Back to Journals » Cancer Management and Research » Volume 7
Utilization of bone densitometry for prediction and administration of bisphosphonates to prevent osteoporosis in patients with prostate cancer without bone metastases receiving antiandrogen therapy
Authors Holt A, Khan M, Gujja S, Govindarajan R
Received 11 September 2014
Accepted for publication 22 October 2014
Published 24 December 2014 Volume 2015:7 Pages 13—18
DOI https://doi.org/10.2147/CMAR.S74116
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Abby Holt,1 Muhammad A Khan,2 Swetha Gujja,3 Rangaswmy Govindarajan3
1Arkansas Department of Health, Little Rock, 2White River Health System, Batesville, 3Division of Hematology/Oncology, University of Arkansas for Medical Sciences, Little Rock, AR, USA
Background: Prostate cancer subjects with prostate-specific antigen (PSA) relapse who are treated with androgen deprivation therapy (ADT) are recommended to have baseline and serial bone densitometry and receive bisphosphonates. The purpose of this community population study was to assess the utilization of bone densitometry and bisphosphonate therapy in men receiving ADT for non-metastatic prostate cancer.
Methods: A cohort study of men aged 65 years or older with non-metastatic incident diagnoses of prostate cancer was obtained from the Surveillance Epidemiology End Results (SEER)-linked Medicare claims between 2004 and 2008. Claims were used to assess prescribed treatment of ADT, bone densitometry, and bisphosphonates.
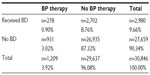
Results: A total of 30,846 incident prostate cancer cases receiving ADT and aged 65 years or older had no bone metastases; 87.3% (n=26,935) on ADT did not receive either bone densitometry or bisphosphonate therapy. Three percent (n=931) of the cases on ADT received bisphosphonate therapy without ever receiving bone densitometry, 8.8% (n=2,702) of the cases on ADT received bone densitometry without receiving intravenous bisphosphonates, while nearly 1% (0.90%, n=278) of the cases on ADT received both bone densitometry and bisphosphonates. Analysis showed treatment differed by patient characteristics.
Conclusion: Contrary to the recommendations, bone densitometry and bisphosphonate therapy are underutilized in men receiving ADT for non-metastatic prostate cancer.
Keywords: prostatic neoplasms, androgen antagonists, bone densitometry, gonadotropin-releasing hormone, osteoporosis
Introduction
Prostate cancer is one of the most common malignancies diagnosed worldwide. Androgen deprivation therapy (ADT) by bilateral orchiectomy or gonadotropin-releasing hormone analogs, with or without antiandrogens, is indicated as front-line treatment in metastatic prostate cancer, as well as in the adjuvant setting following radical prostatectomy with nodal metastasis or radiation therapy, and occasionally in patients with localized disease.1
ADT has influence on many metabolic pathways but the most common side effect is reduction of bone density.2–6 Prostate cancer usually spreads to the bone in metastatic disease, and treatment with intravenous bisphosphonates is commonly prescribed at this stage in order to reduce skeletal-related events.7,8 Loss of BMD correlates with the duration of ADT but is more pronounced during the first year of therapy.7–10 Men initiating ADT are recommended to have an assessment of risk factors for osteoporosis, calcium and vitamin D intake, lifestyle modifications, and baseline and serial BMD assessment while on ADT, along with bisphosphonate therapy.1 There is evidence that loss of BMD is a strong predictor of fracture risk.11 Treatment with bisphosphonates used to prevent osteoporotic fractures has been shown to increase bone mineral density (BMD).12
Although the National Comprehensive Cancer Network and American Society of Clinical Oncology guidelines propose baseline BMD assessment and bisphosphonate therapy, there has been no systematic review of the utilization of these interventions by practicing physicians in the community. The current study evaluated bone densitometry (BD) and bisphosphonate therapy utilization patterns to prevent osteoporosis in this patient population.
Subjects and methods
A retrospective cohort study was conducted using a Surveillance Epidemiology End Results (SEER)-Medicare linked database of men aged 65 years and older with a diagnosis of non-bony metastatic prostate cancer between 2004 and 2007. Individuals with a diagnosis of non-metastatic prostate cancer were identified. Only those treated with ADT were included in the study.
Patient data were obtained from the SEER Patient Entitlement and Diagnosis Summary File (PEDSF), and Medicare inpatient treatment claims were identified using National Claims History noninstitutional physician/supplier part B files (claims for preventive services and coverage for outpatient prescription services) and outpatient part B claims (outpatient services, prescriptions, and durable medical devices) from hospital facilities. SEER-linked patient data from the PEDSF file were unavailable for the year 2008 at the time of the study; so prostate cancer patient data were analyzed for 2004–2007 with linked Medicare treatment claims from 2004 to 2008. In order to limit our analysis to patients with no metastatic disease to bone, only prostate tumors coded as no metastasis and only localized tumors in the PEDSF file were included in the analysis. Medicare claims from the National Claims History physician and outpatient files were matched with PEDSF patient data by SEER case ID number to select prostate cancer cases that had ever received ADT as part of treatment. ADT was identified using Healthcare Common Procedure Coding System (HCPCS) and Current Procedural Terminology Codes (CPT) in the Medicare physician and outpatient claims files. Coding used for orchiectomy included 54520, 54521, 54522, 54530 , and 54535 or ICD-9 code 624, and coding for goserelin, leuprolide, leuprolide implant, or triptorelin were identified as codes J1950, J9202, J9217, J9218, or J9219.13 BMD assessment by dual-energy X-ray absorptiometry was identified using HCPCS or CPT codes 77080 or 77081.
Treatment with intravenous bisphosphonates such as pamidronate or zoledronic acid were identified using Medicare HCPCS codes J2430 and J3487 during the study period 2004–2008. Since the Medicare part D data for outpatient medications including oral bisphosphonates were only available from 2007 in the SEER Medicare files, they could not be utilized for the analysis. Oral bisphosphonates are generally poorly absorbed and have gastrointestinal side effects, leading to low patient adherence with these drugs.14,15 Due to lack of adherence, intravenous bisphosphonates are preferred to oral bisphosphonates in this patient population. Oral bisphosphonate use was not captured because the Medicare database does not collect details of oral therapy.
The one-sided exact binomial test of proportion was used to determine if physician compliance with serial BMD assessment and bisphosphonate treatment in patients undergoing ADT is consistently ≥80%. A survey of practicing physicians with a response rate of 63% found that physician’s self-reported adherence to the clinical guidelines was 77%.16 Therefore, 80% adherence to the clinical guidelines was used in this study. Logistic regression analysis was used to determine treatment differences by patient characteristics such as age group, race, clinical stage, and SEER registry geographic region. SAS 9.3 (SAS Institute, Cary, NC, USA) was used to analyze differences in treatment practices. Further logistic regression analysis was also done to explore the relationship between history of bone fractures and bisphosphonate treatment, age, race, tumor stage at diagnosis, and duration of ADT. The study was approved by the institutional review board at the University of Arkansas for Medical Sciences.
Results
Among 157,974 newly diagnosed prostate cancer cases, 100,865 were aged 65 years and older and had no bone metastases from 2004 to 2007. Subjects who did not have ADT claims were excluded from the analysis. In total, 30,846 prostate cancer patients aged 65 years and older with no bone metastases receiving ADT were eligible for analysis (Table 1). Cases were analyzed by use of BD and parenteral bisphosphonate therapy. Neither BD nor parenteral bisphosphonate therapy were utilized in 87.3% (n=26,935) of subjects on ADT (Table 2). The age group, race, T stage, and geographic distribution are shown in Table 1, and for every covariate (age, race, T stage, and geographic location), the percentage of BD utilization and parenteral bisphosphonate administration was markedly low.
Utilization of BD and intravenous bisphosphonate therapy
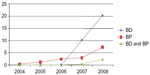
In our study, 8.8% (n=2,707) of subjects on ADT received BD assessments without ever receiving intravenous bisphosphonates. Three percent (n=931) of the cases on ADT received bisphosphonate treatment without ever receiving a BD assessment. Only 0.9% (n=278) of the subjects on ADT received both BD and bisphosphonates. A compliance rate of nearly 1.0% (0.9%), those subjects receiving both BD to screen for bone loss and preventive bisphosphonate therapy, was well below the expected rate of 80% (Table 2). Figure 1 shows the trend of BD and intravenous bisphosphonate utilization during the study period (2004–2008). Bisphosphonates were prescribed more often from 2006 but utilization remained low at below 10% for all years studied.
Utilization of BD and intravenous bisphosphonates by patient characteristics
Odds ratios (ORs) show differences in preventive osteoporotic therapy by treatment category (Table 1). BD utilization increased with advancing age, except for those older than 85 years. BD utilization differed by race. Black men were less likely to receive BD than white men (OR 0.6; 95% confidence interval [CI] 0.54–0.72), and Asian men were more likely to have BD measurement performed than white men (OR 1.6; 95% CI 1.36–1.88). Patients diagnosed at later stages of disease (T3) received BD more often than men diagnosed with earlier stages of disease (T0–T1; OR 1.7; 95% CI 1.49–1.96). BD treatment differed by region. Men with prostate cancer living in the West region were more likely to receive BD than men living in any other region (Table 1). Patients in the Midwest, Northeast, and South regions were significantly less likely to receive BD than patients in the West, (OR 0.7; 95% CI 0.64-0.81, OR 0.8; 95% CI 0.76-0.92, and OR 0.5; 95% CI 0.46-0.58 respectively). Using the Midwest region as the reference group, patients in the West had 20% greater use of BD (OR 1.2; 95% CI 1.02–1.33).
Intravenous bisphosphonate therapy was prescribed more often to older men (80–84 years) than to younger men (aged 65–69 years; OR 1.3; 95% CI 1.07–1.54). Men diagnosed at later stages of disease (T2–T4) were more likely to receive bisphosphonate treatment than men diagnosed at earlier stages (T0–T1; Table 1). Men living in the Northeast and South regions were less likely to receive bisphosphonates than men living in the West region (Table 1).
Among men receiving the recommended treatment of BD and bisphosphonates, there were differences by race, tumor stage, and region. Black men were less likely to receive both BD and bisphosphonates compared with white men (OR 0.6; 95% CI 0.36–0.93). Advanced tumor stage was associated with treatment (Table 1). Men with prostate cancer in the Northwest and South regions were less likely to receive BD and bisphosphonates than men in the West region (Table 1).
Characteristics of study subjects with bone fractures
Only 25 of the 30,846 subjects in this study were identified to have a diagnosis of fracture based on ICD code in the SEER-Medicare database. Diagnosed bone fractures were identified using ICD-9 codes of 733.1 for osteoporotic fractures, and 808-809 for fractures to the spine or trunk. Logistic regression analysis was performed using bone fractures as the outcome variable. When controlling for age (a continuous variable), bisphosphonate treatment (a dichotomous variable), non-white race (a dichotomous variable with white race as the reference group), tumor stage (a dichotomous variable with T4 stage as the reference group), and duration of ADT (a continuous variable), an association between length of therapy and history of bone fractures was found. However, bone fracture was not associated with bisphosphonate therapy in this study population. In multiple studies, it has been demonstrated that ADT results in bone loss which could be a surrogate for increased skeletal-related events.17–21 Although the duration of follow-up after diagnosis was short (≤5 years), continuous ADT increased the risk of bone fracture in this study cohort (P≤0.05). This population-level finding supports the clinical evidence of accelerated bone loss in patients receiving ADT.
Discussion
ADT has been shown to reduce disease progression and increase survival in subjects with prostate cancer.22 BMD loss and osteoporosis is one of the main adverse effects of ADT in this population.2–6,23 In this study, only a small proportion of prostate cancer patients on ADT were evaluated for bone loss, and an even smaller proportion ever received intravenous bisphosphonate therapy. Among the patient characteristics investigated, differences in age, race, tumor stage, and regional treatment were found.
Approximately 12% of men in the general population have osteoporosis.24 Osteoporosis may be asymptomatic, but 20% of men on ADT have skeletal-related events such as fracture.24 ADT has been associated with accelerated bone loss of up to 4.5% per year, and loss of BMD is considered a strong surrogate for increased risk of skeletal-related events.2,4,5,19–22,25 The rate of bone loss is greatest in the first year of ADT, and osteoporosis is prevalent in nearly 50% patients on ADT by 4 years and 80% by 8 years.8 Early BMD assessment and treatment may potentially prevent fragility fractures, as recommended by national organizations such as the National Comprehensive Cancer Network and American Society of Clinical Oncology.1,21,22,25 Prevention of bone loss, resulting in a reduction of skeletal-related events, may maintain good quality of life for patients with prostate cancer on ADT. Concurrent administration of a bisphosphonate or a selective estrogen receptor modulator has been shown to stabilize or increase BMD. In randomized studies, bisphosphonate therapy, including pamidronate, zoledronic acid, or alendronate, has been shown to improve BMD and decrease markers of bone metabolism in men on ADT.4,5,7,20–29 This community-level study supports evidence that BD utilization and bisphosphonate therapy reduce fracture risk in men with prostate cancer receiving ADT. Intravenous bisphosphonate therapy, ie, zoledronic acid, has been evaluated in several studies, and was determined to be the best treatment to prevent bone loss in prostate cancer patients undergoing ADT.30 Treatment with denosumab, which is currently recommended as an alternative to zoledronic acid for patients with nonmetastatic prostate cancer, was approved by the US Food and Drug Administration in 2011, and was not a treatment option during the study years examined.31 Therefore, analysis of guideline compliance using intravenous bisphosphonates as a measure was appropriate for this study.
In addition, the cost of osteoporosis-related fractures among men in the USA is approximately $4.1 billion per year, and the effect on rising health care costs could potentially have an impact on the survival benefit of ADT in patients with metastatic prostate cancer. Subjects on ADT are recommended to be screened for fracture risk, with BMD testing at baseline, after 1 year of ADT, and then every 2 years or as clinically indicated.32,33 In addition, counseling patients for fall risk and applying interventions to reduce falls may consequently reduce fracture risk in this population.
Most evidence supporting the use of bisphosphonates for prevention of ADT-related bone loss in men with prostate cancer is derived from studies evaluating intravenous bisphosphonates.4,5,20,21 This is due to the lack of data available to analyze oral bisphosphonate use in large population studies. Greenspan et al recently demonstrated a significant increase in BMD in men with prostate cancer receiving ADT and once-weekly oral alendronate 70 mg.22 Denosumab, another current treatment and unavailable during the study years examined, is recommended as an alternative to zoledronic acid for nonmetastatic prostate cancer patients.31 The long-term use of oral bisphosphonate therapy could not be evaluated in this study because Medicare part D data are not available during the study period. However, due to lack of patient adherence, oral bisphosphonates have not been the preferred treatment over intravenous bisphosphonates in this patient population, so likely did not significantly affect the outcome.14,15
Our study re-emphasizes the importance of using BD measures to evaluate skeletal integrity and prevent osteoporosis with the utilization of bisphosphonates among patients who are on ADT. There seems to be a lack of understanding regarding the implications of ADT for prostate cancer.
Conclusion
Although the follow-up time was short, continuous ADT was associated with an increased risk of bone fracture, which supports previous clinical studies concerning accelerated bone loss in patients receiving ADT. In this community population study, we demonstrated that a very small proportion of patients underwent evaluation for bone loss and an even smaller proportion of patients received bisphosphonates. In addition, even when overall treatment utilization is low, black men were less likely to receive the recommended treatment of BD and bisphosphonates compared with white men. Further, utilization practices differed by region, with men residing in the West and Midwest regions receiving optimum treatment of BD and bisphosphonates when compared with men in the Northeast and South. Contrary to the recommendations, screening for bone loss and preventive treatment practices among this community population was markedly low for every age group, race, stage at diagnosis (T0–T4), and SEER registries by US geographic region.
Acknowledgments
We would like to thank Youjie Huang, Florida Department of Health, and Robert Delongchamp, University of Arkansas for Medical Sciences, College of Public Health, for their analytical support. The data were analyzed under the direction of Drs Rangaswamy Govindarajan, Youjie Huang, and Robert Delongchamp.
Disclosure
The authors report no conflicts of interest in this work.
References
National Comprehensive Cancer Network. Clinical practice guidelines in oncology. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed August 13, 2012. | |
Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161(4):1219–1222. | |
Diamond T, Campbell J, Bryant C, Lynch W. The effect of combined androgen blockade on bone turnover and bone mineral densities in men treated for prostate carcinoma: longitudinal evaluation and response to intermittent cyclic etidronate therapy. Cancer. 1998;83(8):1561–1566. | |
Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169(6):2008–2012. | |
Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345(13):948–955. | |
Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167(6):2361–2367. | |
Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90(12):6410–6417. | |
Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69(3):500–504. | |
Ryan CW, Huo D, Stallings JW, Davis RL, Beer TM, McWhorter LT. Lifestyle factors and duration of androgen deprivation affect bone mineral density of patients with prostate cancer during first year of therapy. Urology. 2007;70(1):122–126. | |
Kiratli BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57(1):127–132. | |
Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12(12):989–995. | |
Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176(3):972–978. | |
Lu-Yao GL, Albertsen PC, Moore DF, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300(2):173–181. | |
Egerdie B, Saad F. Bone health in the prostate cancer patient receiving androgen deprivation therapy: a review of present and future management options. Can Urol Assoc J. 2010;4(2):129–135. | |
Serpa Neto A, Tobias-Machado M, Esteves MA, et al. A systematic review and meta-analysis of bone metabolism in prostate adenocarcinoma. BMC Urol. 2010;10:9. | |
Maue SK, Segal R, Kimberlin CL, Lipowski EE. Predicting physician guideline compliance: an assessment of motivators and perceived barriers. Am J Manag Care. 2004;10(6):383–391. | |
Higano CS. Androgen-deprivation-therapy-induced fractures in men with nonmetastatic prostate cancer: what do we really know? Nat Clin Pract Urol. 2008;5(1):24–34. | |
Eisenberger MA, Blumenstein BA, Crawford ED, et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med. 1998;339(15):1036–1042. | |
Mittan D, Lee S, Miller E, Perez RC, Basler JW, Bruder JM. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab. 2002;87(8):3656–3661. | |
Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25(9):1038–1042. | |
Israeli RS, Rosenberg SJ, Saltzstein DR, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5(4):271–277. | |
Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146(6):416–424. | |
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166(4):465–471. | |
O’Neill TW, Felsenberg D, Varlow J, Cooper C, Kanis JA, Silman AJ. The prevalence of vertebral deformity in European men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res. 1996;11(7):1010–1018. | |
Orsini LS, Rousculp MD, Long SR, Wang S. Health care utilization and expenditures in the United States: a study of osteoporosis-related fractures. Osteoporos Int. 2005;16(4):359–371. | |
Klotz LH, McNeill IY, Kebabdjian M, et al. A phase 3, double-blind, randomised, parallel-group, placebo-controlled study of oral weekly alendronate for the prevention of androgen deprivation bone loss in nonmetastatic prostate cancer: the Cancer and Osteoporosis Research with Alendronate and Leuprolide (CORAL) study. Eur Urol. 2013;63(5):927–935. | |
Choo R, Lukka H, Cheung P, et al. Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys. 2013;85(5):1239–1245. | |
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353(9156):878–882. | |
Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100(5):892–899. | |
Serpa Neto A, Tobias-Machado M, Esteves MA, et al. Bisphosphonate therapy in patients under androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2012;15(1):36–44. | |
National Cancer Institute. FDA approval of denosumab. Available from; http://www.cancer.gov/cancertopics/druginfo/fda-denosumab#Anchor-BM. Accessed November 20, 2013. | |
Hillner BE, Ingle JN, Chlebowski RT, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–4057. | |
Higano CS. Understanding treatments for bone loss and bone metastases in patients with prostate cancer: a practical review and guide for the clinician. Urol Clin North Am. 2004;31(2):331–352. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.