 |
Figure 2 ROC curve of observer 1 shows the best cut-off for prediction of severe CAD is CAD-RADS 3 with AUC 0.988. |
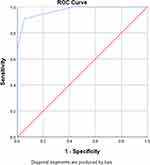 |
Figure 3 The ROC curve of observer 2 shows the best cut-off for prediction of severe CAD is CAD-RADS 3 with AUC 0.972. |
Both observers agreed that CAD-RADS 3 was the best cut-off value for predicting severe CAD based on ROC studies. According to the two observers, AUC was 0.988 for observer 1 and 0.972 for observer 2 Table 5.
Interpretation of the Relation Between CAC-DRS and Calcium Score Grading with ICA Results
Table 7 shows that there was a statistically significant relation between Ca score grading and CAC-DRS with ICA CAD result, with all CAC-DRS 0 cases had non-significant CAD apart from one case but most of cases with significant CAD had mild to severe Ca score.
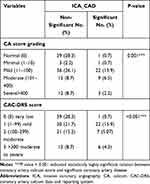 |
Table 7 Relation Between CA Score Grading, CAC-DRS Score, and ICA CAD |
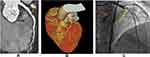
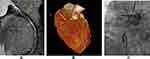
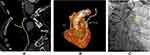
Some of the study representative cases were illustrated in (Figures 4–6A–C).
Discussion
Preventing the progression and consequences of atherosclerotic ischemic heart disease (IHD) requires early CAD detection. Lately, coronary artery imaging may be performed using both invasive and non-invasive methods. CCTA is a non-invasive technique that is beneficial in assessing the early stages of CAD.10
The need for a standardized CCTA report terminology to facilitate information exchange between interpreting and referring doctors resulted in creating a novel report system.6
By providing physicians with a direct link between objective radiological information and evidence-based treatment recommendations, structured reporting systems have become more popular.11 The CAD-RADS categorization is one of these more modern reporting methods. Recently, few papers have described the CAD-RADS in-depth and demonstrated the weak points and limitations of the system.12–14 Few studies on the external validation of the CAD-RADS have been conducted since Cury et al’s study in 2016. The current work aimed to verify the CAD-RADS, compare its results to those obtained using ICA, and evaluate the utility of such reporting system in clinical practice. Overall, the results were promising, indicating that this reporting system effectively categorizes and diagnoses patients with severe CAD.
Furthermore, we noticed that the treating cardiologists highly valued this categorization system. It improved their understanding of the CCTA examination report, clarified the observed findings, and increased their confidence in managing their patients. Another recent system is CAC-DRS, which was introduced in 2018 and was developed on the same lines of CAD-RADS.7 Calcium detection with CT is a sensitive and specific test to predict severe stenotic disease.7
Diagnostic validity was excellent for the CAD-RADS, with exceptionally high sensitivity, specificity, and accuracy (90.9 to 100%, 89.2 to 94.6%, and 93.16 to 93.2%, respectively, according to the reviewer). This conclusion is unsurprising because it is based on CCTA findings that have been thoroughly validated in some recent studies15–17 and demonstrated to be an effective technique for diagnosing coronary artery disease and assessing the severity of coronary artery stenosis. However, due to the relatively high incidence of severe CAD (37.28%) in the current study, the sensitivity and specificity calculations may include a selection bias. The higher prevalence of severe CAD in our patients may be explained by the fact that they were recruited from a major tertiary care center for complicated cases. Numerous variables led to the enhanced sensitivity and negative predictive values in the present study: We used a customized Philips workstation equipped with calcium-removing software. Those with a calcium score >1000 were eliminated. We excluded individuals with poor image quality due to irregular heart rate or inability to hold breath. We excluded those whose examinations were non-diagnostic (CADRADS N) due to motion/respiratory artifacts or because calcified plaques hindered complete plaque formation. Highly trained observers inspected all raw and post-processed CT scans. The CAD-RADS is simple to understand and apply. However, a key limitation of the study was that CAD-RADS is currently uncommon and unfamiliar to many physicians. As a result, before the initiation of the study, the rationale and aim of the CAD-RADS must be explained to referring cardiologists at several scientific meetings.
Without more evidence on CADRADS reliability, the study’s findings are rendered inapplicable in clinical practice, and it remains unclear whether to adopt or not to adopt this form of reporting. As a result, the present study included an inter-reviewer reliability analysis. Overall, the current results were presumed to be quite satisfactory, and the presentation of CAD-RADS classifications and modifiers demonstrated a strong inter-observer agreement (ICC = 0.960 and 0.891, respectively).
These findings are consistent with the overall findings of two prior studies,18,19 which examined the intraclass correlation agreement of CAD-RADS and modifiers. However, the IRA was moderate (ICC = 0.491, 0.570, and 0.496, respectively) in CAD-RADS 1, 4A, and 4B, respectively. This conclusion is inconsistent with the previous work.18,19 It was mostly due to the difficulty in assessing early non-obstructive stenosis and severe occlusion on CCTA.
While ICA is the gold standard for detecting coronary artery disease, it is also invasive and expensive. Although most associated problems are mild, significant complications can occur even in the absence of severe CAD. As a result, non-invasive examinations may be the most convenient to identify intermediate lesions.20 Applying CAD-RADS-guided specific recommendations into clinical practice may assist in reducing over-referral for ICA and promoting further suitable follow-up treatment for cases having CAD. This fact is crucial in stable patients with intermediate lesions (CAD-RADS 3) since significant rates of early ICA were found in these patients immediately after CCTA and probably before appropriate therapeutic trials.
Using the receiver operating characteristic (ROC) curve, both reviewers agreed that the optimum cut-off value for CADRADS for identifying patients with severe CAD was CAD-RADS 3. This cut-off value was correlated with a sensitivity of 90.9 and 100%. However, relatively few previous research have established a CAD-RADS cut-off value. As a result, we recommend conducting more studies to confirm or contradict our cut-off value as recommended before in prior research.21
CACS can be assessed at a low radiation dosage, which would be less harmful to low-risk individuals. If the CACS is 0, CCTA may not be necessary,22 which agrees with Bittner et al.23 They reported no cases of obstructive CAD assigned as CAC-DRS 0.
CACS is strongly predictive of the absence of severe coronary artery stenosis in the general population. Although a CACS of 0–10 was not shown to be significantly associated with coronary stenosis, it warrants additional investigations to evaluate non-calcified plaque burden in symptomatic patients.22
According to a series of investigations, individuals with CACS of zero had a 1% chance of developing severe coronary stenosis.24–26
In symptomatic individuals, a CACS of zero does not have the same strongly negative predictive value as in asymptomatic cases. Thus, obstructive CAD is possible among symptomatic patients with a CACS of zero, as it is associated with obstructive non-calcified plaques and increased CV risk factors.22
In the current study, CAC-DRS 0 was found in 39 cases (28.9%) without severe CAD and found in 1 case with obstructive CAD. A considerable number with CAC-DRS 1 (22 cases, 61.1%) showed severe CAD. Overall, there was a statistically significant association between CAC-DRS scoring and ICA results regarding significant CAD, and this was in line with a study conducted by Tay et al,22 who reported a significant association between significant CAD and mild and moderate calcium score grading with a P-value <0.001.
Based on our results and those of the other authors, it is believed that CAD-RADS categorization is a useful tool for categorizing CCTA data. As a result, we highly recommend the inclusion of the CAD-RADS in the CCTA reports.
Furthermore, the CAD-RADS has practical limitations because critical data (eg, coronary artery anomalies, myocardial perfusion, Agatston calcium score and non-coronary cardiac and extra cardiac CT findings) are not included in the classification of CAD-RADS categories. However, after the assignment of the CAD-RADS score, some researchers, such as Foldyna et al,13 added other imaging findings in their workflow, such as non-coronary and extracardiac data.
As a result, the CAD-RADS requires additional refinement to ensure accuracy, help, and completion in terms of all important definitions. Future large longitudinal studies examining long-term patient outcomes are necessary to demonstrate the added value of this data to the CAD-RADS categorization system and the referring clinicians’ ideas used in this reporting system.
Finally, we agreed with Cury et al, 2016,6 who found several benefits to CADRADS, including creating a common language and communication between physicians and radiologists to improve reporting clarity. This system enables and supports the decisions of the clinicians in addition to the management guidelines.
Regarding CAC-DRS, it showed some pitfalls. As in calcium detection, the net value of a new reporting system is limited unless it is coupled to a few management suggestions. Like the current breast imaging-RADS and CAD-RADS, the new reporting system is likely to be called CAC-RADS. However, because RADS is a signature of the American College of Radiology, it was given the term CAC-DRS.7
Limitations
First and foremost, this investigation was conducted in a single location. As a result, larger multicenter investigations are needed to confirm its validity and reproducibility. Secondly, the relatively increased prevalence of severe CAD in our sample could be a source of selection bias, affecting the computation of sensitivity and specificity. Thirdly, all CCTA examinations were reviewed by two professional specialists, which may impact diagnostic performance. As a result, more research on the performance of this reporting method when used by inexperienced reviewers is required. Fourthly, the omission of patients identified as CADRADS N from the study may have resulted in bias. Fifthly, our analysis contains a large number of CAD-RADS modifiers. CCTA interpretation in patients with previously diagnosed CAD requires considerable experience, particularly in the case of in-stent restenosis, which may be underestimated. Nonetheless, observers in our research have considerable experience. Sixthly, we depended on the Agatston method only in CAC-DRS categorization without evaluating the visual method of calcium detection. The extra coronary calcium (in valves and aorta) was also reported in the scoring system. Finally, the CAD-RADS and CAC-DRS remain novel and uncommon to many physicians, and they are continuously being modified.
Conclusion
The requirement to standardize CCTA report language to facilitate communication between translators and referring physicians created the new reporting system. The CAD-RADS approach is a novel, standardized method for describing the CCTA results. It may be used to triage patients with stable or even acute chest pain. Additionally, CAC-DRS is another recent reporting method that is usually utilized in risk factor categorization.
Institutional Review Board and Informed Consent Statement Statements
The protocol, as well as written consents utilized in this study, were indeed validated by Zagazig University’s IRB. All included individuals provided written consent as well as completed a questionnaire of demographic and clinical information. IRB statement code is 5719, date of approval 12/2/2019. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
2. Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368(21):2004–2013. doi:10.1056/NEJMra1216063
3. Cury RC. President’s page: ten years of innovation in cardiac CT. J Cardiovasc Comput Tomogr. 2014;8(4):338–339. doi:10.1016/j.jcct.2014.07.006
4. Hoffmann U, Ferencik M, Udelson JE, et al. Prognostic value of non-invasive cardiovascular testing in patients with stable chest. Insights from the PROMISE trial. Circulation. 2017;135(24):2320–2332. doi:10.1161/circulationaha.116.024360
5. Hecht HS, Blaha MJ, Kazerooni EA, et al. CAC-DRS: coronary artery calcium data and reporting system. An expert consensus document of the Society of cardiovascular computed Tomography (SCCT). J Cardiovasc Comput Tomogr. 2018;12(3):185–191; MID: 29793848. doi: 10.1016/j.jcct.2018.03.008
6. Cury RC, Achenhach S, Agatston A. Coronary artery disease reporting and data system. JACC Cardiovasc Imaging. 2016;9(9):1099–1113. doi:10.1016/j.jcmg.2016.05.005
7. Ramanathan S. Coronary artery calcium data and reporting system: strengths and limitations. World J Radiol. 2019;11(10):126–133. doi:10.4329/wjr.v11.i10.126
8. Ayad SW, Sobhy MA, El-Sharkawy EM, Abd-Elhamid MS. Role of plaque characterization by 64-slice multi detector computed tomography in prediction of complexity of percutaneous coronary interventions. J Integr Cardiol. 2015;1(5):118–123. doi:10.15761/JIC.1000132
9. Von Ballmoos MW, Haring B, Juillerat P, Alkadhi H. Meta-analysis: diagnostic performance of low-radiation-dose coronary computed tomography angiography. Ann Intern Med. 2011;154(6):413–420. doi:10.7326/0003-4819-154-6-201103150-00007
10. Gitsioudis G, Katus HA, Korosoglou G. Assessment of coronary artery disease using coronary computed tomography angiography and biochemical markers. World J Cardiol. 2014;6(7):663–670. doi:10.4330/wjc.v6.i7.663
11. Goldberg-Stein S, Walter WR, Amis ES
12. Maroules CD, Goerne H, Abbara S, Cury RC. Improving quality and communication in cardiac imaging: the coronary artery disease reporting and data system (CAD-RADS™). Curr Cardiovasc Imaging Rep. 2017;10(6):20. doi:10.1007/s12410-017-9417-1
13. Foldyna B, Szilveszter B, Scholtz JE, Banerji D, Maurovich-Horvat P, Hoffmann U. CAD-RADS–a new clinical decision support tool for coronary computed tomography angiography. Eur Radiol. 2018;28(4):1365–1372. doi:10.1007/s00330-017-5105-4
14. Ramanathan S, Al Heidous M, Alkuwari M. Coronary artery disease reporting and data system (CAD-RADS): strengths and limitations. Clin Radiol. 2019;74(6):411–417. doi:10.1016/j.crad.2019.01.003
15. Neglia D, Rovai D, Caselli C, et al. Detection of significant coronary artery disease by non-invasive anatomical and functional imaging. Circ Cardiovasc Imaging. 2015;8(3):e002179. PMID: 25711274. doi:10.1161/CIRCIMAGING.114.002179
16. Budoff MJ, Li D, Kazerooni EA, Thomas GS, Mieres JH, Shaw LJ. Diagnostic accuracy of non-invasive 64-row computed tomographic coronary angiography (CCTA) compared with myocardial perfusion imaging (MPI): the PICTURE study, a prospective multicenter trial. Acad Radiol. 2017;24(1):22–29. doi:10.1016/j.acra.2016.09.008
17. Takagi H, Tanaka R, Nagata K, et al. Diagnostic performance of coronary CT angiography with ultra-high-resolution CT: comparison with invasive coronary angiography. Eur J Radiol. 2018;101:30–37. PMID: 29571798. doi:10.1016/j.ejrad.2018.01.030
18. Maroules CD, Hamilton-Craig C, Branch K, et al. Coronary artery disease reporting and data system (CAD-RADSTM): inter-observer agreement for assessment categories and modifiers. J Cardiovasc Comput Tomogr. 2018;12(2):125–130; PMID: 29217341. doi: 10.1016/j.jcct.2017.11.014
19. Abdel Razek AAK, Elrakhawy MM, Yossof MM, Nageb HM. Inter-observer agreement of the coronary artery disease reporting and data system (CADRADSTM) in patients with stable chest pain. Pol J Radiol. 2018;83:e151–e159. doi:10.5114/pjr.2018.75641
20. Guerreiro S, Ferreira AM, Abecasis J, et al. Additional cardiac investigation prior to the introduction of the CAD-RADS classification in coronary computed tomography angiography reports. Rev Port Cardiol. 2019; 38(1):45–50. English, Portuguese. PMID: 30661933. 10.1016/j.repc.2018.05.014
21. Basha MAA, Aly SA, Ismail AAA, Bahaaeldin HA, Shehata SM. The validity and applicability of CAD-RADS in the management of patients with coronary artery disease. Insights Imaging. 2019;10(1):117. PMID: 31802266; PMCID: PMC6893004. doi:10.1186/s13244-019-0806-7
22. Tay SY, Chang PY, Lao WT, Lin YC, Chung YH, Chan WP. The proper use of coronary calcium score and coronary computed tomography angiography for screening asymptomatic patients with cardiovascular risk factors. Sci Rep. 2017;7(1):17653. PMID: 29247160; PMCID: PMC5732297. doi:10.1038/s41598-017-17655-w
23. Bittner DO, Mayrhofer T, Budoff M, et al.; PROMISE Investigators. Prognostic value of coronary CTA in stable chest pain: CAD-RADS, CAC, and cardiovascular events in PROMISE. JACC Cardiovasc Imaging. 2020; 13(7):1534–1545. PMID: 31734213; PMCID: PMC7202954. doi:10.1016/j.jcmg.2019.09.012
24. Dedic A, Ten Kate GJ, Roos CJ, et al. Prognostic value of coronary computed tomography imaging in patients at high risk without symptoms of coronary artery disease. Am J Cardiol. 2016;117(5):768–774; PMID: 26754124. doi: 10.1016/j.amjcard.2015.11.058
25. Shaw LJ, Giambrone AE, Blaha MJ, et al. Long-term prognosis after coronary artery calcification testing in asymptomatic patients: a cohort study. Ann Intern Med. 2015;163(1):14–21. PMID: 26148276. doi:10.7326/M14-0612
26. Nakanishi R, Li D, Blaha MJ, et al. The relationship between coronary artery calcium score and the long-term mortality among patients with minimal or absent coronary artery risk factors. Int J Cardiol. 2015;185:275–281. PMID: 25818539. doi:10.1016/j.ijcard.2015.03.146
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.