Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
UVR Promotes Keratinocyte Phagocytosis and Skin Pigmentation Through TRPA1 Channels
Authors Liu Y , Li Z, Wu W , Wang Y, Zhao G, Liu Y, Liu J, Song Z
Received 22 March 2022
Accepted for publication 15 June 2022
Published 27 June 2022 Volume 2022:15 Pages 1183—1193
DOI https://doi.org/10.2147/CCID.S365682
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Ying Liu,1,* Zhou Li,1,* Wei Wu,1,* Yupeng Wang,1 Guangming Zhao,1 Yuejian Liu,2 Jing Liu,3 Zhiqi Song1
1Department of Dermatology, First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 2Central Laboratory, First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China; 3Stem Cell Clinical Research Center, First Affiliated Hospital of Dalian Medical University, Dalian, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhiqi Song, Department of Dermatology, First Affiliated Hospital of Dalian Medical University, 222 Zhongshan Road, Dalian, 116011, People’s Republic of China, Email [email protected]
Purpose: Ultraviolet radiation (UVR) enhances skin pigmentation, which involves the production of melanin by melanocytes and subsequent transfer to keratinocytes. In the epidermis, keratinocyte phagocytosis plays a pivotal role in the process of melanosome transfer to protect DNA of epidermal cells against damage from UVR. Previous research suggested that transient receptor potential channels ankyrin 1 (TRPA1) was required for UVR-induced early melanin synthesis in melanocytes. Currently, there is no evidence that supports the detailed mechanism of TRPA1 for UVR-induced phagocytosis by keratinocytes. Here, we investigated the effect and the possible mechanisms of TRPA1 on keratinocyte phagocytosis and skin pigmentation after UVR exposure.
Methods: Flow cytometry was applied to investigate the effect of TRPA1 on intracellular calcium concentration ([Ca2+]ic) and fluorescent microspheres uptake was carried out to analyze phagocytosis in HaCaT cells (human immortalized keratinocytes). Western blotting was applied to measure the protein expression of calcium/calmodulin-dependent protein kinase II (CaMKII), phosphorylated CaMKII and β-catenin after UVA/UVB exposure. Masson-Fontana staining was applied to observe the effect of XAV-939 (decreasing the expression of β-catenin) on UVB-induced skin pigmentation in guinea pigs.
Results: TRPA1 channels activated by UVR increased the [ca2+]ic and phosphorylation of CaMKII in HaCaT cells. The UVR-induced phagocytosis was regulated by TRPA1 in HaCaT cells. TRPA1 promoted the protein expression of β-catenin after UVR exposure in HaCaT cells. XAV-939, inhibiting β-catenin expression, decreased the UVB-induced skin pigmentation on in vivo guinea pig models.
Conclusion: Taken together, TRPA1 activated by UVR led to the increase of intracellular calcium, which promoted the phosphorylation of CaMKII, enhancing keratinocyte phagocytosis. Moreover, TRPA1 regulated the protein expression of β-catenin to exert a lightening effect on skin pigmentation. Our findings suggest that TRPA1 may be a potential therapeutic target for UVR-induced skin pigmentary diseases.
Keywords: transient receptor potential arkyin 1, ultraviolet radiation, phagocytosis, skin pigmentation, CaMKII, β-catenin
Introduction
Ultraviolet radiation (UVR) is known to be the main extrinsic factor that induces skin pigmentation, which is a vital physiological process to protect nuclear DNA of epidermal cells from UV damage.1 Melanocytes stimulated by UVR synthesize melanin within melanosomes, which are subsequently transferred to adjacent keratinocytes, thus resulting in visually observable skin pigmentation.1 Proposed mechanisms of melanosome transfer include the following models: cytophagocytosis,2 direct membrane fusion,3 shed vesicles,4,5 and coupled exo/phagocytosis.6,7 Recent observations favor the coupled exo/phagocytosis model, which proposed that the melanosome core was released from melanocytes and then phagocytosed by neighboring keratinocytes.8 In this process, keratinocyte phagocytosis serves an important part, however, the detailed mechanism of which has not been fully elucidated.
Transient receptor potential ankyrin 1 (TRPA1), a calcium (Ca2+)-permeable non-selective cationic channel, is a unique member of the mammalian TRP ankyrin subfamily which plays key functions in chemo-, thermo-, and mechano-sensing.9,10 Previous studies indicated that TRPA1 was mainly expressed in sensory neurons, while recent researches confirmed its non-neuronal expressions in lung, brain and vascular endothelial tissues.9 Moreover, TRPA1 was detected to be expressed in human cutaneous cells, including melanocytes and keratinocytes.11 With regard to the biological function of TRPA1 in epidermal cells, it is demonstrated that TRPA1 activated by UVR caused a retinal-dependent current and a rapid calcium influx and was required for the UVR-induced early increase of cellular melanin in melanocytes.12 In mammalian phototransduction, retinal evoked a UVR-sensitive current in melanocytes, probably due to the conversion of trans-retinal to the cis- conformation catalyzed by the retinoid isomerohydrolase RPE65 (retinal pigment epithelium-specific 65kDa protein), which is also expressed in keratinocytes, as in the rod and cone cells of visual cycle.13–15 What is more, TRPA1 was found to be involved in the proliferation and differentiation of keratinocytes.11 However, whether it contributes to keratinocyte phagocytosis for promoting skin pigmentation remains unclear.
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a serine-threonine protein kinase, which plays a crucial role in cell-migration-related cytoskeleton dynamics via auto-phosphorylation mediated mechanism.16 β-catenin is a core component of the canonical Wnt signaling pathway, and it facilitates the process of gene transcription and cell–cell adhesion.17,18 β-catenin binds to the intercellular domain of E-cadherin (Epithelia-cadherin) and links α‑catenin, composing a cadherin-catenin complex, which directly anchors the actin cytoskeleton so that mediates the cell–cell adhesion.19 In the process of phagocytosis, actin cytoskeleton dynamics is required for the localized protrusion of the plasma membrane and the formation of the extended pseudopodia.20 Given the relevance of the CaMKII and β-catenin to the actin cytoskeleton, their involvement in phagocytosis is of great research potential. However, their effect on keratinocyte phagocytosis has not been investigated yet.
Since UVR could activate TRPA1 channels in melanocytes leading to melanin synthesis, the aim of this study was to evaluate whether TRPA1 channels activated by UVR could promote keratinocyte phagocytosis and skin pigmentation in vitro and in vivo, and investigate the possible mechanisms which may involve the phosphorylation of CaMKII and the increased expression of β-catenin in keratinocytes.
Materials and Methods
Main Antibodies and Materials
TRPA1 (19124-1-AP) was purchased from Proteintech Group (Rosemont, IL 60018, USA). CaMKII (4436), pCaMKII (12716), β-catenin (8480) were purchased from Cell Signaling Technology (Danvers, Massachusetts, USA). Retinal (R2500), JT010 (SML1672), HC030031 (H4415) were purchased from Sigma Aldrich (Darmstadt, Germany). Dimethyl sulfoxide (DMSO), RIPA buffer (R0100), BCA protein assay kit (PC0020), poloxamer gel (Polyethylene-polypropylene glycol 407, S7071) and formalin (G2161) were purchased from Solarbio (Beijing, China). Protease inhibitor cocktail (B14011) was purchased from Bimake (Shanghai, China). XAV-939 (S1180) was purchased from Selleck Chemicals (Shanghai, China). Fluo-4 AM (ab142773), DIO Staining Solution (C1038) were purchased from Beyotime (Shanghai, China). Stock solutions were prepared as follows: HC030031, and XAV-939 were dissolved in DMSO, JT010, and retinal in absolute ethanol. Masson-Fontana staining solution (G2032) was purchased from Solarbio (Beijing, China). Opti-MEM medium (31985062), Lipofectamine 2000 (11668030) and chemiluminescence (ECL) were obtained from Thermo Fisher Scientific (Massachusetts, USA). The UVA or UVB source used was a 9W UVA or UVB broadband lamp (Philips, Eindhoven, Netherlands) and radiation energies were measured using a UVX radiometer (UVP, Upland, California, USA).
HaCaT Cell Lines and Transfection
HaCaT cells (human immortalized keratinocytes) were purchased from the CABRI (European Union). Cells were cultured and grown in Minimum Eagle’s Medium (MEM, Gibco, USA), supplemented with 10% fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/streptomycin solution (Gibco, USA) at the humidified incubator with 5% CO2 (v/v) at 37℃. hTRPA1-specific primers were designed based on NM_007332.2: F 5’-GGTTTGGCAGTTGGCGACATTGCTGA3’ and R 5’-CTAAGGCTCAAGATGGTGTGTTTTTG-3’. The primers amplified a DNA band that was sequenced and found to be identical to hTRPA1. TRPA1 full-length cDNA was then recombined into pcDNA3.1-HA vector and expressed using a transient transfection system. HaCaT cells (50×104 cells/well) were overexpressed TRPA1 plasmid in an Opti-MEM medium containing Lipofectamine 2000 at 37℃. Cells were harvested 48 hours after transfection.
Measurement of Intracellular Calcium Concentration
HaCaT cells (50×104 cells/well) were seeded in 6-well plates (Corning Costar, USA) and incubated overnight. Cells were treated with different conditions (Retinal 12 µM, JT010 1 µM, HC-030031 10 µM, XVA-939 10 µM, UVA 225 mJ/cm2 or UVB 25 mJ/cm2) for 2 hours, and then loaded with 5 µM Fluo-4 AM calcium probes in PBS for 30 minutes and then washed twice with PBS. Cells were incubated with 0.8 mM Ca2+-containing solution (140 mM NaCl, 3 mM KCl, 0.4 mM Na2HPO4, 10 mM HEPES, 5 mM Glucose, and 1 mM MgCl2, and 0.8 mM CaCl2 with pH 7.4) by incubation for 30 min at room temperature in the dark. To remove the Ca2+-containing solution, cells were washed two times with PBS solution, harvested, resuspended, and then the fluorescence intensity was measured by flow cytometry (BD Aria II software, USA) with excitation of 340 nm and emission at 510 nm. At least 30,000 cells were collected per sample. Intracellular calcium concentration ([Ca2+]ic) was assessed by the fluorescence intensity ratio of the calcium probe Fluo-4 AM and expressed as relative fluorescence intensity normalized to control cells.
Calcium Imaging
HaCaT cells (20×104 cells/dish) were seeded onto culture glass dishes (Nest, China) and incubated overnight. The following day, cells were treated with JT010 (1 µM), HC-030031 (10 µM), XVA-939 (10 µM), UVA exposure (225 mJ/cm2) or UVB exposure (25 mJ/cm2), and loaded with 5 µM Fluo-4 AM calcium probes as described in intracellular calcium concentration measurement. After incubation with the Fluo-4 AM calcium probe, calcium imaging was measured by Confocal fluorescence microscopy (Lacia, TCS SP8), and randomly taken from 5 microscopic fields in each experiment. Three independent experiments were conducted for each experiment and the fluorescence areas were measured by Image J software.
Microsphere-Based Phagocytosis Assay
HaCaT cells (30×104 cells/well) were seeded onto coverslips in 24-well plates (Corning Costar) and incubated overnight. Cells were serum-starved for 6 hours, then incubated with Fluospheres® carboxylate-modified red fluorescent microspheres (0.5 µm diameter, Invitrogen) for 16 hours, when cells were treated with JT010 (1 µM), HC-030031 (10 µM), XVA-939 (10 µM), UVA exposure (225 mJ/cm2) or UVB exposure (25 mJ/cm2). Cells were vigorously washed 3 times with cold PBS to remove microspheres that had not been internalized. Cells were directly harvested and measured the fluorescence intensity by flow cytometry. In addition, cells were fixed in cold 4% paraformaldehyde for 30 min, stained with Dio for 15 min, and observed by confocal fluorescence microscopy. Quantitative analysis was performed by counting the number of internalized microspheres in 100 cells per condition, which were randomly taken from 6 microscopic fields in three independent experiments.
Western Blotting Analysis
Total protein was solubilized from cell lysates using RIPA buffer supplemented with protease inhibitor cocktail. Protein concentration was measured using the BCA protein assay kit. A total of 40 mg proteins were electrophoresed in 8% SDS-PAGE gels and transferred to polyvinylidene difluoride (PVDF) membranes, and then were blocked in 5% bovine serum albumin (BSA) in Tris-buffered saline containing 0.1% Tween-20 (TBST) for 1 h. Subsequently, membranes were incubated with TRPA1, CaMKII, pCaMKII or β-catenin antibodies (all diluted to 1:1000 with 5%BSA solution) overnight at 4℃, followed by horseradish peroxidase-conjugated secondary antibodies (diluted to 1:2000 with 5%BSA solution) for 1 hour at room temperature. Finally, immunopositive bands were analyzed by enhanced chemiluminescence (ECL) and visualized using Tanon 5200 software (China). Band quantification for three experiments was done using Image J software (USA).
Animals
Experimental procedures were approved by the Animal Ethics Committee of Dalian Medical University (AEE22013), and animal care followed the National Institute of Health guidelines on the care and use of laboratory animals. The dorsal skin of brown guinea pigs (7 weeks old, weighing approximately 500–550 g) was divided into 3 areas, Control, Vehicle (33% poloxamer gel), XAV-939 (10 mM in 33% poloxamer gel). The corresponding areas were treated locally for 30 min every other day, and the UVB lamp was placed 15 cm above the guinea pig. The total energy dose of UVB exposure was 500 mJ/cm2 for 2 weeks. The L value was measured in each application area (automatically averaging 3 times per point) by the colorimeter (Thermo), which was used to evaluate the lightness of skin. Experimental areas of skin were biopsied, processed, and fixed overnight in neutral-buffered 10% formalin, and then embedded in paraffin. They were then applied to Hematoxylin Eosin (H&E) staining and Masson-Fontana (M&F) staining. M&F staining was performed according to the manufacturer’s instructions. The melanin granules were measured by Image J software. Quantitative analysis was performed by counting the positive stained areas of M&F staining in the epidermis, which were randomly taken from 5 microscopic fields in three independent experiments.
Statistical Analysis
All data are presented as means ± standard deviation (SD), and the data are the mean values from at least three independent experiments. Statistical analyses were performed using GraphPad Prism 8.0 software (San Diego, CA). Significant differences between the groups were performed using Brown-Forsythe’s test, Student’s t-test and one-way ANOVA with Tukey’s post hoc test. A P-value <0.05 was considered statistically significant.
Results
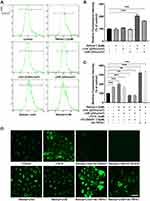
TRPA1 Channels Contributed to UVA/UVB-Induced Ca2+ Responses in HaCaT Cells
To unveil the effect of UVA/UVB on the Ca2+ responses in HaCaT cells, the cells were processed with 225 mJ/cm2 UVA or 25 mJ/cm2 UVB (equivalent to 250 s of full sun exposure).12 In Figure 1A and B, the data from flow cytometry assay showed that the [Ca2+]ic had no change after treatment with retinal (the chromophore required for light activation of opsin G protein-coupled receptors)21 or UVA/UVB compared to the control group, respectively. Furthermore, the [Ca2+]ic was increased 102%/66%, respectively, after UVA/UVB exposure in comparison with the control group, when HaCaT cells were preincubated with retinal (Figure 1A and B, p < 0.001). To investigate the effect of TRPA1 on UVA/UVB-induced Ca2+ responses, HaCaT cells were transfected with plasmids to overexpress TRPA1 or treated with JT010 (1 µM, a selective TRPA1 agonist), HC-030031 (10 µM, a selective TRPA1 antagonist). As shown in Figure 1C, the [Ca2+]ic was significantly increased 70% after treatment with JT010 (p < 0.001), whereas pretreatment with HC-030031 decreased 35%/36% of the UVA/UVB-induced [Ca2+]ic, respectively (p < 0.01), in HaCaT cells. Besides, UVA/UVB exposure significantly increased 224%/232% [Ca2+]ic in HaCaT cells, which were transfected with plasmids overexpressing TRPA1 (Figure 1C, p < 0.001). Subsequently, HaCaT cells were loaded with Fluo4-AM, which showed fluorescence on binding with free calcium, and the fluorescence-positive areas were observed using a fluorescence confocal microscopy. The results of the fluorescence-positive area showed that exposure to UVA/UVB, treatment with JT010, or overexpressing TRPA1 enhanced the Ca2+ responses, but it was significantly decreased after treatment with HC-030031 in HaCaT cells (Figure 1D). These results indicated that UVR-induced Ca2+ responses were regulated by TRPA1 in HaCaT cells.
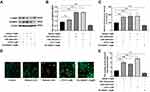
UVR-Induced Phosphorylation of CaMKII and Keratinocyte Phagocytosis Was Regulated by TRPA1 in HaCaT Cells
To further explore the effect of TRPA1 mediated Ca2+ responses on UVR-induced keratinocyte phagocytosis, we detected the expression of phosphorylated CaMKII (pCaMKII) using Western bolt, and measured the fluorescent intensity and the fluorescent microspheres uptake by flow cytometry and confocal fluorescence microscopy. As shown in Figure 2A and B, the results showed that the ratio of pCaMKII/CaMKII was significantly increased 1.41-, 1.82- or 1.93-fold after treatment with JT010 (p < 0.05) or UVA/UVB (p < 0.001), respectively. However, the decline of the ratio of pCaMKII/CaMKII was observed in HaCaT cells after treated with HC-030031, and the fold decreases were 0.5 compared with the control group (p < 0.001) (Figure 2A and B). Furthermore, fluorescent microspheres (0.5 µm, red) are often used as pseudo-melanocores to study keratinocyte phagocytosis,22,23 and the results showed that the fluorescence intensity was increased 82%/81% after UVA/UVB exposure compared with the control group, respectively (Figure 2C, p < 0.001). At the same time, the fluorescent intensity was increased 70% after treatment with JT010, but it was decreased 36% after treatment with HC-030031, in comparison with the control group (Figure 2C, p < 0.001). Moreover, the fluorescent microsphere uptake was increased 2.21-/2.00-fold after UVA/UVB exposure, as well as treatment with JT010 (2.92-fold) (Figure 2D and E, p < 0.001). The fluorescent microsphere uptake decreased 0.5-fold after treatment with HC-030031 compared with the control group (Figure 2D and E, p < 0.05). These discoveries above proved that TRPA1 regulated UVR-induced phosphorylation of CaMKII and the fluorescent microsphere uptake in HaCaT cells.
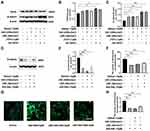
TRPA1 Enhanced the Expression of β-Catenin Promoting Phagocytosis in HaCaT Cells
To determine the effect of β-catenin on UVR-induced phagocytosis mediated by TRPA1, we measured the expression of β-catenin and observed the fluorescent microsphere uptake in HaCaT cells. The results showed that the expression of TRPA1 increased 1.28-/1.30-fold after UVA/UVB exposure in HaCaT cells, which were transfected with HA-TRPA1 plasmids compared to the control group (Figure 3A and B, p < 0.05). Subsequently, compared with the control group, the expression of β-catenin increased 1.64-, 1.72-/1.68-fold after treatment with JT010, UVA/UVB exposure in HaCaT cells (Figure 3A and C, p < 0.05). Interestingly, when HaCaT cells were transfected with HA-TRPA1 plasmids, the protein expression of β-catenin significantly increased 2.54-/2.49-fold after UVA/UVB exposure (Figure 3A–C, p < 0.001). Moreover, the expression of β-catenin significantly decreased 0.48-, 0.61-/0.45-fold in HaCaT cells, which were treated with XAV-939 (10 µM, decreasing β-catenin expression) alone, or before UVA/UVB exposure (Figure 3D and E, p < 0.001). Meanwhile, the fluorescent intensity was decreased 44%, 37%/31% (Figure 3F, p < 0.001), and the fluorescent microsphere uptake decreased 0.32-, 0.27-/0.39-fold compared with the control group (Figure 3G and H, p < 0.001), when HaCaT cells were treated with XAV-939 alone, or before UVA/UVB exposure. These findings indicated that TRPA1 enhanced the protein expression of β-catenin to promote UVR-induced phagocytosis in HaCaT cells.
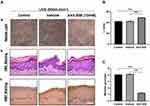
β-Catenin Regulated the UVB-Induced Skin Pigmentation on Guinea Pig Models
To ascertain the effect of β-catenin on pigmentation, we carried out the topical application of XAV-939 in vivo UVB-induced skin pigmentation guinea pig models. In the photograph of dorsal skin, XAV-939 (10 mM) showed depigmenting effects on UVB-induced skin pigmentation after 2 weeks of topical application, compared with the control and vehicle groups (Figure 4A). As for colorimetric measurements, the L value (skin lightness) significantly increased 17% and 16% in XAV-939 areas compared to control and vehicle groups (Figure 4B). The skin biopsy specimens were obtained from treated areas and were processed for light microscopy examination with H&E staining and M&F staining. The results showed no obvious changes of epidermal thickness in the control, vehicle and XAV-939 groups determined by H&E staining (Figure 4A). Furthermore, XAV-939 significantly reduced 69% and 70% the melanin granules revealed by M&F staining, which stains melanin granules as black, comparing with control and vehicle groups (Figure 4A and C). This result indicated that XAV-939 displayed a skin lightening effect on UVB-induced skin pigmentation in guinea pig models.
Discussion
Melanocytes in the epidermis of skin synthesize melanosomes and transfer them to the nuclear area of approximately 36 surrounding keratinocytes under the stimulation of UVR in the sunlight, which causes skin pigmentation, severing as a photo-protecting mechanism.1 In the process of melanosome transfer, keratinocyte phagocytosis has recently been considered as a crucial section.24 Notably, a recent study suggested that melanocores (the melanin core devoid of surrounding membrane) were internalized by phagocytosis, whereas melanosomes (melanin core with intact surrounding membrane) were internalized by macropinocytosis in keratinocytes.25 In this study, we confirmed that UVR enhanced the phagocytic ability of keratinocytes, which was regulated by TRPA1.
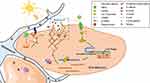
As a Ca2+-permeable cationic channel, TRPA1 was reported to be activated by UVR and increased the [Ca2+]ic in human melanocytes.12 Our results identified similar findings in keratinocytes. Ca2+ is a key second messenger involved in the regulation of numerous cellular functions including adhesion, vesicular trafficking and cytoskeletal rearrangement.20 Calmodulin (CaM), a Ca2+ sensor protein, plays an important role in signal transduction pathways by binding with Ca2+.26 CaMKII is activated by the binding of Ca2+ saturated CaM (Ca2+/CaM) in an auto-phosphorylation manner.27 It is reported that CaMKII is a multi-subunit holoenzyme, which contains a kinase domain, an autoinhibitory/regulatory domain, an actin binding domain and an association domain.27,28 CaMKII possesses two kinds of functional activities including kinase activity and structural function. At basal status, the autoinhibitory domain masks the kinase domain, which inhibits the kinase activity. Meanwhile, the actin binding domain interacts with more than one filament that is composed of filamentous actin (F-actin), and bundles them together, to stabilize the F-actin of cytoskeleton.27 When Ca2+/CaM binds to the regulatory domain, the kinase domain is unmasked and the catalytic activity of the kinase is disinhibited. Once activated, CaMKII not only phosphorylates various substrates but also autophosphorylates itself at the autoinhibitory domain, turning into pCaMKII.27,29 In the meantime, autophosphorylated CaMKII dissociates from F-actin, which is subsequentially unbundled and remodeled by actin regulators, therefore contributing to the cytoskeleton dynamics.30 In the present study, it was demonstrated that UVR increased the [Ca2+]ic in keratinocytes, which was mediated by TRPA1. Moreover, TRPA1 regulated UVR-induced phosphorylation of CaMKII and keratinocyte phagocytosis. Thus, we assumed that UVR enhanced keratinocyte phagocytosis through TRPA1 mediated calcium signaling pathway. The possible mechanism might be that UVR activated TRPA1 and increased the intercellular Ca2+, which phosphorylated CaMKII by binding with CaM, accompanied with the remodeling of F-actin, facilitating phagocytosis in keratinocytes (Figure 5).
Direct cell–cell contact between MC and KC is a requirement for optimal melanosome transfer, which is accomplished by the adhesion ability of the cadherin-catenin complex, where β-catenin binds to E-cadherin and interacts with α-catenin.31,32 And α-catenin bridges these components to actin cytoskeleton, which recruits and organizes actin filaments.33 Actin remodeling at the cell membrane for melanosome uptake and phagosome vesicular trafficking are key processes in keratinocyte phagocytosis.1,34 Previous studies on neuronal cells indicated that β-catenin played a crucial role in the recruitment, localization and distribution of synaptic vesicles in synapses.35 And researches on skeletal muscle cells suggested that β-catenin was involved in regulating glucose transporter 4 containing vesicles recruitment by interacting with cadherin to support cortical actin remodeling at the cell membrane, which provided the physical structure to facilitate the movement of vesicles within the cell.36 Previous work had also identified a role for β-catenin and cadherins in actin remodeling to facilitate insulin vesicle trafficking in pancreatic β-cells.36 Thus, these findings suggested that β-catenin was a regulator of vesicle trafficking and acted as a signaling intermediate controlling actin remodeling in multiple tissues. In keratinocytes, it was demonstrated previously that UVB enhanced the protein expression of β-catenin.37 In the present study, our results confirmed the increased accumulation of β-catenin by UVR in keratinocytes and illustrated that the UVR-induced enhancement of β-catenin was regulated by TRPA1. What is more, the alteration of β-catenin regulated by TRPA1 affected UVR-induced keratinocyte phagocytosis. Therefore, we proposed that UVR interacted with TRPA1 increased the expression of β-catenin which promoted keratinocyte phagocytosis, most likely by enhanced cell–cell adhesion and cytoskeleton dynamics mediated by β-catenin (Figure 5). In addition, our observations on UVB-induced guinea pig skin pigmentation suggested that the inhibition of β-catenin possessed skin lightening effect in vivo, which indicated the therapeutic potential of β-catenin for skin pigmentary diseases (such as melasma).
Conclusion
In conclusion, the data presented herein demonstrated that UVR promoted keratinocyte phagocytosis and skin pigmentation by TRPA1 channels. We speculate that TRPA1 is activated by UVR to promote the increase of intracellular calcium, which causes the activation of CaMKII to affect the remodeling of F-actin, contributing to keratinocyte phagocytosis. Furthermore, TRPA1 activated by UVR upregulates the expression β-catenin to enhance skin pigmentation by cell–cell adhesion and cytoskeleton dynamics. These findings suggest that TRPA1 may be a potential therapeutic target for UVR-induced skin pigmentary diseases.
Acknowledgment
This work was supported by the National Natural Science Foundation of China [No. 81773305 and No. 82073416].
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Moreiras H, Seabra MC, Barral DC. Melanin transfer in the epidermis: the pursuit of skin pigmentation control mechanisms. Int J Mol Sci. 2021;22(9):9. doi:10.3390/ijms22094466
2. Lambert MW, Maddukuri S, Karanfilian KM, Elias ML, Lambert WC. The physiology of melanin deposition in health and disease. Clin Dermatol. 2019;37(5):402–417. doi:10.1016/j.clindermatol.2019.07.013
3. Scott G, Leopardi S, Printup S, Madden BC Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115(Pt 7):1441–1451. doi:10.1242/jcs.115.7.1441
4. Ando H, Niki Y, Ito M, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132(4):1222–1229. doi:10.1038/jid.2011.413
5. Wu XS, Masedunskas A, Weigert R, Copeland NG, Jenkins NA, Hammer JA. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci USA. 2012;109(31):E2101–2109. doi:10.1073/pnas.1209397109
6. Tarafder AK, Bolasco G, Correia MS, et al. Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J Invest Dermatol. 2014;134(4):1056–1066. doi:10.1038/jid.2013.432
7. Hurbain I, Romao M, Sextius P, et al. Melanosome distribution in keratinocytes in different skin types: melanosome clusters are not degradative organelles. J Invest Dermatol. 2018;138(3):647–656. doi:10.1016/j.jid.2017.09.039
8. Takeuchi S, Fukumoto T, Nishigori C, et al. Dynamic visualization of melanosome endo/phagocytosis during melanin transfer using melanosomes pre-stained with carbocyanine dyes. J Dermatol Sci. 2022;105(1):65–67. doi:10.1016/j.jdermsci.2021.12.004
9. Hu F, Song X, Long D. Transient receptor potential ankyrin 1 and calcium: interactions and association with disease (review). Exp Ther Med. 2021;22(6):1462. doi:10.3892/etm.2021.10897
10. Naert R, Lopez-Requena A, Talavera K. TRPA1 expression and pathophysiology in immune cells. Int J Mol Sci. 2021;22(21):21. doi:10.3390/ijms222111460
11. Atoyan R, Shander D, Botchkareva NV. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J Invest Dermatol. 2009;129(9):2312–2315. doi:10.1038/jid.2009.58
12. Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci USA. 2013;110(6):2383–2388. doi:10.1073/pnas.1215555110
13. Fu Y, Zhong H, Wang M-H-H, et al. Intrinsically photosensitive retinal ganglion cells detect light with a vitamin A-based photopigment, melanopsin. Proc Natl Acad Sci USA. 2005;102(29):10339–10344. doi:10.1073/pnas.0501866102
14. Hinterhuber G, Cauza K, Brugger K, et al. RPE65 of retinal pigment epithelium, a putative receptor molecule for plasma retinol-binding protein, is expressed in human keratinocytes. J Invest Dermatol. 2004;122(2):406–413. doi:10.1046/j.0022-202X.2004.22216.x
15. Yau KW, Hardie RC. Phototransduction motifs and variations. Cell. 2009;139(2):246–264. doi:10.1016/j.cell.2009.09.029
16. Cui C, Wang C, Cao M, Kang X. Ca(2+)/calmodulin-dependent protein kinases in leukemia development. J Cell Immunol. 2021;3(3):144–150. doi:10.33696/immunology.3.091
17. Monga SP. Beta-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148(7):1294–1310. doi:10.1053/j.gastro.2015.02.056
18. Shang S, Hua F, Hu ZW. The regulation of beta-catenin activity and function in cancer: therapeutic opportunities. Oncotarget. 2017;8(20):33972–33989. doi:10.18632/oncotarget.15687
19. Ratheesh A, Yap AS. A bigger picture: classical cadherins and the dynamic actin cytoskeleton. Nat Rev Mol Cell Biol. 2012;13(10):673–679. doi:10.1038/nrm3431
20. Nunes-Hasler P, Kaba M, Demaurex N. Molecular mechanisms of calcium signaling during phagocytosis. Adv Exp Med Biol. 2020;1246:103–128.
21. Terakita A. The opsins. Genome Biol. 2005;6(3):213. doi:10.1186/gb-2005-6-3-213
22. Virador VM, Muller J, Wu X, et al. Influence of alpha-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002;16(1):105–107. doi:10.1096/fj.01-0518fje
23. Cardinali G, Bolasco G, Aspite N, et al. Melanosome transfer promoted by keratinocyte growth factor in light and dark skin-derived keratinocytes. J Invest Dermatol. 2008;128(3):558–567. doi:10.1038/sj.jid.5701063
24. Goenka S, Simon SR. Novel Chemically Modified Curcumin (CMC) analogs exhibit anti-melanogenic activity in primary human melanocytes. Int J Mol Sci. 2021;22(11):11. doi:10.3390/ijms22116043
25. Moreiras H, Bento-Lopes L, Neto MV, et al. Melanocore uptake by keratinocytes occurs through phagocytosis and involves protease-activated receptor-2 internalization. Traffic. 2022;23(6):331–345. doi:10.1111/tra.12843
26. Durvanger Z, Harmat V. Structural diversity in calmodulin - peptide interactions. Curr Protein Pept Sci. 2019;20(11):1102–1111. doi:10.2174/1389203720666190925101937
27. Hayashi Y Molecular mechanism of hippocampal long-term potentiation - towards multiscale understanding of learning and memory. Neurosci Res. 2021;175:3–15.
28. Lin YC, Redmond L. Neuronal CaMKII acts as a structural kinase. Commun Integr Biol. 2009;2(1):40–41. doi:10.4161/cib.2.1.7426
29. Hanson PI, Kapiloff MS, Lou LL, Rosenfeld MG, Schulman H. Expression of a multifunctional Ca2+/calmodulin-dependent protein kinase and mutational analysis of its autoregulation. Neuron. 1989;3(1):59–70. doi:10.1016/0896-6273(89)90115-3
30. Kim K, Lakhanpal G, Lu HE, et al. A temporary gating of actin remodeling during synaptic plasticity consists of the interplay between the kinase and structural functions of CaMKII. Neuron. 2015;87(4):813–826. doi:10.1016/j.neuron.2015.07.023
31. Gates J, Peifer M. Can 1000 reviews be wrong? Actin, alpha-Catenin, and adherens junctions. Cell. 2005;123(5):769–772. doi:10.1016/j.cell.2005.11.009
32. Singh SK, Baker R, Sikkink SK, et al. E-cadherin mediates ultraviolet radiation- and calcium-induced melanin transfer in human skin cells. Exp Dermatol. 2017;26(11):1125–1133. doi:10.1111/exd.13395
33. Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123(5):903–915. doi:10.1016/j.cell.2005.09.021
34. Pradhan G, Raj Abraham P, Shrivastava R, Mukhopadhyay S. Calcium signaling commands phagosome maturation process. Int Rev Immunol. 2019;38(2):57–69. doi:10.1080/08830185.2019.1592169
35. Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20(14):3390–3400. doi:10.1091/mbc.e08-12-1172
36. Masson SWC, Sorrenson B, Shepherd PR, Merry TL. beta-catenin regulates muscle glucose transport via actin remodelling and M-cadherin binding. Mol Metab. 2020;42:101091. doi:10.1016/j.molmet.2020.101091
37. Smith KA, Tong X, Abu-Yousif AO, et al. UVB radiation-induced beta-catenin signaling is enhanced by COX-2 expression in keratinocytes. Mol Carcinog. 2012;51(9):734–745. doi:10.1002/mc.20840
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.