Back to Journals » Research and Reports in Tropical Medicine » Volume 14
Using Clinical Vignettes to Understand the Complexity of Diagnosing Type 1 Diabetes in Sub-Saharan Africa
Authors Le Bec E, Kam M, Aebischer Perone S, Boulle P, Cikomola JC, Gandur ME, Gehri M, Kehlenbrink S, Beran D
Received 20 July 2023
Accepted for publication 7 November 2023
Published 13 November 2023 Volume 2023:14 Pages 111—120
DOI https://doi.org/10.2147/RRTM.S397127
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario Rodriguez-Perez
Enora Le Bec,1 Madibele Kam,2 Sigiriya Aebischer Perone,3,4 Philippa Boulle,5 Justin Cirhuza Cikomola,6 Maria Eugenia Gandur,5 Mario Gehri,7 Sylvia Kehlenbrink,8 David Beran3,9
1Internal Medicine, Etablissements Hospitaliers du Nord Vaudois, Yverdon, Switzerland; 2Pediatric University Hospital Charles de Gaulle, Ouagadougou, Burkina Faso; 3Division of Tropical and Humanitarian Medicine, Geneva University Hospitals, Geneva, Switzerland; 4Health Unit, International Committee of the Red Cross, Geneva, Switzerland; 5Médecins sans Frontières, Geneva, Switzerland; 6Faculty of Medicine, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo; 7Faculty of Biology and Medicine, University of Lausanne, Lausanne, Switzerland; 8Division of Endocrinology, Diabetes and Hypertension, Brigham and Women’s Hospital, Boston, MA, USA; 9Faculty of Medicine, University of Geneva, Geneva, Switzerland
Correspondence: Enora Le Bec, Hôpital d’Yverdon-les-Bains, Rue d’Entremonts 11, Yverdon-les-Bains, 1400, Switzerland, Tel +41 24 424 44 44, Fax +41 24 424 43 60, Email [email protected]
Abstract: Lack of awareness, access to insulin and diabetes care can result in high levels of morbidity and mortality for children with type 1 diabetes (T1DM) in sub-Saharan Africa (SSA). Improvements in access to insulin and diabetes management have improved outcomes in some settings. However, many people still present in diabetic ketoacidosis (DKA) in parallel to misdiagnosis of children with T1DM in contexts with high rates of communicable diseases. The aim of this study was to highlight the complexity of diagnosing pediatric T1DM in a healthcare environment dominated by infectious diseases and lack of adequate health system resources. This was done by developing clinical vignettes and recreating the hypothetico-deductive process of a clinician confronted with DKA in the absence of identification of pathognomonic elements of diabetes and with limited diagnostic tools. A non-systematic literature search for T1DM and DKA in SSA was conducted and used to construct clinical vignettes for children presenting in DKA. A broad differential diagnosis of the main conditions present in SSA was made, then used to construct a clinician’s medical reasoning, and anticipate the results of different actions on the diagnostic process. An examination of the use of the digital based Integrated Management of Childhood Illness diagnostic algorithm was done, and an analysis of the software’s efficiency in adequately diagnosing DKA was assessed. The main obstacles to diagnosis were low specificity of non-pathognomonic DKA symptoms and lack of tools to measure blood or urine glucose. Avenues for improvement include awareness of T1DM symptomatology in communities and health systems, and greater availability of diagnostic tests. Through this work clinical vignettes are shown to be a useful tool in analyzing the obstacles to underdiagnosis of diabetes, a technique that could be used for other pathologies in limited settings, for clinical teaching, research, and advocacy.
Keywords: type 1 diabetes, underdiagnosis, sub-Saharan Africa, clinical vignette
Introduction
It is estimated that there are currently 9 million people with type 1 diabetes (T1DM) worldwide.1 A general increase in cases of T1DM is observed worldwide, with an annual rate increase of approximately 3%, predominantly in people under 15 years of age.2 Africa accounts for 8% of prevalent cases of T1DM.1 However, the epidemiological situation of T1DM in sub-Saharan Africa (SSA) is very poorly understood, as estimates are based on a small number of old studies.3,4 In addition people with T1DM in sub-Saharan Africa face a number of challenges in terms of diabetes management.5,6 These relate to both the overall socio-economic context of this region as well more specific health challenges related to access to the delivery of diabetes care and insulin. Sub-Saharan Africa includes over 70% of low-income countries (LMIC), with fragile healthcare systems that already bear a heavy burden of mortality linked to infectious diseases.7 Health systems also need to contend with an estimated 70 to 80% increase in the prevalence of type 2 diabetes by 2050 in the sub-Saharan Africa,8 of which 44% of cases are currently undiagnosed.2
Delayed diagnosis of diabetes, combined with a lack of awareness among the general public and healthcare staff, not only exacerbates the complications associated with chronic hyperglycemia, but is of particular concern for T1DM, where delayed diagnosis and access to care can rapidly lead to diabetic ketoacidosis (DKA), which may be fatal.9 In high income countries (HIC), mortality from DKA is estimated at less than 1%, whereas studies carried out in sub-Saharan Africa estimate a mortality rate of over 40% on initial presentation.8 The rate of DKA at first presentation in sub-Saharan Africa is extremely high, ranging from 20% to 100% depending on the study.10–12 Several authors suggest that the main cause of mortality in children with T1DM is under-diagnosis of the condition.13,14 Atkinson and Ogle,15 assert that:
the most common cause of death for a child with type 1 diabetes is more likely to be mortality from ketoacidosis that has been mistakenly managed as an infection or other disorder.
DKA is the result of the combination of a relative or absolute insulin deficiency and an increase in counter-regulatory hormones including glucagon, catecholamines, growth hormone, and cortisol. The latter induce a state of glycogenolysis, gluconeogenesis, lipolysis and proteolysis that worsens hyperglycemia and dehydration, leading to ketoacidosis and plasma hyperosmolarity. If left untreated, profound dehydration and metabolic acidosis, potentially aggravated by concomitant sepsis, can lead to death.
The classically recognized signs and symptoms of DKA include polyuria, polydipsia, and weight loss; tachypnea with a Kussmaul breathing pattern; nausea, vomiting, and abdominal pain (which may mimic an acute abdomen) and ultimately impaired consciousness and coma. This spectrum of signs and symptoms overlaps with those of other more common conditions in SSA, such as infections and malnutrition, and can make clinical suspicion of DKA difficult.
Given the increased DKA mortality and likely under-diagnosis of T1DM in SSA, the aim of this study was to explore the reasons for under-diagnosis and to suggest possible improvements by observing the clinical course of using vignettes of fictitious cases of DKA in the epidemiological and health system context of Burkina Faso.
Methodology
Different clinical vignettes of children in moderate DKA were developed. A vignette is a description of a clinical situation that a clinician may encounter in real life, intended for training and reflexion.16 These vignettes included detailed descriptions of the symptoms, signs and potential laboratory results for a fictitious patient. These were created using a non-systematic review of existing literature on the epidemiology of T1DM and DKA in Africa on PubMed. This was complemented by examining reports from the International Diabetes Federation (IDF), International Society for Pediatric and Adolescent Diabetes (ISPAD) focusing first on the pathophysiology of T1DM and DKA, its symptomatology and risk factors, and recommendations for the management of DKA. From this review, three initial vignettes of children with moderate to severe DKA were established, according to the ISPAD classification.17 The age of the hypothetical patients, as well as the symptoms and signs presented, classified by physiological system, are based on eight retrospective studies of T1DM and DKA cases hospitalized in countries in the African region, focusing on patients’ symptomatology prior to diagnosis.10,11,18–23 Laboratory values shown are the disturbances that ISPAD classifies as “moderate to severe” in a DKA.24
These vignettes were then compared with clinical practice observed during six weeks of fieldwork during the summer of 2018 at the Charles de Gaulle Pediatric University Hospital, in Ouagadougou, Burkina Faso. Burkina Faso’s healthcare system is organized in a pyramidal structure, with primary health care centers run by community health workers, clinics and peripheral hospitals in the middle staffed with general practitioners and surgeons, and university hospitals at the tip. This country faces a high infant mortality (42.6/1000 < 1 years, 81.6/1000 < 5 years in 2015), linked to the heavy burden of malaria, as well as malnutrition. Malaria prevalence in children under 5 is estimated at 46%, with a case-fatality rate of 1.4%.25–27 The IDF estimates that there are 254 people with type 1 diabetes aged 0–19 years.2 There are no national statistics on the actual prevalence of T1DM. The pediatric endocrinology consultation at the University Hospital Yalgado, in Ouagadougou, monitored 91 children in 2019, the majority of whom were reported being diagnosed with severe DKA.
During this period, unstructured meetings took place with a pediatric endocrinologist, ten children, and their care givers from the T1DM pediatric consultations, during a diabetes education session, with collection of their testimonies concerning the diagnosis and difficulties of day-to-day treatment. Observation of the work of clinicians working in the pediatric emergency department of the Charles de Gaulle Pediatric University Hospital shed light on the functioning of the healthcare system, assessment and treatment habits in emergency situations, and resources available.
Based on clinical pediatric reference textbooks,28,29 a broad pediatric differential diagnosis of the main complaints of 2 of the 3 vignettes created (dyspnea/abdominal pain) was established. The most significant clinical elements for the diagnosis found in the literature search were noted, either from specific sources (meningitis,30–34 malaria,35–39 pneumonia,40,41 enteric fever,42 appendicitis43,44), or general textbook references,28,29 with an assessment of sensitivity, specificity of said clinical elements if present. The basic medical examinations necessary to confirm or deny the diagnosis were added. When such information existed, their prevalence in Burkina Faso was added.25,26 The vignettes and differential diagnosis were then validated by external experts specialized in tropical medicine and pediatrics, from Democratic Republic of Congo, USA and Switzerland, including co-authors (SK and JC) on this paper. In addition, using existing records from the emergency department at the Charles de Gaulle Pediatric University Hospital, the initial vignettes were adapted to reflect different “real-life” challenges actual children diagnosed with T1DM had faced. In the development of the vignettes the presence or absence of certain diagnostic tools in Burkina Faso, such as tools to measure blood or urine glucose or ketones, was also included in the clinical pathway. With these elements, and the assessment and treatments habits seen during field work, it was possible to establish a differential diagnosis, and create a hypothesis of the clinician’s hypothetico-deductive process.
For each differential diagnosis, the presence or absence of identification of symptoms in the vignette was included, enabling the likelihood of a given diagnosis to be established. This process resulted in a more restricted differential diagnosis, which can be easily investigated via the list of complementary examinations required by each differential diagnosis previously outlined. Once this process was completed, it was possible to consider the patient’s complete clinical course, with the impact of each clinician’s decision and laboratory results on their prognosis. More broadly, the assessment of the patient’s journey from home to diagnosis has been evaluated, with a search for the various obstacles to proper care. For the purpose of this exercise the identification of traditional symptoms of polyuria/polydipsia and weight loss were excluded.
The third case (impaired consciousness) went through another process. This case was presented to a computer algorithm based on Integrated Management of Childhood Illness (IMCI),45–47 a project currently being deployed in certain regions of Burkina Faso to guide community health workers without advanced medical training in their management, and observed the treatment suggestions offered by the algorithm, as well as their impact on the patient’s prognosis.
Results
The first part of the literature search focused on DKA symptomatology and its risks factor and identified six articles on inaugural T1DM and DKA clinical presentation in SSA.10,11,18,20,22,48 The most frequent symptoms noted outside the classical symptoms (polydipsia 70–98%, polyuria 40–100%, loss of weight 50–82%, asthenia 50–70%) were respiratory distress/tachypnea (10–90%), abdominal or nausea and vomiting (0.2–50%), and altered state of consciousness (37–90%), and fever in 7–10% (Appendix 1).
Three articles were found regarding the risk factors of DKA.12,49,50 The risk factors for DKA at diagnosis, were mainly based on data from HICs, and identified low socioeconomic status, lack of health insurance, delayed treatment and misdiagnosis. In one review, almost 40% of children with DKA were seen at least once by a physician in the period preceding diagnosis, illustrating the degree of difficulty of clinical suspicion.12 Another study found that DKA frequency is inversely associated with gross domestic product, latitude and background incidence of type 1 diabetes.49
Based on this literature search, three vignettes were created, illustrating cases of moderate to severe DKA (Appendix 2). Vignette A and B are summarized in Figure 1. Vignette A is that of an 8-year-old girl, brought to hospital because of a deterioration in general condition with labored breathing. The hypothetical management, presented in vignette A (Figure 2), shows that after a physical examination, the working diagnostic hypothesis, which is based on the differential diagnosis developed in Appendix 3, includes pneumonia, meningitis, sepsis of undetermined origin, malaria, or severe anemia (Appendix 3). The practitioner then performs a baseline biological workup and chest X-ray, while starting hydration (Glucose 5% versus other isotonic fluids). Laboratory results would be expected to show an inflammatory syndrome with hyperglycemia and a chest X-ray within normal limits, raising the suspicion of DKA, or sepsis of undetermined origin, needing further investigation. At the end of the process, two options are possible: a correct diagnosis of DKA, or a mistaken/incomplete diagnosis of an infectious event, leading to a significant delay in management, or death, with an obvious risk of deterioration in the event of glucose infusion.
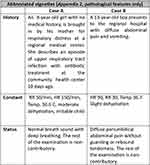 |
Figure 1 Abbreviated description of vignettes. |
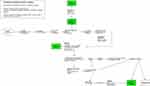 |
Figure 2 Hypothetical management scheme vignette A - DKA in the form of respiratory distress. |
Child B is a 13-year-old boy presenting to a hospital with abdominal pain and vomiting. After an examination, the working diagnosis includes gastroenteritis, infectious colitis, appendicitis, typhoid fever, or sickle-cell crisis. (Appendix 4) Two possible diagnostic pathways are possible depending on the clinician’s assessment. Outpatient investigations may be possible, with a delay inherent in the tests and a risk of DKA deterioration, or inpatient work-up. After a biological workup without major abnormality, in the absence of any suspicion of diabetes, the clinician could decide on conservative care with antibiotic therapy, or surgical care, both of which would result in delayed management with a significant risk of complications and death, as shown in Figure 3.
 |
Figure 3 Hypothetical management scheme vignette B - DKA in the form of abdominal pain. |
In both cases, the hypothetical management generated by these differential diagnoses and additional investigations assume that DKA is not part of the clinician’s primary differential diagnosis. The frequent absence of capillary glycemia, linked to a lack of resources, limits its regular/systemic use, and makes blood glucose testing a deliberate act by the clinician, implying pre-existing clinical suspicion. This highlights the difficulty of diagnosing DKA in the absence of awareness or recognition of the pathognomonic symptom triad of diabetes, and the life-threatening danger associated with delayed diagnosis of diabetes. Intravenous glucose infusions were routinely given during clinical care witnessed in Burkina Faso for the prevention of hypoglycemia associated with severe malaria, which would be an aggravating and potentially confounding factor in cases of DKA.
Child C is a 4.5-year-old, in hypovolemic shock, consulting in a community health center for altered consciousness. Clinical examination reveals poor general condition, severe dehydration (>10%) with tachypnea and fruity breath. A thorough history could have revealed weight loss with polyuria/polydipsia for two weeks, and lethargy over the past 48 hours. This vignette was used to observe the effectiveness of an IMCI-based computer algorithm in the diagnosis and management of DKA.47 The flowchart Figure 4 describes the steps performed on the software with the clinical elements for Child C. The computer algorithm makes two proposals, depending on the possibility of transfer, involving broad-spectrum antibiotic therapy and oral sugar intake. These proposals are based on the high frequency of infectious and parasitic etiology, as well as the danger of hypoglycemia in this population, but would consequently exacerbate DKA.
 |
Figure 4 Hypothetical management scheme management of vignette C – impaired consciousness. |
Discussion
The aim of this study was to highlight the complexity of DKA diagnosis in SSA using clinical vignettes. These vignettes demonstrate that the vast differential diagnosis of DKA is overly complex due to the intrinsic variability of DKA’s clinical presentation, the variability in disease presentation across age groups from toddler to adolescent, the low prevalence of T1DM in sub-Saharan African contexts, the high prevalence of other conditions with similar modes of presentation and symptomology, the lack of awareness, clinical suspicion, and training on T1DM, and the lack of access, or limited access to simple diagnostic tools like capillary glucose, making systematic screening impossible. In this context, a high degree of suspicion is required for an early diagnosis. The vignettes also allow for the identification of the key places where the diagnosis of T1DM is missed.
The presence or absence of diagnostic equipment (dipstick, capillary glucose, blood gases) in each vignette is based on observation in the field in Burkina Faso. Access to, or lack of diagnostic tools, some of which are absent in the cases illustrated, may alter the potential management of these cases. Easy access to capillary blood glucose can make a diagnosis of diabetes obvious, if used. However, in the absence of systematic capillary blood glucose monitoring, it is the practitioner’s clinical suspicion that allows a diagnosis to be made, regardless of the tools used.
More generally, this work has demonstrated that clinical vignettes with their hypothetical management can be used as tools in the multi-level analysis of the causes of under-diagnosis of rare pathologies, highlighting expected and unexpected obstacles to the management of these conditions. These can also serve as demonstrations and arguments for better management of these diseases, as well as accessible and cost-effective training tools in the medical training of the countries concerned.
From a clinical perspective in the SSA context, it might be imagined that the implementation of diagnostic algorithms, as an aid to healthcare staff, would improve survival of children presenting in DKA. However, the IMCI algorithm used in vignette C proved deleterious to the patient. Theoretically, the introduction of an item looking for the cardinal signs of T1DM is possible but has its challenges. These algorithms are based on the local burden of disease and adapted to the level of training of health workers. Adding an item looking for signs of T1DM could be considered but would need to balance this with treating the most common, rapidly fatal diseases in these areas. Similarly, discontinuing the systematic use of glucose infusion will certainly be detrimental to a majority of patients in the epidemiological context of SSA. An easier solution might be to add glucose check in the algorithm.
In looking at these vignettes from a more global perspective, based on the biopsychosocial model, there is a need to contextualize these barriers to diagnosis within the wider barriers to access to healthcare. In 1994, Thaddeus and Maine51 described the “Three delays model (deciding to seek care, accessibility to care, quality of care)”, initially used in public health to study maternal mortality, and since applied to other public health issues. This same concept can be applied to T1DM in SSA, as shown in Figure 5.
 |
Figure 5 Hypothetical global management scheme for vignettes A and B. |
The care process begins at home, in the child’s environment and, in the pediatric context more specifically, in their family. In SSA the use of traditional healers as a first point of provision of healthcare should also be considered. The first task is to observe symptoms and seek care, before even taking the diagnostic steps described above. In the case of DKA, the “atypical” clinical presentation, which may mimic or exist in parallel with more common diseases, may lead to a delay in seeking care, as the symptoms encountered are not those most frequently experienced by parents compared to other childhood pathologies in tropical environments (cough, fever, diarrhea). Numerous factors can influence the decision to seek care, including economic factors (consultation costs, transport, treatment, loss of earnings, etc.).38,52 The perception of the health system, the family’s previous experiences and their health literacy are also important parameters.
The next barrier, access to care facilities, encompasses broad socio-economic issues, such as geographical distance from the place of care and the patient’s ability to travel, which are not specific to T1DM.53 The third barrier, quality of care, includes first and foremost the diagnosis. This study demonstrated the complexity of diagnosing DKA in a resource-poor environment, in view of the wide range of differential diagnoses and in the absence of simple diagnostic equipment. If the diagnosis is made, optimal management of the condition remains a major challenge, as DKA is often associated with severe electrolyte and infectious disorders. As these challenges are present in many LMICs this approach and its findings might be relevant in providing insights into similar contexts with weak health systems and high rates of infectious diseases in children.
In the long term, the survival of children with T1DM depends on increased awareness of the condition, its symptoms and presentation, and access to insulin, diagnostics, and appropriate follow-up. In Burkina Faso, treatment costs can represent 500% of the average annual salary of the lowest economic classes, with a very low number of specialized facilities for diabetes care provision.5 At the time of the field study, the only two official health facilities for the management of T1DM were in Ouagadougou and Bobo-Dioulasso, the country’s two largest cities.
Some improvements in the management of T1DM in SSA are due to an increase in the number of trained endocrinologists,14 and follow-up programs and access to insulin via philanthropic associations.23,54,55 More generally, several public health measures could contribute to a reduction in T1DM mortality in LMICs, such as awareness campaigns for the general public and healthcare personnel which are known to be effective in decreasing the percentage of DKA in HICs,56,57 and improved access to blood or urine glucose testing in health facilities.54 These are theoretically relatively simple measures, but practically, are complex due to the size of the territories and the costs involved.
Limitations
A generalization to the entire African continent is obviously not possible based on this approach, but the hypothetico-deductive process in this work was an attempt to recreate the thinking of a clinician faced with children presenting in DKA. It was deliberately decided to exclude identification of the pathognomonic triad of polyuria/polydipsia and weight loss from the elements given in the vignettes, given that in clinical reality, we postulate it is highly possible that these symptoms are not systematically recognized, reported by the patient, or specifically sought by the clinician, as shown in Appendix 1. This assumption needs to be formally assessed by further studies. This choice also made it possible to consider a broader differential diagnosis in the process described, to create a worst-case scenario. Other management pathways exist, shortening or nullifying the delay in diagnosis, but were not presented in this article. The cases presented here are fictious, and it’s important to note that DKA management, or mismanagement were not observed during fieldwork.
Conclusion
The challenge of diagnosing T1DM in low-resourced health systems remains a significant barrier to improving care. Mortality at initial presentation for these children is driven by health system, economic and global health factors. These include facilities lacking the appropriate equipment to appropriately diagnose this condition, trained staff, challenges in accessing the health system, absence of universal health coverage, high rates of poverty and the high burden of communicable diseases in children. However, with persisting barriers to access to insulin, self-monitoring equipment and other resources, a comprehensive approach is needed to decrease the mortality and morbidity of T1DM in low resource settings, by ensuring access to proper diagnosis as well as continuous quality management. The use of these clinical vignettes offers an interesting approach to disentangle the complexity of diagnosing T1DM and allows for recommendations to be formulated. Based on this work, a health system response to the challenges presented above would need to include increased availability of diagnostic tools and increased awareness of T1DM symptomology in communities and health systems.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Green A, Hede SM, Patterson CC, et al. Type 1 diabetes in 2017: global estimates of incident and prevalent cases in children and adults. Diabetologia. 2021;64(12):2741–2750. doi:10.1007/s00125-021-05571-8
2. International Diabetes Federation. IDF Diabetes Atlas.
3. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi:10.1016/j.diabres.2018.02.023
4. Patterson C, Guariguata L, Dahlquist G, Soltesz G, Ogle G, Silink M. Diabetes in the young - a global view and worldwide estimates of numbers of children with type 1 diabetes. Diabetes Res Clin Pract. 2014;103(2):161–175. doi:10.1016/j.diabres.2013.11.005
5. Ogle GD, Kim H, Middlehurst AC, Silink M, Jenkins AJ. Financial costs for families of children with Type 1 diabetes in lower-income countries. Diabet Med. 2016;33(6):820–826. doi:10.1111/dme.12997
6. Beran D, Yudkin JS. Diabetes care in sub-Saharan Africa. Lancet. 2006;368(9548):1689–1695. doi:10.1016/S0140-6736(06)69704-3
7. Boutayeb A. The Impact of Infectious Diseases on the Development of Africa. In: Preedy V, Watson R, editors. Handbook of Disease Burdens and Quality of Life Measures. Springer Nature; 2010:1171–1188.
8. Pastakia SD, Pekny CR, Manyara SM, Fischer L. Diabetes in sub-Saharan Africa - from policy to practice to progress: targeting the existing gaps for future care for diabetes. Diabetes Metabol Syndr Obes. 2017;10:247–263. doi:10.2147/DMSO.S126314
9. Bui H, To T, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156(3):472–477. doi:10.1016/j.jpeds.2009.10.001
10. Ameyaw E, Asafo-Agyei SB, Thavapalan S, Middlehurst AC, Ogle GD. Clinical profile of diabetes at diagnosis among children and adolescents at an endocrine clinic in Ghana. World J Diabetes. 2017;8(9):429–435. doi:10.4239/wjd.v8.i9.429
11. Iddi S, Francis B, Jaka H, Mirambo M, Mushi M. Clinical presentation and precipitating factors of diabetic ketoacidosis among patients admitted to intensive care unit at a tertiary hospital in Mwanza, Tanzania. Tanzan J Health Res. 2017;19(1). doi:10.4314/thrb.v19i1.6
12. Usher-Smith JA, Thompson MJ, Sharp SJ, Walter FM. Factors associated with the presence of diabetic ketoacidosis at diagnosis of diabetes in children and young adults: a systematic review. BMJ. 2011;343:d4092. doi:10.1136/bmj.d4092
13. Murunga AN, Owira PM. Diabetic ketoacidosis: an overlooked child killer in sub-Saharan Africa? Trop Med Int Health. 2013;18(11):1357–1364. doi:10.1111/tmi.12195
14. Piloya-Were T, Sunni M, Ogle GD, Moran A. Childhood diabetes in Africa. Curr Opin Endocrinol Diabetes Obes. 2016;23(4):306–311. doi:10.1097/MED.0000000000000262
15. Atkinson MA, Ogle GD. Improving diabetes care in resource-poor countries: challenges and opportunities. Lancet Diabetes Endocrinol. 2013;1(4):268–270. doi:10.1016/S2213-8587(13)70172-4
16. Piryani RM, Piryani S. Clinical vignette-based interactive discussion sessions: feedback from residents. Adv Med Educ Pract. 2019;10:829–833. doi:10.2147/AMEP.S218157
17. Virmani A, Brink SJ, Middlehurst A, et al. ISPAD Clinical Practice Consensus Guidelines 2022: management of the child, adolescent, and young adult with diabetes in limited resource settings. Pediatr Diabetes. 2022;23(8):1529–1551. doi:10.1111/pedi.13456
18. Atkilt HS, Turago MG, Tegegne BS. Clinical characteristics of diabetic ketoacidosis in children with newly diagnosed type 1 diabetes in Addis Ababa, Ethiopia: a Cross-Sectional Study. PLoS One. 2017;12(1):e0169666. doi:10.1371/journal.pone.0169666
19. Omoy MN, Ngoy DM, Ilunga EK, et al. Le diabete Sucre de type I chez l’enfant de moins de 5 ans: a propos d’une observation aux cliniques universitaires de Lubumbashi et revue de la litterature [Type I diabetes mellitus in children less than 5 years: case study conducted at the university clinics of Lubumbashi and review of the literature]. Pan Afr Med J. 2017;26:170. doi:10.11604/pamj.2017.26.170.11876
20. Onyiriuka AN, Ifebi E. Ketoacidosis at diagnosis of type 1 diabetes in children and adolescents: frequency and clinical characteristics. J Diabetes Metab Disord. 2013;12(1):47. doi:10.1186/2251-6581-12-47
21. Reddy Y, Ganie Y, Pillay K. Characteristics of children presenting with newly diagnosed type 1 diabetes. South Afr J Child Health. 2013;7:46–48.
22. Ibekwe M, Ibekwe R. Pattern of type 1 diabetes mellitus in Abakaliki, Southeastern, Nigeria. Pediatr Oncall J. 2011;8:59–62.
23. Marshall SL, Edidin D, Arena VC, et al. Prevalence and incidence of clinically recognized cases of Type 1 diabetes in children and adolescents in Rwanda, Africa. Diabet Med. 2015;32(9):1186–1192. doi:10.1111/dme.12701
24. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155–177. doi:10.1111/pedi.12701
25. Ministère de la Santé Burkina Faso. Profil sanitaire complet du Burkina Faso: module 2; 2017. Available from: https://www.afro.who.int/sites/default/files/2018-08/Profil%20sanitaire%20du%20Burkina%20%202.pdf.
26. Ministère de l’Urbanisme et de l’habitat. Annuaire statistique 2016; 2016. Available from: http://www.cns.bf/IMG/pdf/annuaire_statistique_2016.pdf.
27. Ministère de la Santé Burkina Faso. Plan Pluri- Annuel Complet. 2012. Available from: https://extranet.who.int/countryplanningcycles/sites/default/files/country_docs/Burkina%20Faso/ppac_2011_2015_dpv_revise_30_aout_2012_1.pdf.
28. Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, Steinbach WJ. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases.
29. Farrar J, Hotez P, Junghanss T, Kang G, Lalloo D, White N. Manson’s Tropical Diseases.
30. Curtis S, Stobart K, Vandermeer B, Simel DL, Klassen T. Clinical features suggestive of meningitis in children: a systematic review of prospective data. Pediatrics. 2010;126(5):952–960. doi:10.1542/peds.2010-0277
31. Koutangni T, Boubacar Mainassara H, Mueller JE. Incidence, carriage and case-carrier ratios for meningococcal meningitis in the African meningitis belt: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0116725. doi:10.1371/journal.pone.0116725
32. Fayyaz J, Rehman A, Hamid A, Khursheed M, Zia N, Feroze A. Age related clinical manifestation of acute bacterial meningitis in children presenting to emergency department of a tertiary care hospital. J Pak Med Assoc. 2014;64(3):296–299.
33. Kim KS. Acute bacterial meningitis in infants and children. Lancet Infect Dis. 2010;10(1):32–42. doi:10.1016/S1473-3099(09)70306-8
34. Aipit J, Laman M, Hwaiwhanje I, et al. Accuracy of initial clinical diagnosis of acute bacterial meningitis in children from a malaria-endemic area of Papua New Guinea. Trans R Soc Trop Med Hyg. 2014;108(7):444–448. doi:10.1093/trstmh/tru067
35. Makani J, Matuja W, Liyombo E, Snow RW, Marsh K, Warrell DA. Admission diagnosis of cerebral malaria in adults in an endemic area of Tanzania: implications and clinical description. QJM. 2003;96(5):355–362. doi:10.1093/qjmed/hcg059
36. Jiya N, Airede K, Ahmed H. Cerebral malaria: presentation and outcome in children in Sokoto. Niger Med Pract. 2006;50(3):55–61.
37. Organisation mondiale de la Santé. La prise en charge du paludisme grave: guide pratique. Organisation mondiale de la Santé. Available from: https://apps.who.int/iris/handle/10665/87012.
38. Romay-Barja M, Jarrin I, Ncogo P, et al. Rural-Urban differences in household treatment-seeking behaviour for suspected malaria in children at Bata District, Equatorial Guinea. PLoS One. 2015;10(8):e0135887. doi:10.1371/journal.pone.0135887
39. Imananagha K. The presenting complaints of children with severe malaria. Ann Trop Med Public Health. 2010;3:68–71.
40. Schot MJC, Dekker ARJ, Giorgi WG, et al. Diagnostic value of signs, symptoms and diagnostic tests for diagnosing pneumonia in ambulant children in developed countries: a systematic review. NPJ Prim Care Respir Med. 2018;28(1):40. doi:10.1038/s41533-018-0104-8
41. Shah SN, Bachur RG, Simel DL, Neuman MI. Does this child have pneumonia? The rational clinical examination systematic review. JAMA. 2017;318(5):462–471. doi:10.1001/jama.2017.9039
42. Azmatullah A, Qamar FN, Thaver D, Zaidi AK, Bhutta ZA. Systematic review of the global epidemiology, clinical and laboratory profile of enteric fever. J Glob Health. 2015;5(2):020407. doi:10.7189/jogh.05.020407
43. Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE. Does this child have appendicitis? JAMA. 2007;298(4):438–451. doi:10.1001/jama.298.4.438
44. Kalan M, Talbot D, Cunliffe WJ, Rich AJ. Evaluation of the modified Alvarado score in the diagnosis of acute appendicitis: a prospective study. Ann R Coll Surg Engl. 1994;76(6):418–419.
45. Bessat C, Zonon NA, D’Acremont V. Large-scale implementation of electronic Integrated Management of Childhood Illness (eIMCI) at the primary care level in Burkina Faso: a qualitative study on health worker perception of its medical content, usability and impact on antibiotic prescription and resistance. BMC Public Health. 2019;19(1):449. doi:10.1186/s12889-019-6692-6
46. Blanchet K, Lewis JJ, Pozo-Martin F, et al. A mixed methods protocol to evaluate the effect and cost-effectiveness of an Integrated electronic Diagnosis Approach (IeDA) for the management of childhood illnesses at primary health facilities in Burkina Faso. Implement Sci. 2016;11(1):111. doi:10.1186/s13012-016-0476-5
47. Sarrassat S, Lewis JJ, Some AS, Somda S, Cousens S, Blanchet K. An Integrated eDiagnosis Approach (IeDA) versus standard IMCI for assessing and managing childhood illness in Burkina Faso: a stepped-wedge cluster randomised trial. BMC Health Serv Res. 2021;21(1):354. doi:10.1186/s12913-021-06317-3
48. Mbugua PK, Otieno CF, Kayima JK, Amayo AA, McLigeyo SO. Diabetic ketoacidosis: clinical presentation and precipitating factors at Kenyatta National Hospital, Nairobi. East Afr Med J. 2005;82(12 Suppl):S191–6. doi:10.4314/eamj.v82i12.9381
49. Usher-Smith JA, Thompson M, Ercole A, Walter FM. Variation between countries in the frequency of diabetic ketoacidosis at first presentation of type 1 diabetes in children: a systematic review. Diabetologia. 2012;55(11):2878–2894. doi:10.1007/s00125-012-2690-2
50. Munoz C, Floreen A, Garey C, et al. Misdiagnosis and diabetic ketoacidosis at diagnosis of type 1 diabetes: patient and caregiver perspectives. Clin Diabetes. 2019;37(3):276–281. doi:10.2337/cd18-0088
51. Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med. 1994;38(8):1091–1110. doi:10.1016/0277-9536(94)90226-7
52. Romay-Barja M, Cano J, Ncogo P, et al. Determinants of delay in malaria care-seeking behaviour for children 15 years and under in Bata district, Equatorial Guinea. Malar J. 2016;15:187. doi:10.1186/s12936-016-1239-0
53. Dawkins B, Renwick C, Ensor T, Shinkins B, Jayne D, Meads D. What factors affect patients’ ability to access healthcare? An overview of systematic reviews. Trop Med Int Health. 2021;26(10):1177–1188. doi:10.1111/tmi.13651
54. Sandy JL, Besancon S, Sidibe AT, Minkailou M, Togo A, Ogle GD. Rapid increases in observed incidence and prevalence of Type 1 diabetes in children and youth in Mali, 2007–2016. Pediatr Diabetes. 2021;22(4):545–551. doi:10.1111/pedi.13191
55. Hogerzeil HV, Recourt S. The importance of insulin donations for children in 43 low- and middle-income countries. J Public Health Policy. 2019;40(2):253–263. doi:10.1057/s41271-018-00159-w
56. Choleau C, Maitre J, Elie C, et al. Effet a un an de la campagne nationale de prevention de l’acidocetose au moment du diagnostic de diabete de type 1 chez l’enfant et l’adolescent [Ketoacidosis at time of diagnosis of type 1 diabetes in children and adolescents: effect of a national prevention campaign]. Arch Pediatr. 2015;22(4):343–351. doi:10.1016/j.arcped.2014.11.001
57. Cherubini V, Marino M, Carle F, Zagaroli L, Bowers R, Gesuita R. Effectiveness of ketoacidosis prevention campaigns at diagnosis of type 1 diabetes in children: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;175:108838. doi:10.1016/j.diabres.2021.108838
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
