Back to Journals » Medical Devices: Evidence and Research » Volume 11
Validation of BP devices QardioArm® in the general population and Omron M6 Comfort® in type II diabetic patients according to the European Society of Hypertension International Protocol (ESH-IP)
Authors Chahine MN, Topouchian J, Zelveian P, Hakobyan Z , Melkonyan A, Azaki A , Diab R , Harb A, Asmar R
Received 18 May 2017
Accepted for publication 12 September 2017
Published 27 December 2017 Volume 2018:11 Pages 11—20
DOI https://doi.org/10.2147/MDER.S142126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mirna N Chahine,1,2 Jirar Topouchian,3,4 Parounak Zelveian,3 Zoya Hakobyan,3 Arevik Melkonyan,3 Alaa Azaki,1 Reem Diab,1 Aya Harb,1 Roland Asmar1,2
1Faculty of Medical Sciences, Lebanese University, Hadath, Lebanon; 2Foundation-Medical Research Institutes (F-MRI), Beirut, Lebanon; 3Preventive Cardiology Center, Yerevan, Armenia; 4Diagnostic Center, Hotel-Dieu Hospital, Paris, France
Background: Following the European Society of Hypertension International Protocol (ESH-IP) Revision 2010, QardioArm® and Omron M6 Comfort IT® oscillometric devices were evaluated in the general population and in patients with type II diabetes, respectively, for self-blood pressure (BP) measurement.
Methods: Both devices, QardioArm® and Omron M6 Comfort®, measure BP at the brachial level. The ESH-IP Revision 2010 includes a total number of 33 subjects. For each measure, the difference between observer and device BP values was calculated. In all, 99 pairs of BP differences are classified into three categories (≤5, ≤10, and ≤15 mmHg). The protocol procedures were followed precisely.
Results: QardioArm® and Omron M6 Comfort® fulfilled the requirements of the ESH-IP and passed the validation process successfully. For QardioArm®, a total of 69 out of 99 comparisons for systolic blood pressure (SBP) showed an absolute difference within 5 mmHg and 82 out of 99 for diastolic blood pressure (DBP). As for Omron M6 Comfort®, a total of 83 out of 99 comparisons for SBP showed an absolute difference within 5 mmHg and 77 out of 99 for DBP. The mean differences between the device and mercury readings were 0.7±5.9 mmHg for SBP and 0.3±4.1 mmHg for DBP for QardioArm® and −1.4±4.7 mmHg for SBP and −2.1±4.3 mmHg for DBP for Omron M6 Comfort®. With regard to part 2 of ESH-IP 2010, 27 out of 33 subjects had a minimum of two out of three measurements within 5 mmHg difference for SBP and 31 out of 33 subjects for DBP for the QardioArm®, and 29 out of 33 patients had a minimum of two out of three measurements within 5 mmHg difference for SBP and 26 out of 33 patients for DBP for Omron M6 Comfort®.
Conclusion: QardioArm® and Omron M6 Comfort® readings differing from the mercury standard by <5, 10, and 15 mmHg fulfill the ESH-IP Revision 2010 requirements. Consequently, these two devices are suitable for use in the general population and non-insulin-dependent type II diabetic patients, respectively.
Keywords: QardioArm®, Omron M6 Comfort®, validation, blood pressure measurement, type II diabetes, International Protocol
Introduction
Over the last years, many automated blood pressure (BP) measuring devices have been developed to gradually supplant the standard mercury sphygmomanometer due to mercury limitations.1,2 A validation process for these devices is therefore needed to verify their accuracy following different protocols, such as the European Society of Hypertension International protocol (ESH-IP),3 the British Hypertension Society (BHS) protocol,4 and the Association for the Advancement of Medical Instrumentation (AAMI) protocol.5 This validation process is required in the general population and in specific populations such as type II diabetic patients, for example, where accurate BP measurement is crucial, so the effective management of hypertension can delay disease complications.
Type II diabetes is a major health problem that has higher prevalence in the older population.6,7 It is well known that atherosclerotic disease is the major complication of type II diabetes.8,9 Atherosclerosis leads to the degeneration of arterial elasticity and subsequent arterial wall stiffening which is a strong risk factor for early mortality in patients with type II diabetes.8–10 Arterial wall stiffening is exacerbated with diabetes11 and hypertension.10,12,13 It is estimated that high BP occurs in over two-thirds of patients with type II diabetes.14 Recent evidence has showed that BP control decreases the risk of developing diabetes-related microvascular, and microvascular complications.15 Knowing that arterial stiffness is more critical in type II diabetic patients with hypertension than those without hypertension,16 and that increase in arterial stiffness may affect the accuracy of automatic BP measurements,17,18 accurate BP measurements in such populations is therefore crucial for the diagnosis and management. However, only a limited number of studies have tested the accuracy of automated BP monitors in the type II diabetic population,19–22 and the majority of these validations did not adhere to existing validation standards according to specific protocols cited above.3–5 Therefore, the issue of BP measurements accuracy remains controversial.23 Because of the high prevalence of type II diabetes, and the importance to reduce morbidity and mortality associated to hypertension, it is important to assess the accuracy of automatic BP measurements in this specific population. Most importantly, measuring BP accurately will facilitate the establishment of a threshold level of BP above which antihypertensive treatment can be recommended especially in type II diabetic patients, highly prone to developing hypertension and its complications if misdiagnosed.
In 2014, the Omron M6 Comfort® BP device was only validated in the general population following the ESH-IP;24 and QardioArm® device has never been validated previously in any population. The aim of this study is to check the validity of the QardioArm® BP measurement device in the general population and Omron M6 Comfort® oscillometric BP measurement device in non-insulin-dependent type II diabetic patients, following the ESH-IP 2010.
Materials and methods
Ethical information
The study was approved by the local ethic committees, namely National Institute of Health, Ministry of Health, Republic of Armenia and Institutional Review Board and Ethical Committee Geitaoui Hospital, Beirut, Lebanon.
All eligible subjects included in this study signed an informed consent form prior to any BP measurement.
Tested devices
The QardioArm® device
The QardioArm® device, provided by Qardio Inc (San Francisco, CA, USA), is a home BP measurement automatic device at the arm level. It needs a device with Bluetooth 4.0, iOS 7.0 (or later), Android 4.4 “KitKat” (or later), and is compatible with iPhone, iPod, iPad, and Apple Watch as well as with Android phones and tablets. In order to operate the device, a specific free Qardio application can be downloaded from the Apple App Store or Google Play (or www.getqardio.com). The unit records BP using the oscillometric method, with an automatic inflation and a controlled pressure release valve, with a pressure range of 40–250 mmHg and pulse rate range of 40–200 beats/min. The unit weighs ~310 g including batteries. Four 1.5 V AAA batteries are needed. The included single cuff is easy-fit Lycra, applicable to arm circumferences ranging from 22 to 37 cm. The dimensions are 140×68×38 mm, when closed.
The Omron M6 IT Comfort® device
This device (HEM-7322U-E) was provided and randomly selected by the manufacturer (Omron Healthcare, Kyoto, Japan). It is a home BP measurement digital automatic device at the arm level. The monitor uses a Fuzzy-logic inflation system controlled by an electric pump and an automatic pressure release valve for the deflation. BP is recorded using the oscillometric method with a pressure range of 0–299 mmHg and pulse rate range of 40–180 beats/min. A digital LCD screen displays the measured BP and pulse rate. The unit weighs ~380 g without batteries. The device requires four AA alkaline batteries with an approximate capacity of 1000 measurements. A semirigid nylon/polyester single cuff is included with dimensions of 145 mm ×532 mm (air tube: 750 mm) and weights ~163 g; it is applicable to arm circumferences ranging from 22 to 42 cm.
Study protocol
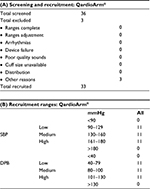
Subjects and patients who were included fulfilled the main criteria (age, gender, and recruitment BP requirements) of the ESH-IP with age ≥25 years, at least 10 men and 10 women, with 10–12 participants in each of the three BP recruitment ranges (Tables 1–4).
General population
A total of 33 participants fulfilled the ESH-IP recruitment criteria and were included in the validation of the QardioArm® device. All participants’ arm circumference was between 22 and 37 cm and none of them had atrial fibrillation or any other arrhythmia. Subjects were preselected from the general population in Yerevan, Armenia.
Non-insulin-dependent type II diabetic population
A total of 33 participants fulfilled the ESH-IP recruitment criteria and were included in the validation of the Omron M6 IT Comfort® device. All participants’ circumference was between 22 and 42 cm and none of them had atrial fibrillation or any other arrhythmia or used insulin as treatment for type II diabetes. Patients were preselected from outpatient clinics and from inpatients at the Lebanese University affiliated hospitals (Mount-Lebanon, Lebanese Hospital Geitaoui, Rafik Hariri University Hospital, Governmental Hospital of Baabda, Rasoul el-Aazam Hospital, and Bahman Hospital).
For BP measurement, three persons were included in the validation team: two observers trained in accurate BP measurement and a supervisor, all of them having completed a training based on a CD-ROM specifically developed by the French Society of Hypertension to certify observers involved in clinical studies and to familiarize them with the use of tested devices.
Each device validation study was assessed in different populations (general population/type II diabetic populations), separately from the other device validations and at another time. Therefore, each patient participated in only one study device validation. Evaluation of the devices was done according to the ESH-IP Revision 2010.
In our study, the two observers used two parallel connected mercury sphygmomanometers and a teaching stethoscope as a reference standard. They also measured the circumference of the arm to make certain that the cuff size being used is adequate for the subject. The agreement between the two observers, blinded from each other’s result, was checked all over the evaluation period by the supervisor to ensure that the difference between the two observers is ≤4 mmHg for systolic and diastolic BP values. Otherwise, the measurement was repeated. Measurements through the mercury sphygmomanometer were performed according to the “same arm, consecutive measurements” supported at the heart level. Measurements by both devices were on the same arm supported at the heart level, as recommended by the manufacturers asking the subject to relax for 5–10 minutes, while making sure that the subject is seated with uncrossed legs and supported back. Two cuffs were used for the standard mercury sphygmomanometer measurements: standard cuff for 22–32 cm and large cuff for 32–42 cm arm circumference. In total, in each patient, nine consecutive BP measurements were performed using the mercury sphygmomanometers (five times) and the tested device (four times), and were recorded as follows:
- BPA: entry BP, observers 1 and 2 each with the mercury standard
- BPB: device detection BP, supervisor
- BP1: observers 1 and 2 with mercury standard
- BP2: supervisor with the test instrument
- BP3: observers 1 and 2 with mercury standard
- BP4: supervisor with the test instrument
- BP5: observers 1 and 2 with mercury standard
- BP6: supervisor with the test instrument
- BP7: observers 1 and 2 with mercury standard
Certainly, the interval between one BP measurement and the next one was 30–60 seconds.
Data analysis
The accuracy of a device according to the ESH-IP is based on a comparison between the device and reference (mercury) measurements. Results were analyzed and expressed according to the ESH-IP requirements to conclude if the device passed or failed to pass the validation protocol. Part 1 and part 2 of the validation process concern the number of differences in the requested ranges for individual measurements (99 measurements) and for individual subjects (33 subjects), respectively.3 A detailed explanation is provided in the “Results” section.
A specific analysis software developed by the International Society for Vascular Health was used for the statistical analysis. Bland–Altman plots were used to represent the relation between delta systolic blood pressure (SBP; device – reference) and mean SBP (device and reference) or delta diastolic blood pressure (DBP; device – reference) and mean DBP (device and reference). BP1, BP3, BP5, and BP7 were the means of the two observer measurements. To calculate the means, for each subject, the device measurements BP2, BP4, and BP6 were first compared to observer measurements BP1, BP3, and BP5, respectively, and then to observer measurements BP3, BP5, and BP7, respectively. Comparisons more favorable to the device were used, which means that since there were two means of observers’ measurements that might be used, only the observers’ mean that was the closest to the device measurements value was used. To calculate the differences, the observer measurement was subtracted from the device measurement. Differences were classified, separately for both SBP and DBP, according to whether their values were within 5, 10, or 15 mmHg. Data were expressed as mean ± standard deviation.
Results
The QardioArm® device
Subjects’ details
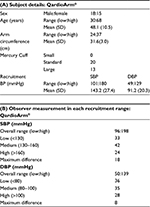
A total of 36 subjects were screened to recruit 33 participants who met the ESH-IP criteria and three were excluded for reasons mentioned in Table 1A. The 33 remaining participants were recruited following the three different BP ranges, such as 11 subjects in each of the ranges in Table 1B. There were 18 males and 15 females; the mean age of the participants was 48±10.5 years (from 30 to 68 years). Their arm circumference ranged from 24 to 37 cm, with a total of 13/33 participants having an arm circumference ≥32 cm, where one easy-fit Lycra cuff (22–37 cm) with the QardioArm® device was used, and the standard (22–32 cm) and large (32–42 cm) cuffs were used with the mercury sphygmomanometer, according to patients’ arm size. The mean recruitment SBP was 143.2±27.4 mmHg (101–180 mmHg) and the mean recruitment DBP was 91.2±20.3 mmHg (49–129 mmHg). More details about the participants’ characteristics are described in Table 2A.
BP requirements
Observer measurements in each recruitment range are described in Table 2B. The number of observer test measurements in each pressure range is, as required by the ESH-IP, between 22 and 44; more specifically, it is between 24 and 42 measurements. The difference between the range with the highest count and that with the lowest count is, as required by the ESH-IP, ≤19; more specifically, it is 18 for SBP and 8 for DBP. The overall SBP range is, as required by the ESH-IP, from ≤100 to ≥170 mmHg; more specifically, it ranges from 96 to 198 mmHg. The overall DBP range is, as required by the ESH-IP, from ≤50 to ≥120 mmHg; more specifically, it varies from 50 to 139 mmHg.
BP measurements
The difference between two observers was −0.3±1.7 and −0.3±1.7 mmHg for SBP and DBP, respectively (−4 to +4 mmHg). The mean differences between the observers and the tested device were 0.7±5.9 mmHg for SBP and 0.3±4.1 mmHg for DBP. The numbers of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 5. Indeed, a total of 69 out of 99 comparisons for SBP showed an absolute difference within 5 mmHg and 82 out of 99 for DBP (vs at least 73 for SBP and 65 for DBP following ESH-IP requirements). In addition, a total of 91 out of 99 comparisons for SBP showed an absolute difference within 10 mmHg and 97 out of 99 for DBP (vs at least 87 for SBP and 81 for DBP following ESH-IP requirements). Furthermore, a total of 98 out of 99 comparisons for SBP showed an absolute difference within 15 mmHg and 98 out of 99 for DBP (vs at least 96 for SBP and 93 for DBP following ESH-IP requirements). Since “two” out of these three absolute differences of 5 or 10 or 15 mmHg for SBP are within the required range mentioned above and “all” (three out of three) of them for DBP, part 1 device validation was successfully completed.
As for part 2 of ESH-IP 2010, 27 out of 33 subjects had a minimum of two out of three measurements within 5 mmHg difference for SBP and 31 out of 33 subjects for DBP (vs at least 24 out of 33 subjects for SBP and DBP following ESH-IP requirements). In addition, three out of 33 subjects had a minimum of zero out of three measurements within 5 mmHg difference for SBP and two out of 33 subjects for DBP (vs a maximum of three subjects for SBP and DBP following ESH-IP requirements). Since these two conditions mentioned above were validated, part 2 device validation was successfully completed. Therefore part 3 of the QardioArm® device validation passed, since parts 1 and 2 were both validated.
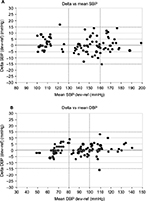
Bland–Altman plots of the differences between BP measurements obtained with the QardioArm® device and the sphygmomanometer are shown for SBP (Figure 1A) and for DBP (Figure 1B). These results are in concordance with the requested criteria of the ESH-IP and the device succeeded in fulfilling the validation criteria of the ESH-IP Revision 2010.
The Omron M6 IT Comfort® device
Patients’ details
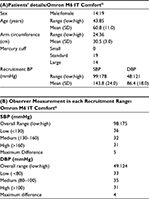
In all, 58 non-insulin-dependent type II diabetic patients were screened to recruit 33 participants who met the ESH-IP criteria and 25 were excluded for reasons mentioned in Table 3A. The 33 remaining participants were recruited following the three different BP ranges, such as 11 subjects in each of the ranges in Table 3B. There were 14 males and 19 females; the mean age of the participants was 61±11 years. Their arm circumference ranged from 24 to 36 cm, with a total of 14/33 participants having an arm circumference ≥32 cm, where one universal rigid cuff (22–42 cm) with the Omron M6 IT Comfort® device was used, and the standard (22–32 cm) (n=19 patients) and large (32–42 cm) (n=14 patients) cuffs were used with the mercury sphygmomanometer, according to patients’ arm size. The mean recruitment SBP was 143.8±24 mmHg (99–178 mmHg) and the mean recruitment DBP was 86.4±18 mmHg (48–121 mmHg). More details about participants’ characteristics are described in Table 4A.
BP requirements
Observer measurements in each recruitment range are described in Table 4B. The number of observer test measurements in each pressure range is, as required by the ESH-IP, between 22 and 44; more specifically, it is between 31 and 36 measurements. The difference between the range with the highest count and that with the lowest count is, as required by the ESH-IP, ≤19; more specifically, it is 5 for SBP and 4 for DBP. The overall SBP range is, as required by the ESH-IP, from ≤100 to ≥170 mmHg; more specifically, it varies from 98 to 175 mmHg. The overall DBP range is, as required by the ESH-IP, from ≤50 to ≥120 mmHg; more specifically, it ranges from 49 to 124 mmHg.
BP measurements
The difference between the two observers was −0.2±2.2 and +0.3±2.1 mmHg for SBP and DBP, respectively (−4 to +4 mmHg). The mean differences between the observers and the tested device were −1.4±4.7 mmHg for SBP and −2.1±4.3 mmHg for DBP. The numbers of measurements differing from the mercury standard by 5, 10, and 15 mmHg or less are shown in Table 6. Indeed, a total of 83 out of 99 comparisons for SBP showed an absolute difference within 5 mmHg and 77 out of 99 for DBP (vs at least 73 for SBP and 65 for DBP following ESH-IP requirements). In addition, a total of 94 out of 99 comparisons for SBP showed an absolute difference within 10 mmHg and 93 out of 99 for DBP (vs at least 87 for SBP and 81 for DBP following ESH-IP requirements). Furthermore, a total of 97 out of 99 comparisons for SBP showed an absolute difference within 15 mmHg and 99 out of 99 for DBP (vs at least 96 for SBP and 93 for DBP following ESH-IP requirements). Since “two” out of these three absolute differences of 5 or 10 or 15 mmHg for SBP are within the required range mentioned above and “all” (three out of three) of them for DBP, part 1 device validation was successfully completed.
As for part 2 of ESH-IP 2010, 29 out of 33 subjects had a minimum of two out of three measurements within 5 mmHg difference for SBP and 26 out of 33 subjects for DBP (vs at least 24 out of 33 subjects for SBP and DBP following ESH-IP requirements). In addition, none of the 33 subjects had a minimum of zero out of three measurements within 5 mmHg difference for SBP and two out of 33 subjects for DBP (vs a maximum of three subjects for SBP and DBP following ESH-IP requirements). Since these two conditions mentioned above were validated, part 2 device validation was successfully completed. Therefore, part 3 of the Omron M6 IT Comfort® validation passed since parts 1 and 2 were both validated.
Bland–Altman plots of the differences between BP measurements obtained with the Omron M6 IT Comfort® device and the sphygmomanometer are shown for SBP (Figure 2A) and for DBP (Figure 2B). These results are in concordance with the requested criteria of the ESH-IP and the device succeeded in fulfilling the validation criteria of the ESH-IP Revision 2010.
Discussion
This study is the first to provide information on the accuracy of both the QardioArm® device for BP measurement in the general population and the Omron M6 IT Comfort® device for BP measurement in the non-insulin-dependent type II diabetic population. Omron M6 Comfort® BP device was previously validated by our team in the general population following ESH-IP24; however, the QardioArm® device has never been validated previously in any population. The results of the study showed that QardioArm® and Omron M6 Comfort® device successfully passed the validation requirements in the general population and in non-insulin-dependent type II diabetic patients, respectively, according to the ESH-IP Revision 2010.
There are two important points related to both the devices (QardioArm® and Omron M6 Comfort®) and the validation protocol (ESH-IP) that need to be discussed.
First, QardioArm® has never been validated in any type of population and Omron M6 Comfort® has only been validated previously in the general population.12 In this study, QardioArm® has been validated successfully in the general population and Omron M6 Comfort® has been validated successfully in a specific population (type II diabetic patients). However, other specific populations such as obese, elderly, and pregnant women have not been addressed. Therefore, the results of our study cannot be extrapolated to other specific populations.
Second, in the present study, regarding the type II diabetic population, the patients recruited were known to have non-insulin-dependent type II diabetes; however, this was regardless of checking the presence of microalbuminuria (MAU). Whether the presence of MAU may affect the BP measurement25 accuracy remains questionable.
Third, we presumed that diabetes type II measurements involve stiffer arteries; however, this has not been investigated as it was not the main objective of our study. Certainly, measuring arterial stiffness would have been an added value to analyze the accuracy of the BP monitor accordingly.
In terms of the validation protocol, ESH-IP was used in this study. It has been published in 2002 and revised in 2010.3 The aim of this protocol was to simplify the previous protocols (BHS4 and AAMI5) without violating their integrity.3 Its main advantage is that the sample size required is smaller (n=33) compared to the two other protocols (n=85). However, some limitations were noted.
First, the ESH-IP does not specify the number of validation studies needed to approve the accuracy of the device although experts agreed that a device should be validated in two different centers separately, at least. Therefore, it is important to check the validity of BP measuring devices in specific populations as an add-on step in the validation process before widespread application in clinics/homes.
Second, there are specific requirements needed for the subjects recruited; their age should be at least 25 years and children and young adults are excluded from being studied. Moreover, since adult population is a part of a larger heterogeneous population affected by hypertension, extrapolation of the validation studies’ results in this selected population to a more specific population is risky and may negatively affect clinical practice. However, in our study, standard and large arm circumferences were covered.
Finally, there is no explicit criteria mentioned for a validation process in specific populations in the ESH-IP, and this is highly recommended to be taken into consideration in its next revision. Actually, the presence of several protocols for validation such as AAMI,5 BHS,4 ESH-IP3 is problematic for several reasons: 1) manufacturers cannot perform all three protocols; 2) experts are recognizing mainly their national protocols; for example, AAMI in the USA, ESH-IP in Europe; 3) impossibility to compare various studies; therefore, there is an ultimate need for a unified international protocol.26
Conclusion
This study showed that the QardioArm® device and Omron M6 Comfort® device successfully passed the validation requirements in the general population and in type II diabetic patients, respectively, according to the ESH-IP Revision 2010.
Acknowledgments
Qardio Inc and Omron Healthcare have financially supported this study. They initially provided three devices each; one of them was chosen randomly and selected for the study.
Disclosure
The authors report no conflicts of interest in this work.
References
O’Brien E, Asmar R, Beilin L, et al; European Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self-blood pressure measurement. J Hypertens. 2005;23:697–701. | ||
Stergiou GS, Parati G, Asmar R, O’Brien E ; European Society of Hypertension Working Group on Blood Pressure Monitoring. Requirements for professional office blood pressure monitors. J Hypertens. 2012;30:537–542. | ||
O’Brien E, Atkins N, Stergiou G, et al; Working Group on Blood Pressure Monitoring of the European Society of Hypertension. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. | ||
O’Brien E, Petrie J, Littler WA, et al. The British Hypertension Society protocol for the evaluation of blood pressure measuring devices. J Hypertens. 1993;11(Suppl 2):S43–S62. | ||
Association for the Advancement of Medical Instrumentation. 2013. ANSI/AAMI/ISO 11137-2:2013. | ||
Quinn L. Type 2 diabetes: epidemiology, pathophysiology, and diagnosis. Nurs Clin North Am. 2001;36:175–192. | ||
Wilkinson E, Waqar M, Sinclair A, Randhawa G. Meeting the challenge of diabetes in ageing and diverse populations: a review of the literature from the UK. J Diabetes Res. 2016;2016:8030627. | ||
Kochkina MS, Zateishchikov DA, Sidorenko BA. [Measurement of arterial stiffness and its clinical value]. Kardiologiia. 2005;45:63–71. Russian. | ||
Cardoso CR, Salles GF. Aortic stiffness as a surrogate endpoint to micro- and macrovascular complications in patients with type 2 diabetes. Int J Mol Sci. 2016;17:2044–2055. | ||
Stehouwer C, Ferreira HRM. Arterial stiffness in diabetes and the metabolic syndrome: a pathway to cardiovascular disease. Diabetologia. 2008;51:527–539. | ||
Lehmann ED, Gosling RG, Sonksen PH. Arterial wall compliance in diabetes. Diabet Med. 1992;9:114–119. | ||
Lim HS, Li GYH. Arterial stiffness in diabetes and hypertension. J Human Hypertens. 2004;18:467–468. | ||
Bruno RM, Penno G, Daniele G, et al. Type 2 diabetes mellitus worsens arterial stiffness in hypertensive patients through endothelial dysfunction. Diabetologia. 2012;55:1847–1855. | ||
Ferrannini E, Cushman WC. Diabetes and hypertension: the bad companions. Lancet. 2012;380:601–610. | ||
Salanitro AH, Roumie CL. Blood pressure management in patients with diabetes. Clin Diabetes. 2010;28:107–114. | ||
Chen Y, Huang Y, Li X, et al. Association of arterial stiffness with HbA1c in 1,000 type 2 diabetic patients with or without hypertension. Endocrine. 2009;36:262–267. | ||
van Popele NM, Bos WJW, de Beer NAM, et al. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36:484–488. | ||
Hamilton PK, Lockhart CJ, Quinn CE, McVeigh GE. Arterial stiffness: clinical relevance, measurement and treatment. Clin Sci. 2007;113(4):157–170. | ||
van Ittersum FJ, Wijering RM, Lambert J, Donker AJ, Stehouwer CD. Determinants of the limits of agreement between the sphygmomanometer and the Space Labs 90207 device for blood pressure measurement in health volunteers and insulin-dependent diabetic patients. J Hypertens. 1998;16:1125–1130. | ||
Lauszus FF, Rosgaard A, Lousen T, Rasmussen OW, Klebe TM, Klebe JG. Precision, consistency, and reproducibility of blood pressure in diabetic and non-diabetic pregnancy: the appraisal of repeated measurements. Acta Obstet Gynecol Scand. 2007;86:1063–1070. | ||
Theilade S, Joergensen C, Persson F, Lajer M, Rossing P. Ambulatory tonometric blood pressure measurements in patients with diabetes. Diabetes Technol Ther. 2012;14:453–456. | ||
Laugesen E, Rossen NB, Peters CD, et al. Assessment of central blood pressure in patients with type 2 diabetes: a comparison between SphygmoCor and invasively measured values. Am J Hypertens. 2014;27:169–176. | ||
de Greeff A, Shennan A. Blood pressure measuring devices: ubiquitous, essential but imprecise. Expert Rev Med Devices. 2008;5:573–579. | ||
Topouchian J, Agnoletti D, Blacher J, et al. Validation of four devices: Omron M6 Comfort, Omron HEM-7420, Withings BP-800, and Polygreen KP-7670 for home blood pressure measurement according to the European Society of Hypertension International Protocol. Vasc Health Risk Manag. 2014;10:33–44. | ||
Shin DI, Seung KB, Yoon HE, et al. Microalbuminuria is independently associated with arterial stiffness and vascular inflammation but not with carotid intima-media thickness in patients with newly diagnosed type 2 diabetes or essential hypertension. J Korean Med Sci. 2013;28:252–260. | ||
Ng KG. Clinical validation protocols for noninvasive blood pressure monitors and their recognition by regulatory authorities and professional organizations: rationale and considerations for a single unified protocol or standard. Blood Press Monit. 2013;18(5):282–289. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.